Abstract
Background
In Coronavirus disease 2019 (COVID-19), some patients have low oxygen saturation without any dyspnea. This has been termed “happy hypoxia.” No worldwide prevalence survey of this phenomenon has been conducted. This review aimed to summarize information on the prevalence, risk factors, and outcomes of patients with happy hypoxia to improve their management.
Methods
We conducted a systematic search of electronic databases for all studies published up to April 30, 2022. We included high-quality studies using the Newcastle-Ottawa Scale (NOS) tool for qualitative assessment of searches. The prevalence of happy hypoxia, as well as the mortality rate of patients with happy hypoxia, were estimated by pooled analysis and heterogeneity by I2.
Results
Of the 25,086 COVID-19 patients from the 7 studies, the prevalence of happy hypoxia ranged from 4.8 to 65%. The pooled prevalence was 6%. Happy hypoxia was associated with age > 65 years, male sex, body mass index (BMI)> 25 kg/m2, smoking, chronic obstructive pulmonary disease, diabetes mellitus, high respiratory rate, and high d-dimer. Mortality ranged from 01 to 45.4%. The pooled mortality was 2%. In 2 studies, patients with dyspnea were admitted to intensive care more often than those with happy hypoxia. One study reported that the length of stay in intensive care did not differ between patients with dyspnea and those with happy hypoxia at admission. One study reported the need for extracorporeal membrane oxygenation (ECMO) in patients with happy hypoxia.
Conclusion
The pooled prevalence and mortality of patients with happy hypoxia were not very high. Happy hypoxia was associated with advanced age and comorbidities. Some patients were admitted to the intensive care unit, although fewer than dyspneic patients. Its early detection and management should improve the prognosis.
Keywords:
Introduction
Coronavirus Disease 2019 (COVID-19) is a contagious disease that first appeared in Wuhan, China in late December 2019. It is caused by the virus called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a highly transmissible virus.Citation1,Citation2 The disease has spread all over the world, considered a pandemic by the WHO since March 11, 2020.Citation3 As of August 30, 2022, the world has 599,071,265 confirmed cases and 6,467,023 deaths.Citation4 The clinical forms can be asymptomatic, mild, moderate, severe, and critical.Citation5 Although pulmonary manifestations are common, the disease can affect several organs of the body.Citation6
The main symptoms of Coronavirus disease 2019 (COVID-19) are fever, cough, and dyspnea.Citation7 Some patients present with dyspnea in the setting of severe respiratory distress with a drop in oxygen saturation or oxygen partial pressure.Citation8 Despite the absence of dyspnea, some patients with COVID-19 may have a markedly reduced oxygen saturation as measured by pulse oximetry. This phenomenon is referred to as “silent hypoxia or happy hypoxia”.Citation9 Each time there has been a major wave of COVID-19, medical facilities have been overwhelmed, resulting in a rapid increase in the number of patients receiving home treatment. As a result, several deaths were recorded among patients treated at home, which became a social problem. Happy hypoxia has been one of the causes of death in COVID-19 patients receiving home care, as the absence of respiratory difficulty despite the presence of hypoxemia delays the seeking of medical care.Citation10
The prevalence of COVID-19 patients with happy hypoxia was variable depending on the definitions of happy hypoxia used, the age of the patients, comorbidities, and the regions where the studies were conducted.Citation11 The prevalence ranged from 31.9 to 65% in EuropeCitation11,Citation12 and from 4.8 to 21. 5% in Asia.Citation10,Citation13,Citation14 The prevalence was 4.8 in one American countryCitation15 and 6% in one African country.Citation16 A systematic review would be beneficial in aggregating these disparities in prevalence. In addition, some studies have shown that patients with both COVID-19 and happy hypoxia are known to have poor outcomes.Citation11,Citation16 Therefore, hypoxemia in patients with COVID-19 without dyspnea should be identified and monitored carefully.
It is important to identify risk factors for hypoxemia in patients with COVID-19 without dyspnea. No worldwide prevalence survey of this phenomenon has been conducted. This review aimed to summarize information on the prevalence, risk factors, and outcomes of patients with happy hypoxia in order to improve their management.
Methods
Search Strategy and Study Selection
Relevant studies will be identified through a search of MEDLINE, Europe PMC, and the Cochrane Library. The following will be the primary search terms in MEDLINE: ((“COVID-19” [Title/Abstract]) OR (“SARS-CoV-2” [Title/Abstract]) OR (“coronavirus” [Title/Abstract]), which will be cross-referenced to the terms (“happy hypoxia” [Title/Abstract]) OR (“silent hypoxia” [Title/Abstract]) The search will be in the English language. The search period runs from December 1st, 2019 to April 1st, 2022. The site preprints.org will search for preprints using the terms “COVID-19” or “Coronavirus.” Official reports from medical societies, governmental institutes, and registries will also be manually searched and included if they match the inclusion criteria. The protocol was recorded on PROSPERO CRD42022293727.
Inclusion Criteria
Design
All observational studies report the prevalence, risk factors, and outcomes of happy hypoxia in COVID-19.
Study setting
Worldwide.
Population
All hospitalized patients infected with COVID-19
Publication status
All published and unpublished articles.
Language
Only studies reported using the English language.
Publication date
Published from the December 1st, 2019 to April 30, 2022
Exclusion Criteria
Patients who had received oxygen prior to hospitalization.
Data Extraction
Two independent investigators assessed the results of the initial search for the title and abstract relevancy. The whole text was checked to see if it met the eligibility criteria. Duplicate articles, reviews, editorials, case reports, family studies, and publications that exclusively report on pediatric cases will be eliminated. Clinical studies that did not explicitly state death as a possible outcome will be ruled out. Furthermore, if a single author published two or more studies on the same patient sample, only the highest-quality publication was considered. Authors, year of publication, nation, study design, study location (number of study sites), sample size, age, sex, outcome, the definition of happy hypoxia, and proportion of happy hypoxia will be all included on data extraction forms. Two investigators (researchers with a master’s degree in medicine or the humanities and clinical research experience) independently obtained this information. A third investigator double-checked the list of papers and data to make sure there were no duplicates and to rule out any inconsistencies.
Risk of Bias (Quality) Assessment
The Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of the included retrospective cohort studies based on three primary components: study patient selection which is worth up to 4 points, and adjustment for potential confounding variables which are worth up to 2 points, and outcome measurement which is worth up to 3 points.Citation17 Each study can receive a maximum of nine points based on this scale. Articles with a NOS score of 5 were deemed high-quality publications in this study. The quality assessment was conducted by two reviewers. Disagreements were handled by discussion among reviewers, with the assistance of a third party if necessary to reach a consensus.
Statistical Analysis and Data Synthesis
We will extract the authors, year of publication, nation, study design, study location (number of study sites), sample size, age, sex, the definition of happy hypoxia and proportion of happy hypoxia, gender (male/female), patient comorbidities, and outcome. We performed a meta-analysis of proportions (and 95% CI) for the prevalence of COVID-19 patients with happy hypoxia. The statistical heterogeneity among the included studies will be measured by the Cochran’s Q with the p-value, and the extent of heterogeneity attributable to heterogeneity will be measured by the I2 statistic. The descriptive analyses will be performed using Stata version 14.
Results
Search Results and Study Selection
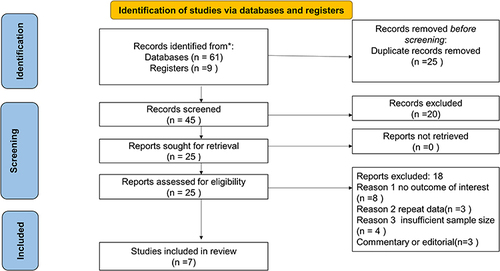
Through electronic database searches and registries, a total of 70 records were collected, with 25 records being eliminated before screening owing to duplication. Then, out of the 45 articles found, 20 were eliminated due to irrelevant titles, abstracts, or texts. A total of 25 papers were chosen for the full-text review, with 18 being deleted due to the lack of a result of interest, repeat data, or insufficient sample size. Finally, the research looked at seven studies ().
Quality Assessment
The methodological quality was high and the risk of bias was low, with a median Newcastle-Ottawa scale score of 77% (extreme values 77–88%). The detailed quality assessment of all included studies can be found in Appendix A.
Study Characteristics
In total, 7 studiesCitation10–16 were included in the review. The time period for the studies was 2020–2021. All studies were published between 2020 and 2021. The studies had sample sizes ranging from 141 to 21,544. One study was conducted in Africa (DRC);Citation16 two studies in Europe, France, and Italy,Citation11,Citation12 three studies in Asia (Japan, India, and Saudi Arabia);Citation10,Citation13,Citation14 and one study in the Americas (Mexico).Citation15 In addition, only one study was prospective,Citation15 and the rest were retrospective cohorts. Six studies took place in a single hospital, while one study in Japan involved Japanese national registries.Citation10 Two studies defined happy hypoxia with an oxygen saturation threshold < 90%, two studies with a threshold < 94%, one study with a threshold< 95% also combining Pa O2 and PCO2, one study used the saturation threshold < 80%, one used the PaO2/Fi02 ratio < 300 mm Hg ()
Table 1 Study Characteristics of COVID-19 Patients with Happy Hypoxia
Prevalence
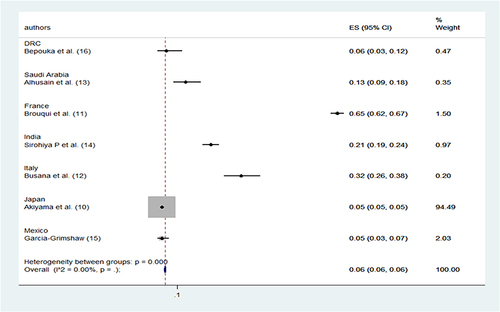
All studies reported the prevalence of happy hypoxia (). The prevalence varied from 4.8 to 65% for all definitions. In a 2020 study, Brouqui et al used an oxygen saturation of 93% as a definition. et al reported in the 2nd largest cohort a very high prevalence of 65% of happy hypoxia situations (). The pooled prevalence of the 7 studies is 6% ().
Table 2 Prevalence of Happy Hypoxia in COVID-19
Risk Factors
Brouqui et alCitation11 discovered risk factors for poor clinical outcomes during follow-up (death/transfer to ICU) in patients without dyspnea. Hypoxemia/hypocapnia syndrome (yellow dots) was clustered with death/ICU, elevated NEWS score, age, male, and elevated D-dimers. Hypoxia/hypocapnia was linked to aging, maleness, and chronic heart disease but not to type 2 diabetes. Death/ICU was strongly associated with hypoxemia/hypocapnia syndrome (OR 95% CI: 4.37; 2.12–9.03) (p= 0.0001), as were elevated D-dimers > 2.5 mg/l (OR 95% CI: 6.26; 1.99–19.75) (p = 0.002). Sirohiya et alCitation14 found that multivariable logistic regression models were fitted to calculate the odds of death with silent hypoxia as the explanatory variable and other clinical, laboratory, and treatment parameters as covariates. We found that though these models showed a higher odds of death among patients with silent hypoxia, none of them were statistically significant. Akiyama et alCitation10 found that hypoxemia without dyspnea was associated with age > 65 years (95% CI: 2.920–4.350, p < 0.001), male sex (95% CI: 1.070–1.600, p = 0.0087), BMI > 25 kg/m2 (95% CI: 1.160–1.500, p = 0.036), chronic obstructive pulmonary disease (COPD) (95% CI: 1.300–3.100, p = 0.002), other chronic lung disease (95% CI: 1.060–3.400, p = 0.031), and diabetes mellitus (CI: 1.240–1.850, p < 0.001). The hypoxemia without dyspnea group had a greater median respiratory rate (RR) than the control group (31/min vs 18/min, p=0.001).
Mortality
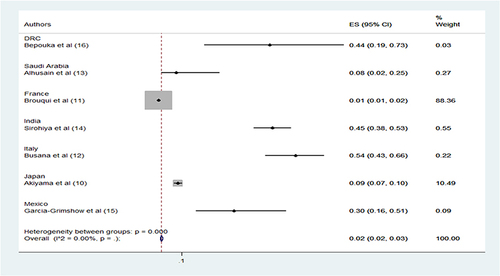
All studies revealed mortality rates among patients with happy hypoxia. Mortality ranged from 1 to 45.4%. The study with a mortality of 45.4% used SpO2 < 94% as a criterion (). The pooled mortality rate of the studies was 2% ().
Table 3 Mortality of Patients with Happy Hypoxia
Other Outcomes of Patients with Happy Hypoxia
Five studies reported other outcomes.Citation10–13,Citation15 Four studies reported admission to ICU.Citation11-13,Citation15 According to studies by Alhusain et al, patients with dyspnea were admitted to ICU more frequently than those with happy hypoxia (107 (64%) versus 9 (36%), p = 0.007); and Brouqui et al (31(5.1%) versus 16 (1.4%), p=0.001). For the other 3 studies, the difference was not significant.Citation5,Citation8 Alhusain et alCitation13 reported that the length of stay in the ICU did not differ between dyspnea and happy hypoxia on admission. Patients with dyspnea had a longer length of stay, though the difference was not statistically significant (2 (22%) vs 37 (35%), p=0.783). One study reported the need for ECMO.Citation10 ECMO was used more frequently in patients with happy hypoxia in Japan, 57 (5.1%) vs 221 (1%) (Akiyama et al). ().
Table 4 Other Outcomes of Patients with Happy Hypoxia
Discussion
To our knowledge, this was the first large-scale systematic review on the prevalence and outcome of COVID-19 patients with happy hypoxia. This is an understudied topic, with only eight studies specifically reporting the prevalence, risk factors, and outcome of COVID-19 patients with happy hypoxia. Of these, by far the largest cohort was from Japan.
The prevalence of happy hypoxia depends on the definition of happy hypoxia used. Considering all the definitions used, the prevalence of happy hypoxia ranged from 4.8% to 65%. The pooled prevalence was 6%. The highest prevalence of 65% was reported in the study in France, where oxygen saturation of less than 95% was considered in the definition of happy hypoxia. In the same study, in the subset of patients with at least one blood gas analysis (n = 161) who did not have dyspnea on admission, 28.1% had hypoxemia/hypocapnia syndrome, defining asymptomatic hypoxia.Citation11 This value is still higher than the pooled prevalence in this systematic review. There were 2 studies reporting a low prevalence of 4.8%.Citation10,Citation15 One of these studies from Japan had happy definitions of hypoxia with a value of less than 94% while the other study from Mexico had a threshold of less than 80%, which could also explain the low prevalence. Compared with the results of a recent systematic review and meta-analysis on hypoxia in children infected with COVID-19 in low and moderate resource settings, considering the definition of hypoxia with saturation below 90%, the pooled prevalence was 31%.Citation18 When compared to patients with hypoxia and dyspnea who were intubated, the prevalence of patients with hypoxia who were intubated was 28% (95% CI 20%-38%, I 2 = 63%). with a mortality rate of 14% (95% CI 7.4–24.4%) among these patients.Citation19
Early intubation in COVID-19 has not shown many benefits. The literature does not find significant differences in mortality between the early intubation group and never intubated patients.Citation20
Akiyama et al found that hypoxemia without dyspnea was associated with age > 65 years, male sex, BMI > 25 kg/m2, smoking history, chronic obstructive pulmonary disease (COPD), another chronic lung disease, and diabetes mellitus.Citation10 These same factors are associated with severe forms and mortality related to COVID-19. Patients with COVID-19 with any of these characteristics may have hypoxemia and remain non-dyspneic. Thus, close monitoring of these patients is necessary. Specifically, they should be provided with transcutaneous oximeters so that they can monitor their own SpO2 regularly. Brouqui et al also found that patients with happy hypoxemia were elderly and chronically ill. Diabetic patients were 1.8 times more likely to have poor respiratory perception than non-diabetic controls and therefore had the lowest scores.Citation11 It is well recognized that chronic conditions like diabetes and aging can desensitize the respiratory center, which can lead to happy hypoxia.Citation21
The hypoxemia without dyspnea group had a greater median respiratory rate (RR) than the control group (31/min vs 18/min, p= 0.001). This finding implies that tachypnoea is an important indicator of hypoxemia, even in the absence of dyspnea. Furthermore, RR is an indicator of severe dysfunction in many-body systems, not just the respiratory system.Citation22 It is therefore important that COVID-19 patients and their families know how to predict hypoxemia, even without transcutaneous oximetry, to ensure prompt medical management before the disease becomes severe.Citation10 Brouqui et al found that factors associated with poor clinical outcomes during follow-up (death/transfer to ICU) among patients without dyspnea Hypoxemia/hypocapnia syndrome were clustered with death/ICU, elevated NEWS score, age, male, and elevated D-dimers.Citation11 Hypoxemia and elevated D-dimers strongly suggest that the resulting lung damage is due in part to arterial microemboli and might explain the severity of clinical presentation and the subsequent death. These findings reinforce the recommendation to apply thrombosis prophylaxis in these patients.Citation23
Anticoagulants are crucial for treating microvascular and microvascular thrombosis and inflammation in COVID-19 patients.Citation24–26 They also prevent the development of DIC,Citation27 and they help to reduce mortality.Citation28,Citation29 The 28-day mortality was consistently lower in those who got anticoagulation compared to those who did not use.Citation30,Citation31
All studies showed mortality rates among patients with happy hypoxia. Mortality ranged from 1 to 45.4%. The study with a mortality of 45.4% used SpO2 < 94% as a criterion. The pooled mortality of the studies was 2%. A high mortality of 45.4% was found in the Sirohoya study in India.Citation14 Similarly, a study in the UK reported room air oxygen saturation as a significant predictor of patient outcome and mortality.Citation32 This is also confirmed by a Peruvian study reporting that oxygen saturation below 90% on admission was a significant predictor of in-hospital mortality in patients with COVID-19.Citation33 Another study concluded that low oxygen levels on admission were strongly associated with more critical illness and mortality.Citation34 The mortality rate for COVID-19 patients with severe disease can reach 61%.Citation35,Citation36 The primary factor is progressive hypoxia, which damages multiple associated organs, including the lungs.Citation7 The use of standard mechanical ventilation in COVID-19 patients can result in mortality of up to 86%, in contrast to usual ARDS.Citation37–39 Before the advanced stage of COVID-19, when edema and shunt develop, High flow nasal oxygen (HFNO) should be taken into account as a superior option for early oxygen therapy. Supraglottic jet oxygenation and ventilation (SJOV) is an option, but more research is required to substantiate it.Citation40
Four studies reported admission to the ICU.Citation11–13,Citation15 According to studies by Alhusain et al (107 (64%) versus 9 (36%), p = 0.007) and Brouqui et al (31(5.1%) versus 16 (1.4%), p=0.001),Citation11,Citation13 patients with dyspnea were admitted to ICU more frequently than those with happy hypoxia. For the other 3 studies, the difference was not significant. According to Alhusain et al, the length of stay in the intensive care unit did not differ between dyspnea and happy hypoxia on admission. Patients with dyspnea had a longer length of stay, though the difference was not statistically significant (2 (22%) versus 37 (35%), p= 0.783). Two studies reported the need for ECMO.Citation4,Citation7 In Japan, ECMO was used more often in patients with happy hypoxia. 57(5.1) vs 221(1).Citation10 The use of ECMO in severe COVID-19 patients seems to be the same as it is in ARDS patients that are not COVID-19.
The length of ECMO seems to be longer than in non-COVID-19 ARDS, and older age is a determinant in death.Citation41
This lack of breathlessness deserves medical attention and should not be taken as a good sign of well-being. We suggest that for these patients with “mild clinical presentation”, it is particularly important to routinely achieve oxygen saturation by full pulse oximetry with blood gas analysis, if necessary, to allow early diagnosis of asymptomatic hypoxia and more appropriate management to reduce the poor outcome.Citation11
Limitations
Our systematic review had several limitations. First, we only included studies written in English. Secondly, another limitation in assessing prevalence is that the definition of happy hypoxia was inconsistent as there is not yet a standardized and validated definition. Some studies used different values of saturation, others used either PaO2 or the PaO2/FiO2 ratio. Finally, because the articles included are limited to a few nations, the global figure may not be accurate.
Conclusions
The pooled prevalence and mortality of patients with happy hypoxia were not very high. Happy hypoxia was associated with advanced age and comorbidities. Some patients were admitted to the intensive care unit, although fewer than dyspneic patients. Its early detection and management should improve the prognosis.
Abbreviations
COVID-19, Coronavirus Disease 1; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; NEWS, National Early Warning Score; BMI, body mass index; NOS, Newcastle–Ottawa Scale; CI, confidence interval; OR, odds ratio.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no competing interests in this work.
References
- Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi:10.1001/jama.2020.4344
- Gable L, Courtney B, Gatter R, et al. Global public health legal responses to H1N1. J Law Med Ethics. 2011;39(Suppl1):46–50. doi:10.1111/j.1748-720X.2011.00565.x
- Ucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Bio Med Atenei Parmensis. 2020;91(1):157‐160.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. Accessed August 30, 2022.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. PMID: 32109013; PMCID: PMC7092819. doi:10.1056/NEJMoa2002032
- Johnson KD, Harris C, Cain JK, Hummer C, Goyal H, Perisetti A. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front Med. 2020;7:526. PMID: 32903492; PMCID: PMC7438449. doi:10.3389/fmed.2020.00526
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi:10.1056/NEJMc2010419
- Gallo Marin B, Aghagoli G, Lavine K, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10. doi:10.1002/rmv.2146
- Levitan R. The infection That’s silently killing coronavirus patients; 2010. Available from: https://www.nytimes.com/2020/04/20/opinion/sunday/coronavirus-testing-pneumonia.html/. Accessed April 12, 2022.
- Akiyama Y, Morioka S, Asai Y, et al. Risk factors associated with asymptomatic hypoxemia among COVID-19 patients: a retrospective study using the nationwide Japanese registry, COVIREGI-JP. J Infect Public Health. 2022;15(3):312–314. doi:10.1016/j.jiph.2022.01.014
- Brouqui P, Amrane S, Million M, et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis. 2021;102:233–238. doi:10.1016/j.ijid.2020.10.067
- Busana M, Gasperetti A, Giosa L, et al. Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol. 2021;87(3):325–333. doi:10.23736/S0375-9393.21.15245-9
- Alhusain F, Alromaih A, Alhajress G, et al. Predictors and clinical outcomes of silent hypoxia in COVID-19 patients, a single-center retrospective cohort study. J Infect Public Health. 2021;14(11):1595–1599. doi:10.1016/j.jiph.2021.09.007
- Sirohiya P, Elavarasi A, Sagiraju HKR, et al. Silent Hypoxia in Coronavirus disease-2019: is it more dangerous? -A retrospective cohort study. medRxiv preprint. 2021. doi:10.1101/2021.08.26.21262668
- García-Grimshaw M, Flores-Silva FD, Chiquete E, et al. Characteristics and predictors for silent hypoxemia in a cohort of hospitalized COVID-19 patients. Auton Neurosci. 2021;235:102855. doi:10.1016/j.autneu.2021.102855
- Bepouka B, Situakibanza H, Odio O, et al. Happy Hypoxia in COVID-19 patients at Kinshasa University Hospital (the Democratic Republic of the Congo): frequency and vital outcome. J Biosci Med. 2021;9:12–20. doi:10.4236/jbm.2021.92002
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi:10.1007/s10654-010-9491-z
- Rahman AE, Hossain AT, Nair H, et al. Prevalence of hypoxemia in children with pneumonia in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob Health. 2022;10(3):e348–e359. doi:10.1016/S2214-109X(21)00586-6
- Cardona S, Downing J, Alfalasi R, et al. Intubation rate of patients with hypoxia due to COVID-19 treated with awake proning: a meta-analysis. Am J Emerg Med. 2021;43:88–96. doi:10.1016/j.ajem.2021.01.058
- Mohammadi M, Khafaee Pour Khamseh A, Varpaei HA. Invasive Airway “Intubation” in COVID-19 patients; statistics, causes, and recommendations: a review article. Anesth Pain Med. 2021;11(3):e115868. PMID: 34540642; PMCID: PMC8438719. doi:10.5812/aapm.115868
- O’Donnell CR, Friedman LS, Russomanno JH, Rose RM. Diminished perception of inspiratory-resistive loads in insulin-dependent diabetics. N Engl J Med. 1988;319(November(21)):1369–1373. doi:10.1056/NEJM198811243192102
- Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust. 2008;188(11):657–659. doi:10.5694/j.1326-5377.2008.tb01825.x
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi:10.1016/j.thromres.2020.04.041
- Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. 2014;12:1010–1019. doi:10.1111/jth.12596
- Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015:507151. doi:10.1155/2015/507151
- Hoppensteadt D, Fareed J, Klein AL, Jasper SE, Apperson-Hansen C, Lieber EA. Comparison of anticoagulant and anti-inflammatory responses using enoxaparin versus unfractionated heparin for transesophageal echocardiography-guided cardioversion of atrial fibrillation. Am J Cardiol. 2008;102:842–846. doi:10.1016/j.amjcard.2008.05.025
- Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi:10.1111/jth.14854
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi:10.1111/jth.14817
- Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. doi:10.1136/bmjopen-2017-017046
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi:10.1111/jth.14830
- Hadid T, Kafri Z, Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2021;47:100761. PMID: 33067035; PMCID: PMC7543932. doi:10.1016/j.blre.2020.100761
- Gupta RK, Marks M, Samuels THA, et al. Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: an observational cohort study. Eur Respir J. 2020;56:2003498. doi:10.1183/13993003.03498-2020
- Mejía F, Medina C, Cornejo E, et al. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020;15:e0244171. doi:10.1371/journal.pone.0244171
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369. doi:10.1136/bmj.m1966
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult in-patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi:10.1016/S0140-6736(20)30566-3
- Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi:10.1001/jama.2020.1585
- Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi:10.1007/s00134-020-06033-2
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi:10.1016/S2213-2600(20)30079-5
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi:10.1164/rccm.202003-0817LE
- Jiang B, Wei H. Oxygen therapy strategies and techniques to treat hypoxia in COVID-19 patients. Eur Rev Med Pharmacol Sci. 2020;24(19):10239–10246. PMID: 33090435; PMCID: PMC9377789. doi:10.26355/eurrev_202010_23248
- Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25(1):211. PMID: 34127027; PMCID: PMC8201440. doi:10.1186/s13054-021-03634-1