Abstract
Introduction
Patients with delayed intensive phase sputum conversion have a higher risk of multidrug resistant-tuberculosis (MDR-TB) and poorer treatment outcomes. Both, host (immune response and comorbidity) and pathogen factors play important roles in determining sputum conversion after treatment initiation. Impaired host immune response, especially the cellular components, as defined by the increased pre-treatment level of neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR) and other additional factors, were associated with severe active TB.
Purpose
To evaluate whether impaired immune responses (high pre-treatment level of NLR and MLR) and other factors associate with delayed sputum conversion at the end of the intensive phase treatment.
Patients and Methods
This was a case–control study from 2016 to 2020, which retrospectively analyzed the pre-treatment level of NLR, MLR and other factors among patients with new cases of pulmonary tuberculosis (PTB).
Results
A total of 62 patients (31 cases and 31 control). The cut-off value of high pretreatment level of NLR and MLR was 5.065 and 0.585, respectively. Bivariate analysis showed that pretreatment NLR ≥5.065 (OR 8.23, CI 95% 2.48–27.32, p < 0.001), MLR ≥0.585 (OR 10.18, 95% CI 3.13–33.18, p < 0.001) and BMI <18.5 (OR 2.91, 95% CI 1.03–8.20, p = 0.041) were associated with an increased risk of delayed sputum conversion. Multivariate analysis, however, showed that pretreatment NLR ≥5.065 was not significantly associated with delayed sputum conversion (AOR 3.370, 95% CI 0.71–15.91, p value 0.125). A high pretreatment of MLR (AOR 30.802, 95% CI 3.22–287.55, p value 0.003) and lower BMI (AOR 10.942, 95% CI 1.121–98.563, p value 0.033) were significantly associated with an increased risk of delayed intensive phase sputum conversion.
Conclusion
High MLR pretreatment and a low BMI were significantly associated with an increased risk of delayed sputum conversion at the end of the PTB intensive phase treatment. High NLR pretreatment, smoking, diabetes, and HIV were not associated with sputum conversion.
Introduction
Tuberculosis (TB) is the leading cause of death from an infectious disease and remains as a major health problem worldwide, with a persistently high annual incidence and mortality rate, according to the World Health Organization (WHO) annual report. Another disease burden was the significant proportion (7.3–25.4%) of pulmonary tuberculosis (PTB) patients with positive sputum who did not achieve sputum conversion at the end of intensive phase treatment.Citation1–3 Delayed intensive phase sputum conversion has a high risk of multidrug resistant-tuberculosis (MDR-TB) and poorer treatment outcome.Citation4 Immune response, comorbidity and pathogen factors play important roles in determining sputum conversion after treatment initiation. An adequate host immune response and other factors such as body mass index (BMI), diabetes, human immunodeficiency virus (HIV), and smoking were paramount to contain the infection as well as to achieve the target of treatment. Impaired host immune response, especially the cellular components, as defined by increased pre-treatment level of neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR) and other additional factors were associated with severe active TB,Citation5,Citation6 higher retreatment rate,Citation7 and higher mortality rate.Citation8,Citation9 Furthermore, if high pre-treatment levels of NLR and MLR are associated with delayed sputum conversion at the end of intensive phase treatment, it could be preferred in practice as a simple, cheap and effective alternative method to monitor routinely for TB patients.
Methods
Study Design and Ethics
This was a matched case–control study, which retrospectively evaluated the pre-treatment level of NLR and MLR of new PTB cases at a pulmonary outpatient clinic in Sanglah Hospital and Wangaya Hospital from 2016 to 2020. This study was approved by the Faculty of Medicine Udayana University’s ethical review board with register No: 349/UN14.2.2.VII.14/LT/2021. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants.
Patient and Public Involvement
It was not appropriate or possible to involve patients or the public in the design, conduct, reporting or dissemination plans of our research.
Population
The inclusion criteria of cases was patients aged ≥18 years old, who were treated as new case PTB with WHO standard regimen (fixed dose combination) for 2 months (intensive phase) with good adherence, and did not show sputum conversion at the end of the intensive phase treatment. The inclusion criteria for controls are similar with the cases, except for the observed sputum conversion at the end of the intensive phase treatment. The exclusion criteria included patients that did not receive a WHO standard TB regimen, patients who were resistant to rifampicin, patients with secondary bacterial infections, heart diseases, autoimmune diseases, malignancy, and incomplete data of pre-treatment NLR and MLR. Control subjects were matched with case subjects (1:1), according to age (5-year age range) and sex. The confounding factors that were controlled by analysis including, diabetes, HIV, body mass index (BMI), and smoking habit.
Procedures
Patients’ characteristics as well as the pre-treatment level of NLR and MLR were obtained from medical records. The initial sputum evaluation to diagnose PTB is done using the Gene-Xpert MTB/RIF assay. Sputum evaluation after the intensive phase treatment is done using the acid-fast bacilli smear.
Statistical Analysis
The cut-off point of high pre-treatment levels of NLR and MLR was determined from ROC curve analysis. The Chi-square test was done and statistical significance was declared at p < 0.05. Multiple logistic regression was used to evaluate high pre-treatment levels of NLR, MLR and other associated factors with sputum conversion. All of the statistical analysis was analyzed with the software Statistical Package for the Social Sciences (SPSS) version 20.0.
Result
We found a total of 73 patients with delayed intensive phase sputum conversion from 2016 to 2020. Exclusion was made for 2 patients due to having secondary bacterial infections, 1 patient with a malignancy, and 39 patients without complete data of pre-treatment NLR, MLR and other factors. The number of patients with delayed sputum conversion that enrolled to this study was 31.
Thirty-one subjects with sputum conversion and matched by age and gender were enrolled as controls ().
Patients’ characteristics are shown in . The majority of our patients were male, with the mean age in the case group being 40.55 ± 16.06 (19–75) and 40.71 ± 15.26 (18–76) in the control group. The mean of body mass index of patients in the case group was 18.18 ± 2.92 (13.1–23.7) and it was 19.24 ± 4.89 (18.3–27.67) in the control group. The mean of pre-treatment NLR in the case group was 8.74 ± 3.87 (1.47–17.4) and the patients in the control group had a lower mean of pre-treatment NLR of 6.24 ±4.50 (1.3–23.3); this difference was statistically significant. The case group also had a significantly high mean of pre-treatment MLR (0.97 ±0.77 (0.28–4.70)) compared to the control group (0.45±0.19 (0.13–0.90)).
Table 1 The Characteristics of Subjects According to Case and Control Groups
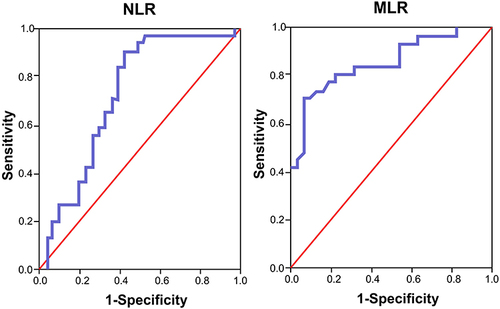
The cut-off point of high pre-treatment NLR and MLR was determined using the analysis of the ROC curve. The best cut-off point for high pre-treatment NLR and MLR was 5065 (sensitivity 83.9%, specificity 61.3%, AUC 0.725, IK 95% 0.594–0.856, p value 0.002) and 0.585 (sensitivity 80.6%, specificity 77.4%, AUC 0.855, 95% CI 0.760–0.950, p value 0.049), respectively ().
Figure 2 Receiver operating characteristics (ROC) curve of high pre-treatment NLR and MLR as risk factors of delayed intensive phase sputum conversion.
A high pre-treatment NLR was found in 83.9% of the case group and in 38.7% of the control group and was associated with a delayed intensive phase sputum conversion (OR 8.23, 95% CI 2.482–27.317, p value <0.001). A high pre-treatment MLR was found in 80.6% of the case group and showed a significant association with the delay of intensive phase sputum conversion (OR 10.18, 95% CI 3.125–33.188, p value <0.001). Body mass index was further categorized into <18.5 and ≥18.5 (kg/m) and we found BMI <18.5 was associated with a delay in the intensive phase sputum conversion (OR 2.908,95% CI 1031–8204, p value 0.041). Diabetes mellitus, HIV infection, and smoking were not significantly associated with a delay in the intensive phase sputum conversion. The data were analyzed using the Chi-square test to determine the association between the dependent and independent variables with a significant value <0.05. The results of the analysis can be seen in .
Table 2 Bivariate Analysis of NLR, MLR, and Other Variables as Risk Factors for Delayed Intensive Phase Sputum Conversion
Multivariate analysis was done by multiple logistic regressions to consider factors such as NLR, MLR, BMI, smoking, diabetes and HIV in order to deduce the extent to which each of these variables correlates with risk factors for delayed intensive phase sputum conversion. We found high pre-treatment NLR was not associated with a delayed intensive phase sputum conversion, after adjustment by confounding factors. A high pre-treatment MLR showed consistent association with a delayed intensive phase sputum conversion in multivariate analysis (AOR 30.80, 95% CI 3.299–287.558, p value 0.003). A low level BMI (<18.5 kg/m) also showed significant association with a delayed intensive phase sputum conversion in multivariate analysis (AOR 10.942, 95% CI 1.121–98.563, p value 0.033) ().
Table 3 Multivariate Analysis of High Pre-treatment NLR and MLR as Risk Factors for Delayed Intensive Phase Sputum Conversion
Discussion
A high NLR on active PTB was associated with high neutrophil and low lymphocyte counts, that were further associated with extensive pulmonary destruction and persistent infection.Citation10 In our study, the cut-off point of a high pretreatment NLR (5.065) was found in 83.9% of the case group and significantly associated with a delayed intensive phase sputum conversion in bivariate analysis (OR 8.23, 95% CI 2.482–27.317, p value <0.001). This cut-off point was higher than those found by Yin et al in 2017 (2.53), which was associated with retreatment in PTB patients. A study to evaluate the prognostic performance of hematologic indices to predict the outcome of intensive phase treatment was done previously by Stefanescu et al,Citation11 however in their study the cut-off point of NLR was showing inadequate performance (AUC<7). Based on an ROC curve analysis, our cut-off point showed a fair value of AUC (0.725). However, in our study high pre-treatment NLR was, not showing consistent association with delayed intensive phase sputum conversion in multivariate analysis. This finding signified the non-specific nature of NLR as an inflammatory marker, in which the high level of NLR in our study might be affected by other condition such as malnutrition, HIV, or diabetes.
All of our subjects with low BMI (54.8% of the case group) showed high pre-treatment levels of NLR. This finding is supported by previous studies which found high NLR in pulmonary TB patients who were was associated with malnutrition and pulmonary cavities.Citation12,Citation13 Patients with diabetes showed significantly higher levels of NLR compared to the non-diabetic patients, which represented the continuous low grade inflammation in patients with diabetes.Citation14 In our study, 84% patients with diabetes also showed high pretreatment NLR, nonetheless, diabetes was not associated with delayed intensive phase sputum conversion in our study. This result was possibly due to a smaller sample size than the previous studiesCitation15 and the possible role of the degree of glycemic control in our patients. Patients with HIV-TB co-infections, as well as HIV patients with uncontrolled disease also showed a high NLR level.Citation16 HIV infection by itself has been shown as a strong predictor of active TB disease and a poor TB treatment outcome.Citation17,Citation18 On the contrary, in our study HIV was not associated with a delayed intensive phase sputum conversion, which is possibly due to a smaller number of HIV patients involved in our study, therefore it was inadequate to detect the different outcomes of TB treatment between HIV patients and non-HIV patients.
In our study we found that high pre-treatment MLR (≥0.585) was significantly associated with an increased risk of delayed sputum conversion at the end of the intensive phase treatment (OR 10.18, 95% CI 3.125–33.188, p value <0.001), even after adjustment for confounding factors (AOR 30.80, 95% CI 3.299–287.558, p value 0.003). This result is supported by the results of a prior molecular study of monocyte which showed that the function of monocyte as a defence mechanism against MTB was mainly described by the level of MLR, rather than its absolute count.Citation19 High levels of MLR in patients with active TB is associated with an increased level of type I IFN, which lead to a poor phagocytosis function, necrosis of macrophage, and bacterial expansion. An extreme level of immune response, both inadequate and excessive, was associated with the growth of mycobacterium.Citation20 A previous study showed that patients with active TB disease were having either lower (<9th percentile) or higher (>25th percentile) levels of MLR compared to healthy subjects.Citation21 On the other hand, infection by MTB per se would cause a change of monocyte subsets balance in peripheral blood, which favoured the expansion of CD16+ subsets, resulting in the growth of bacteria and progression of the disease.Citation22 A study by Stefanescu et al,Citation11 was also aimed at evaluating the prognostic performance of MLR to predict the outcome of intensive phase treatment, which found patients with delayed intensive phase sputum conversion showed persistently high MLR after two months of treatment. Nonetheless, pre-treatment MLR was not associated with the outcome of intensive phase treatment in that study, due to the inadequate performance of MLR based on the result of ROC curve analysis.
Low BMI (<18.5 kg/m2) at diagnosis was associated with an increased risk of delayed intensive phase sputum conversion (OR 2.90, 95% CI 1031–8204, p value 0.041, AOR 10,942, 95% CI 11,215–98,563, p value 0.033). This result is similar with the findings of a previous study that also showed poor nutrition (BMI <18.5 kg/m2) at diagnosis and after 2 months of treatment was associated with poor treatment outcome (treatment failure, relapse).Citation23 Nutrition plays a paramount role in maintaining and enhancing the immune response, both innate and adaptive, against pathogens, including MTB. The presence of malnutrition would impair the function of macrophages, the complement system, dendritic cells, and lymphocytes, which would further cause an increase of susceptibility to tuberculosis infection and reactivation of any latent infection.Citation24 Patients with low BMI appeared to have a low level of pro-inflammatory cytokines and a high level of regulatory cytokine, the opposite condition was found in patients with higher BMI, which conferred protective benefits of high BMI to TB disease progression.Citation25 However, high BMI was also associated with an increased risk of diabetes, which subsequently negates the beneficial effect of higher BMI to reduced the risk of TB infection and disease progression.Citation26,Citation27
A limitation of our study was the small sample size, owing to the design of this study, which obtained the result of hematologic examination from medical records, meanwhile this examination was not routinely performed in Wangaya Hospital. This caused substantial exclusions of subjects with delayed intensive phase sputum conversion in whom pretreatment complete blood count examination was not performed. A further prospective study with a bigger sample size is needed in the future to evaluate the factors associated with delayed sputum conversion to enhance the cure rate of PTB.
Conclusion
High pretreatment levels of MLR and a low BMI were significantly associated with an increased risk of delayed sputum conversion at the end of the intensive phase treatment of pulmonary TB. High pretreatment levels of NLR, smoking, diabetes, and HIV were not associated with an increased risk of a delay in the intensive phase sputum conversion.
Disclosure
The authors report no conflicts of interest in this work.
References
- Calderwood CJ, Wilson JP, Fielding KL, et al. Dynamics of sputum conversion during effective tuberculosis treatment: a systematic review and meta-analysis. PLoS Med. 2021;18(4):e1003566. doi:10.1371/journal.pmed.1003566
- Gunda DW, Nkandala I, Kavishe GA, Kilonzo SB, Kabangila R, Mpondo BC. Prevalence and risk factors of delayed sputum conversion among patients treated for smear positive PTB in northwestern rural Tanzania: a retrospective cohort study. J Trop Med. 2017;2017:1–5. doi:10.1155/2017/5352906
- Su WJ, Feng JY, Chiu YC, Huang SF, Lee YC. Role of 2-month sputum smears in predicting culture conversion in pulmonary tuberculosis. Eur Respir J. 2011;37(2):376–383. doi:10.1183/09031936.00007410
- Shah NS, Wright A, Bai GH, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13(3):380–387. doi:10.3201/eid1303.061400
- Wang W, Wang L, Liu Y, Yang F, Zhu L. Value of the ratio of monocytes to lymphocytes for monitoring tuberculosis therapy. Can J Infect Dis Med Microbiol. 2019;2019:3270393.
- Eliaw A. The utility and validity of immunological, inflammatory, and nutritional-based scores and indices in active pulmonary tuberculosis. Int Clin Pathol J. 2018;6:199–213. doi:10.15406/icpjl.2018.06.00188
- Kayigamba FR, Bakker MI, Mugisha V, et al. Adherence to tuberculosis treatment, sputum smear conversion and mortality: a retrospective cohort study in 48 Rwandan clinics. PLoS One. 2013;8(9):e73501. doi:10.1371/journal.pone.0073501
- Yin Y, Kuai S, Liu J, et al. Pretreatment neutrophil-to-lymphocyte ratio in peripheral blood was associated with pulmonary tuberculosis retreatment. Arch Med Sci. 2017;13(2):404–411. doi:10.5114/aoms.2016.60822
- Han Y, Kim SJ, Lee SH, et al. High blood neutrophil-lymphocyte ratio associated with poor outcomes in miliary tuberculosis. J Thorac Dis. 2018;10(1):339–346. doi:10.21037/jtd.2017.12.65
- La Manna MP, Orlando V, Dieli F, et al. Quantitative and qualitative profiles of circulating monocytes may help identifying tuberculosis infection and disease stages. PLoS One. 2017;12(2):e0171358. doi:10.1371/journal.pone.0171358
- Stefanescu S, Cocos R, Turcu-Stiolica A, et al. Evaluation of prognostic significance of hematological profiles after the intensive phase treatment in pulmonary tuberculosis patients from Romania. PLoS One. 2021;16(4):e0249301. doi:10.1371/journal.pone.0249301
- Nakao M, Muramatsu H, Arakawa S, et al. Immunonutritional status and pulmonary cavitation in patients with tuberculosis: a revisit with an assessment of neutrophil/lymphocyte ratio. Respir Investig. 2019;57(1):60–66. doi:10.1016/j.resinv.2018.08.007
- Duman TT, Aktas G, Atak BM, Kocak MZ, Erkus E, Savli H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afri Health Sci. 2019;19(1):1602–1606. doi:10.4314/ahs.v19i1.35
- Chiang CY, Bai KJ, Lin HH, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. 2015;10(3):e0121698. doi:10.1371/journal.pone.0121698
- Caetano Mota P, Carvalho A, Valente I, Braga R, Duarte R. Predictors of delayed sputum smear and culture conversion among a Portuguese population with pulmonary tuberculosis. Rev Port Pneumol. 2012;18(2):72–79. English, Portuguese. doi:10.1016/j.rppneu.2011.12.005
- Cailleaux-Cezar M, Loredo C, Silva JRLE, Conde MB. Impact of smoking on sputum culture conversion and pulmonary tuberculosis treatment outcomes in Brazil: a retrospective cohort study. J Bras Pneumol. 2018;44(2):99–105. doi:10.1590/s1806-37562017000000161
- Merriman RC, Dissanayake O, Alnjar S, Burns F, Miller RF. Incidence and significance of elevated platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios among hospitalized HIV-positive adult patients. Int J STD AIDS. 2019;30(13):1329–1332. doi:10.1177/0956462419868881
- Raviglione MC. Tuberculosis. In: Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 20th ed. New York: McGraw Hill; 2018:1236–1243.
- Rees CA, Pineros DB, Amour M, et al. The potential of CBC-derived ratios (monocyte-to-lymphocyte, neutrophil-to-lymphocyte, and platelet-to-lymphocyte) to predict or diagnose incident TB infection in Tanzanian adolescents. BMC Infect Dis. 2020;20:609. doi:10.1186/s12879-020-05331-w
- MbatchouNgahane BH, Ebenezer AT, Eveline ND, et al. Diagnostic value of leukocyte count abnormalities in newly diagnosed tuberculosis patients. Open J Respir Dis. 2020;10:1–10. doi:10.4236/ojrd.2020.101001
- Moreira-Teixeira L, Mayer-Barber K, Sher A, O’Garra A. Type 1 interferons in tuberculosis: foe and occasionally friend. J Exp Med. 2018;215(5):1273–1285. doi:10.1084/jem.20180325
- Tobin DM, Roca FJ, Oh SF, et al. Host-genotype-specific therapies can optimize the inflammatory response to mycobacterial infection. Cell. 2012;148(3):434–446. doi:10.1016/j.cell.2011.12.023
- Wang J, Yin Y, Wang X, et al. Ratio of monocytes to lymphocytes in peripheral blood in patients diagnosed with active tuberculosis. Braz J Infect Dis. 2015;19(2):125–131. doi:10.1016/j.bjid.2014.10.008
- Lastrucci C, Benard A, Balboa L, et al. Tuberculosis is associated with expansion of a motile permissive and immunomodulatory CD16+ monocyte population via the IL-10/STAT3 axis. Cell Res. 2015;25(12):1333–1351. doi:10.1038/cr.2015.123
- Sahile Z, Tezera R, Marlam DH, Collins J, Ali JH. Nutritional status and TB treatment outcomes in Addis Ababa, Ethiopia: an ambi-directional cohort study. PLoS One. 2021;16(3):e0247945. doi:10.1371/journal.pone.0247945
- Chandrasekaran P, Saravanan N, Bethunaickan R, Tripathy S. Malnutrition: modulator of immune response in tuberculosis. Front Immunol. 2017;8:1316. doi:10.3389/fimmu.2017.01316
- Kubiak RW, Sarkar S, Horsburgh CR, et al. Interaction of nutritional status and diabetes on active and latent tuberculosis: a cross-sectional study. BMC Infect Dis. 2019;19:267. doi:10.1186/s12879-019-4244-4