Abstract
Purpose
The accurate detection of antibiotic susceptibility of Neisseria gonorrhoeae (N. gonorrhoeae) is of great importance for the treatment of patients with gonorrhea as well as to hinder the progress of drug resistance. To promote the application of gonococcal antibiotic susceptibility monitoring in primary hospitals and remote medical institutions, this study evaluated the effect of alternative growth supplements on the antibiotic susceptibility testing of N. gonorrhoeae isolates.
Methods
We divided the antimicrobial-containing media into three groups by adding different growth supplements (sterile defibrinated sheep blood, bovine hemoglobin, and Vitox). We tested the antimicrobial susceptibility of 80 N. gonorrhoeae isolates in different groups against eight antibiotics. Nonparametric signed-rank tests were utilized to compare the minimum inhibitory concentration (MIC) results of each group. Taking the MIC results of Vitox group as expected, the essential agreement (EA) and category agreement (CA) of the other two groups were calculated.
Results
For the group using sheep blood as growth supplements, the EA values and CA values of each antibiotic were above 90.00% and minor error rates were less than 7.00%. No very major error and major error were observed. For the group using hemoglobin as growth supplements, the EA values of the susceptibility results of zoliflodacin, penicillin, and ceftriaxone were lower than 90.00%. The overall MIC results of using hemoglobin as a growth supplement were higher than those of sheep blood and Vitox in the susceptibility testing of these three antibiotics.
Conclusion
Compared with the expected results, sheep blood may be considered for the use as an alternative material for N. gonorrhoeae antibiotics susceptibility surveillance, while hemoglobin may not be suitable for supplement to antimicrobial-containing medium.
Introduction
Neisseria gonorrhoeae (N. gonorrhoeae) is the etiologic agent of gonorrhea, a sexually transmitted infection (STI) that remains a major global public health concern.Citation1 According to the WHO estimation, there were 87 million global incidents of gonorrhea annually.Citation2 In 2021, China reported 127,803 new cases of gonorrhea.Citation3 Currently, the treatment of N. gonorrhoeae infection mainly depends on a variety of antibiotics.Citation4 Notably, as the susceptibility of N. gonorrhoeae to various antibiotics decreases with time, multidrug-resistant (MDR) N. gonorrhoeae cases have been reported intermittently.Citation5 Hence, the detection of antibiotic susceptibility of N. gonorrhoeae is of great importance for the accurate treatment of patients with gonorrhea as well as to hinder the progress of drug resistance.Citation6
In 1990, the WHO Global Gonococcal Antimicrobial Surveillance Program (WHO GASP) was established to monitor gonococcal antimicrobial resistance (AMR) worldwide.Citation7 China-GASP was initiated in 1987 by the National Center for Sexually Transmitted Disease Control (NCSTD).Citation8 According to the laboratory diagnosis guidelines of WHO and EUCAST,Citation9,Citation10 the methods currently used for determining MICs include the agar dilution method and E-test method. In the CLSI guidelines, disk diffusion or agar dilution MIC tests were used for routine clinical testing.Citation11 Disk diffusion only enabled qualitative testing of antimicrobial resistance.Citation12 Agar dilution is the most widely used method and the “gold standard” method for the determination of the MIC of gonococcal isolates against antimicrobial drugs.Citation13
In the agar dilution method, the MIC determination is conducted in an antimicrobial-containing medium, which is a mix of GC agar base supplemented with a 1% defined growth supplement and antimicrobial solutions.Citation8,Citation11 From the data uploaded by various monitoring points in China, methodological differences among local laboratories and research institutions were summarized. In the standard operation of the agar dilution method, 1% Vitox was recommended as a growth supplement, while sterile defibrinated sheep blood and bovine hemoglobin were used as substitutes for Vitox in some hospitals and research institutions.Citation14,Citation15 For those institutions, the possible reasons for choosing sheep blood or hemoglobin as growth supplements were that these are more accessible and less costly. Hemoglobin was also used as a nutrient supplement in the Thayer Martin Agar used to isolate and culture N. gonorrhoeae.Citation16
To assess possible systematic errors among the monitoring points and to find suitable growth supplement alternatives, we conducted this study to evaluate the accuracy for various experimental materials of antibiotic susceptibility testing of N. gonorrhoeae.
Methods
Bacterial Isolates
The N. gonorrhoeae isolates we used were 80 clinical gonococcal isolates from China Gonococcal Resistance Surveillance Program (China-GRSP). WHO N. gonorrhoeae reference isolates and ATCC 49226 were used for quality assurance. All the strains were previously determined to be N. gonorrhoeae through standardized methodologies.Citation8 The Medical Ethics Committee at the Institute of Dermatology, the Chinese Academy of Medical Sciences & Peking Union Medical College and the National Center for Sexually Transmitted Disease Control all gave their approval to this project (2014-LS-026). This declaration of Helsinki complied in this study. Study participants signed an informed consent form before inclusion in the study. Before the antimicrobial agent susceptibility testing, all of the strains were preserved in skim milk at −80℃.
Resuscitation and Antimicrobial Susceptibility Testing
The effect of different growth supplements on the MIC of eight antibiotics (zoliflodacin, penicillin, gentamicin, tetracycline, spectinomycin, azithromycin, ciprofloxacin, and ceftriaxone) on N. gonorrhoeae isolates was determined. For this GC agar base (OXOID, USA) supplemented with 10% sterile defibrinated sheep blood (Lezhen, China) or 2% bovine hemoglobin (BD, USA) or 1% Vitox (OXOID, USA) were added separately to the media containing different concentrations of the antibiotics. Thereafter, 1 µL of bacterial suspension was transferred onto the agar surface using a replicator. Eventually, each medium was cultured for 18–24 hours at 36°C in a 5% CO2-enriched atmosphere. The growth of N. gonorrhoeae in different groups was observed and recorded. All steps strictly followed the WHO standard operation of the agar dilution method except for the other two additional growth supplements (sheep blood and hemoglobin).Citation9 Each test was repeated for each bacteria strain to confirm the results.
Statistical Analysis
The MIC results of N. gonorrhoeae to the eight antibiotics were recorded. Graphpad Prism ver.6 Software (GraphPad Software, San Diego, CA, USA) was utilized to analyze all statistics. The phenotype characteristics of reference strains that were applied as the quality assessment of this experiment were gathered in the WHO global GASP.Citation10 The percentage of MIC results of each antibiotic in different growth supplement groups was calculated. To assess the accuracy of each group, the MIC results of Vitox group were used to calculate the essential agreement (EA) and category agreement (CA). EA was defined as the percentage of strains with MIC results within plus or minus one doubling dilution step from the expected MIC result. CA was defined as the agreement of susceptible, intermediate and resistant results between MIC test and reference MIC result. The reproducibility was addressed with EA values and the performance of each group with CA values.Citation11 Very major errors were resistant strains being misclassified as susceptible. Major errors were susceptible strains being misclassified as resistant. Minor errors occurred when one method categorized an isolate as intermediate while the other method defined it as susceptible or resistant.Citation12 Breakpoints from Clinical and Laboratory Standards Institute (CLSI) Antimicrobial Susceptibility Testing GuidelinesCitation13 were used to classify the N. gonorrhoeae isolates as sensitive, intermediately resistant, or resistant to antibiotics. The non-parametric Mann–Whitney U-test was implemented to examine the differences between MICs of eight antibiotics in different growth supplement.Citation14
Results
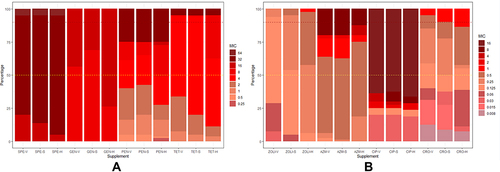
A total of 80 N. gonorrhoeae isolates were tested for antibiotic susceptibility on antimicrobial-containing media supplemented with different growth supplements. During each batch of drug susceptibility testing, the MIC values of the reference strains were within 1 doubling dilution of those previously reported. The overall performances for eight antimicrobials are shown in . For the group using sheep blood as growth supplements, the EA values and CA values of each antibiotic were above 90.00% (EA values ranged from 90.00% to 100.00%, CA values ranged from 93.75% to 100.00%). No very major error and major error was observed, and minor error rates were less than 7.00% for the sheep blood group (minor error rates ranged from 0.00% to 6.25%). The EA values and CA values of gentamicin and spectinomycin were 100.0%. For the group using hemoglobin as growth supplements, the essential agreement values of the susceptibility results of zoliflodacin, penicillin, and ceftriaxone were 38.75%, 81.25%, and 60.00%, all lower than 9000%. A major error was observed in the susceptibility results of ciprofloxacin.
Table 1 Categorical and Essential Agreements of Two Different Growth Supplements for 8 Antibiotics
Comparing the results of the three drugs (zoliflodacin, penicillin, and ceftriaxone) with large differences between the two supplements in , the overall MIC results of using hemoglobin as a growth supplement were higher than those of sheep blood and Vitox in the drug susceptibility testing of zoliflodacin, penicillin, and ceftriaxone (P ≤ 0.05). For example, in the susceptibility results of zoliflodacin, the percentage of ≥0.5 mg/L in the hemoglobin group was 42.50%, while those in the Vitox and sheep blood group were 0.00% and 5.00%. An isolate with an MIC value of 1mg/L was observed in the results of the hemoglobin group. In the susceptibility results of ceftriaxone, the percentage of ≥0.5 mg/L in the hemoglobin group was 42.60%, while those in the Vitox and sheep blood group were 15.00% and 22.50%. Similar results were found for the results of penicillin ().
Table 2 The Percentage (%) of N. Gonorrhoeae Isolates with Different MICs (Mg/L) for Eight Antibiotics in Different Groups
Figure 1 (A and B) The proportions of N. gonorrhoeae isolates with different MICs for eight antibiotics in different groups. The MIC50 (yellow-dotted line), MIC90 (red-dotted line) and number of isolates per province are shown. The block color represents the MIC value and the block length represents the percentage. The vertical coordinate represents the antibiotics of different growth supplement groups.
Discussion
As a result of the significant decline in the efficacy of available antimicrobials, N. gonorrhoeae has been identified as an emerging public health problem. Consequently, it is necessary to detect the trend of N. gonorrhoeae antimicrobial resistance (AMR) for controlling the gonorrhea epidemic.Citation15 In China, the N. gonorrhoeae resistance surveillance program has been carried out since 1987. By comparing the susceptibility testing methods at various participating clinics in China, we found that there were differences among STD laboratories. Previous studies have proven that the results of the agar dilution method can be affected by many factors: the composition of the agar medium, pH-value, and culture parameters.Citation16 Thus, despite the MICs estimated by different laboratories being comparable, random errors and systematic errors in the values due to technical nuances can also affect the clinical interpretation. Therefore, exploring the effect and the accuracy of different experimental materials on the MIC testing of N. gonorrhoeae isolates is of great meaning for the accurate detection of N. gonorrhoeae AMR.
In this study, we aimed to compare the effect of different growth supplements in the antimicrobial-containing media to the MIC results of N. gonorrhoeae and to find suitable growth supplement alternatives. The results demonstrate that sheep blood as an alternative nutrient showed good categorical and essential agreements in multiple antibiotics’ susceptibility testing results, and no very major error and major error was observed in the results of the sheep blood group. While in the hemoglobin group, there were three groups of antibiotics susceptibility results less than accepted percentages (defined as >90% for categorical agreements and <7% for minor errors).Citation12 After comparing the results of the three antibiotics with large differences between two supplements, we also found that the MIC results of the hemoglobin group were higher than those of the sheep blood group and the Vitox group. The results suggested that sheep blood as an alternative growth supplement showed good reproducibility in the susceptibility testing of N. gonorrhoeae, while hemoglobin may not be suitable for a supplement to antibiotics-containing media.
Such a discrepancy between hemoglobin and Vitox in these three antibiotics could arise because of two reasons. The first is the characteristics of the drugs. The main component of Vitox is vitamin B12, adenine, L-glutamine, guanine, aminobenzoic acid, cystine, cysteine, etc., while the main component of sheep blood is hemoglobin, serum protein, vitamin B, lysine, glutamic acid, folic acid, etc.Citation17 In hemoglobin supplements, it is the only substance, which is mainly composed of aspartic acid, leucine and glutamic acid. Therefore, when sheep blood and Vitox are added, some of the components may interact with the drug. Another reason is the impact of resistance mechanisms. According to the guidelines of CLSI,Citation18 the use of a cysteine-free growth supplement is required for agar dilution tests with carbapenems and clavulanate. The main reason is that cysteine is related to the drug resistance of these two kinds of antibiotics.Citation19 Therefore, we suspect that there is a similar situation in this experiment.
During our retrieval of relevant literature, this is the first study comparing the accuracy of alternative growth supplements like sheep blood and hemoglobin with the standard material in the antibiotic susceptibility testing in N. gonorrhoeae. The results of this study provided a database for the use of sheep blood as an alternative growth supplement. Considering that sheep blood is less expensive and more easily available than Vitox, for some primary or remote institutions, the use of sheep blood may be suitable for use as an experimental material for N. gonorrhoeae antibiotics susceptibility surveillance. Under strict quality assurance, including the use of reference strains as quality control in each experiment and the MIC results of the reference strains within 1 doubling dilution of the panel, sheep blood could be used as an alternative growth supplement to Vitox.
In this study, we provided the first exploratory results. Nevertheless, there were also some limitations in our study. First, the sample size of this study is limited, a large panel of clinical isolates and more antibiotic-resistant isolates are needed to verify the trend we have found. Second, the function of specific components of each growth supplement is out of the scope of this study. We did not analyze the possible reasons for the results differences among the three growth supplements in terms of the composition and background. A possible cause of the higher results in hemoglobin medium may be the lower concentration of antibiotics.Citation20,Citation21 According to the CLSI M100 Performance Standards, cysteine-containing defined growth supplement will significantly alter dilution test results with carbapenems and clavulanate. Hence, the role of the specific components in different growth supplements by the control variable method will be our next focus.
Conclusion
In conclusion, this study highlights the effects of different growth supplements on antibiotic susceptibility testing of N. gonorrhoeae. Sheep blood may be considered for use as an alternative material for Vitox in the N. gonorrhoeae antibiotics susceptibility surveillance, while hemoglobin may not be suitable for a supplement to antimicrobial-containing medium.
Data Sharing Statement
The data that supports the findings of this study are available in the submitted article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgments
This work was supported by the National Science and Technology Major Project (2018ZX101010 01-004-003), the Nanjing Incubation Program for National Clinical Research Center (20190600010) and the Natural Science Foundation of Jiangsu Province (BK20180156). The sponsors of this study had no role in the study design, data collection, data analysis, data interpretation or writing of the article.
References
- Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018;16(4):226–240. doi:10.1038/nrmicro.2017.169
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548–562p. doi:10.2471/BLT.18.228486
- NHCot PRC. Overview of the national epidemic of infectious diseases in 2021 [EB/OL]; 2022. Avaialble from: http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml. Accessed September 13, 2022.
- Unemo M, Seifert HS, Hook EW, Hawkes S, Ndowa F, Dillon JR. Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79. doi:10.1038/s41572-019-0128-6
- Dong HV, Klausner JD. Neisseria gonorrhoeae resistance driven by antibiotic use. Nat Rev Urol. 2019;16(9):509–510. doi:10.1038/s41585-019-0206-2
- Seña AC, Bachmann L, Johnston C, et al. Optimising treatments for sexually transmitted infections: surveillance, pharmacokinetics and pharmacodynamics, therapeutic strategies, and molecular resistance prediction. Lancet Infect Dis. 2020;20(8):e181–e191. doi:10.1016/S1473-3099(20)30171-7
- Unemo M, Ison CA, Cole M, Spiteri G, van de Laar M, Khotenashvili L. Gonorrhoea and gonococcal antimicrobial resistance surveillance networks in the WHO European Region, including the independent countries of the former Soviet Union. Sex Transm Infect. 2013;89(Suppl 4):iv42–iv46. doi:10.1136/sextrans-2012-050909
- Unemo M, Ballard R, Ison C, et al. Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus; 2013.
- Wagenlehner FM, Brockmeyer NH, Discher T, Friese K, Wichelhaus TA. The presentation, diagnosis, and treatment of sexually transmitted infections. Dtsch Arztebl Int. 2016;113(1–2):11–22. doi:10.3238/arztebl.2016.0011
- Unemo M, Golparian D, Sánchez-Busó L, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother. 2016;71(11):3096–3108. doi:10.1093/jac/dkw288
- Chen SC, Liu JW, Wu XZ, et al. Comparison of microdilution method with agar dilution method for antibiotic susceptibility test of neisseria gonorrhoeae. Infect Drug Resist. 2020;13:1775–1780. doi:10.2147/IDR.S253811
- Xu WQ, Liu JW, Zhu XY, et al. Evaluation of the accuracy of various disks and strips for rapid culture-based gonococcal antimicrobial susceptibility screening tests in China. Infect Drug Resist. 2021;14:5131–5136. doi:10.2147/IDR.S340074
- Humphries R, Bobenchik AM, Hindler JA, Schuetz AN, McAdam AJ. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st edition. J Clin Microbiol. 2021;59(12):Jcm0021321. doi:10.1128/JCM.00213-21
- Xu W, Zhou Q, Liu J, et al. In vitro study of the interaction of gentamicin with ceftriaxone and azithromycin against neisseria gonorrhoeae using agar dilution method. Antibiotics. 2022;11(8):1083. doi:10.3390/antibiotics11081083
- Hook EW, Kirkcaldy RD, Brief A. History of evolving diagnostics and therapy for gonorrhea: lessons learned. Clin Infect Dis. 2018;67(8):1294–1299. doi:10.1093/cid/ciy271
- Wang F, Liu J, Liu H, et al. Evaluation of the accuracy of molecular assays targeting the mutation A2059G for detecting high-level azithromycin resistance in Neisseria gonorrhoeae: a systematic review and meta-analysis. Infect Drug Resist. 2019;12:95–104. doi:10.2147/IDR.S183754
- Anand C, Gordon R, Shaw H, Fonseca K, Olsen M. Pig and goat blood as substitutes for sheep blood in blood-supplemented agar media. J Clin Microbiol. 2000;38(2):591–594. doi:10.1128/JCM.38.2.591-594.2000
- Patel J, Weinstein M, Eliopoulos G, et al. M100 Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute; 2017:240.
- Gupta R, Al-Kharji N, Alqurafi MA, et al. Atypically modified carbapenem antibiotics display improved antimycobacterial activity in the absence of β-lactamase inhibitors. ACS Infect Dis. 2021;7(8):2425–2436. doi:10.1021/acsinfecdis.1c00185
- Steixner SJM, Spiegel C, Dammerer D, Wurm A, Nogler M, Coraça-Huber DC. Influence of nutrient media compared to human synovial fluid on the antibiotic susceptibility and biofilm gene expression of coagulase-negative staphylococci in vitro. Antibiotics. 2021;10(7):790. doi:10.3390/antibiotics10070790
- Bergeron MG, Simard P, Provencher P. Influence of growth medium and supplement on growth of Haemophilus influenzae and on antibacterial activity of several antibiotics. J Clin Microbiol. 1987;25(4):650–655. doi:10.1128/jcm.25.4.650-655.1987
