Abstract
Purpose
Carbapenem-resistant Enterobacteriaceae (CRE) infection has become a concerning threat, especially in hospital settings; however, its phenotypic characterization, association with rectal colonization and subsequent bloodstream infections (BSI) remain to be clarified. This study aimed to investigate the incidence and risk factors of CRE infection in rectal CRE carriers and to understand the clonality of carbapenem-resistant Klebsiella pneumoniae (CRKP) strains and their association with subsequent BSI in these patients.
Patients and Methods
This was a prospectively designed cohort study. Hospitalized patients treated at our institution from April 2019 to October 2020 with intestinal CRE carriage were screened at admission and weekly thereafter until death or discharge from the hospital. Stool and blood samples were obtained for strain growth and mass spectrometry. The colonization and clinical infection isolates were analyzed by antimicrobial susceptibility testing to identify CRE. The clonality of the CRE strains and their corresponding clinical infection strains was studied by whole-genome sequencing to explore the mechanism of drug resistance and evaluate possible transmission. CRE-associated risk factors were analyzed in combination with epidemiological data.
Results
Of the 1203 patients, 85 were colonized by CRE and 21 developed CRE infection, of whom 13 developed CRE bloodstream infection (BSI). Ninety-one CRE strains were isolated from the rectal specimens of the 85 patients. Tracheotomy and chemotherapy in the past three months were independent risk factors for CRE infection in intestinal CRE carriers. ST11-KL64 (92.3%, 24/26) was the most dominant capsule and multilocus sequence typing (MLST) type among clonal CRKP isolates. Single-nucleotide polymorphism clustering showed homology of representative colonization and infection CRKP strain pairs (n=13) in the same patient. One group of leading clones was endemic in surgical intensive care units (ICUs). Twenty-four CRKP strains carried β-lactamase K. pneumonia carbapenemase 2, and 73.1% (19 strains) of CRKP carried mucoid phenotype regulator genes A2 and iucABCD.
Conclusion
In summary, intestinal CRE colonization was detectable at an elevated rate among hospitalized patients and prevalent in ICU patients, with potential rapid horizontal transmission, providing evidence that CRE BSI infection in hospitalized patients might be due to their colonized strains and indicates the correlation between intestinal colonization and BSI.
Introduction
In recent years, infection with carbapenem-resistant Enterobacteriaceae (CRE), especially carbapenem-resistant Klebsiella pneumoniae (CRKP), has been a major public health challenge worldwide due to the widespread use of carbapenems.Citation1 In a nationwide study comprising 25 hospitals across 14 provinces in China, an increasing burden of CRE infection was reported, with an overall CRE infection incidence of 4.05/10,000 discharges, which differed significantly by region, whereby the highest incidence was observed in the Jiangsu province (14.97) and the lowest in Qinghai (0.34).Citation2 The scale of clinical and public health problems caused by multidrug-resistant bacterial infections has escalated, and descriptions such as superbugsCitation3 and nightmare bacteriaCitation4 have reflected the magnitude of the problem of antibiotic resistance.
The intestinal tract of hospitalized patients represents a large repository of antibiotic-resistant bacteriaCitation5 that can migrate to extraintestinal tissues, leading to serious extraintestinal in-hospital systemic infections and death.Citation6 However, considering that not all intestinal CRKP could cause further infection,Citation7 phenotypic and molecular characterization of highly pathogenic CRE with strong virulence and resistance against the host’s immunity should be clarified to prevent their spread, improve their identification and offer timely treatment to high-risk infected patients.
Isolates from ICU departments showed higher or similar resistance rates among Enterobacteriaceae pathogens compared to other wards.Citation8 Over the past decade, CRKP has spread rapidly in several countries, including Greece, and CRKP-related bloodstream infections (BSIs) are increasingly reported worldwide.Citation9 In 2007, the first K. pneumoniae carbapenemase (KPC)-producing CRE strain in China was reported, and blaKPC-2 has since become the most widespread carbapenemase-encoding gene in China and even globally. More and more CRKP isolates were found to carry various carbapenemase genes and virulence genes.Citation10,Citation11 CRE can survive in the gastrointestinal tract of hospitalized patients without causing any symptoms of infection.Citation12 However, colonization is generally thought to precede infection,Citation13 so asymptomatic colonization as an important link in the transmission route may eventually lead to further transmission and outbreaks of pathogens.Citation14
Fecal CRE carriage is reported to be an important risk factor for developing CRE infection, so understanding the rate of fecal CRE carriage is critical for controlling CRE infections. Therefore, the objective of this study was to evaluate the rate of CRE colonization among patients hospitalized at our institutions, identify risk factors associated with rectal CRE colonization, clarify the phenotypic and molecular characterization of CRE, determine the association between rectal CRE colonization and subsequent BSI, and explore the clonality of CRKP strains.
Materials and Methods
Study Design
This study was a prospective study conducted for 48 months at the Second Affiliated Hospital of Anhui Medical University, a tertiary university hospital in Anhui, China. The hospital is a teaching hospital with 3200 beds. A high prevalence of CRE carriers in intensive care units (ICUs) and hematology wards was observed in a previous baseline investigation, so the above departments were considered high-risk sites for CRE transmission and treated as key observation departments.
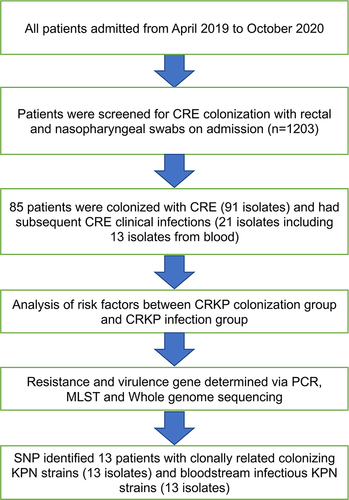
The study inclusion criteria were: (1) patients aged ≥ 18 years old (excluding pregnant women) who agreed to be screened for carbapenem-resistant gram-negative bacteria rectal colonization, (2) hospitalized for at least two days, (3) routinely screened for CRE by rectal swabs on admission and once a week until discharge or infection, (4) diagnosed with CRE based on blood cultures examinations. Patients with a hospital stay ≤ 48 hours and those known to be colonized by CRE prior to admission or identified as colonized within the first 24 hours were excluded from this study. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (YX2022083). The patients or family members provided signed informed consent for study participation.
Patient profiles were collected through questionnaires, and demographic and medical characteristics were recorded, including antibiotic use (at least 1 course of β-lactamase inhibitor + β-lactam combination), broad-spectrum antibiotic use over the past 30 days (cephalosporins, carbapenems, quinolones, and clotrimazole), surgery (past 6 months), invasive procedures (endotracheal intubation and tracheostomy), chronic diseases (such as diabetes, malignancy, blood disorders, and diseases of the central nervous system) and placement of a central venous catheter.
Sample Collection
In this study, the following samples were obtained from eligible patients: perianal swabs, stool and blood. The perianal swabs or stool samples were obtained within 24 hours of admission to detect CRE colonization and then once per week until discharge or death. They were used to assess if the patient carried a CRE strain during admission. The blood samples were obtained when patients had symptoms of infection such as fever and were used for determining whether CRE infection occurs.
The samples were immediately transferred to the hospital’s laboratory for processing and analysis. Screening with only perianal swabs was performed to prevent severe infection in patients with agranulocytosis. Patients with CRE strains in their intestinal specimens were considered CRE carriers or colonizers ().
Figure 1 Hospitalized patients were screened for CRE colonization between 2019 and 2020 (n=1203). The patients were divided into “colonized” and “uncolonized” groups, then further divided into “infected” and “uninfected” groups.
Bacterial Isolates
Stool samples were inoculated on the mSuperCARBA agar (CHROMagarTM, France), and a susceptibility test was performed after the growing strain was confirmed to be Enterobacteriaceae. Blood cultures were obtained from all patients with clinical symptoms of BSI. Only the first positive blood culture sample from each patient was included in the analysis to prevent duplication of data. Bacterial isolates were identified by mass spectrometry (Bruker Autoflex MALDI TOF-MS, Germany).
Antimicrobial Susceptibility
The minimum inhibitory concentrations (MICs) of antimicrobial agents were analyzed using a Vitek 2 Compact instrument (bioMérieux, Marcy L’Étoile, France), except for colistin. Colistin-susceptibility analysis was performed by the broth microdilution method. The quality control strains were ATCC 700603 K. pneumoniae and ATCC BAA-1705 K. pneumoniae. The results were interpreted using the Clinical and Laboratory Standards Institute (CLSI) guidelinesCitation15 breakpoints for all the antimicrobial agents except tigecycline and colistin, which were interpreted using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (10th edition) (http://www.eucast.org/clinical_breakpoints/, accessed on Apr 21, 2021).
Carbapenemase Phenotype and Genotype
ATCC BAA-1705 K. pneumoniae was used as a positive control, and ATCC BAA-1706 K. pneumoniae was used as a negative control. Carbapenemase-encoding genes (blaKPC, blaNDM, blaIMP, blaVIM and blaOXA-48) were identified by PCR.Citation16 PCR products were sequenced by Sangon (Shanghai, China) and analyzed using the BLAST programs (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Whole-Genome Sequencing, Assembly, and Comparative Analysis
A single colony of K. pneumoniae was placed in LB broth, and the culture was shaken overnight at 37°C. Genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB) methodCitation17 with minor modification, then the DNA concentration, quality and integrity were determined by using a Qubit fluorometer (Invitrogen, USA) and a NanoDrop spectrophotometer (Thermo Scientific, USA). Sequencing libraries were generated using a TruSeq DNA Sample Preparation Kit (Illumina, USA) and a Template Prep Kit (Pacific Biosciences, USA). Genome sequencing was then performed by Personal Biotechnology Company (Shanghai, China) using the Pacific Biosciences platform and the Illumina MiSeq platform. Data assembly was performed after adapter contamination removal and data filtering using AdapterRemovalCitation18 and SOAPec.Citation19 The filtered reads were assembled by SPAdesCitation20 and A5-miseqCitation21 to construct scaffolds and contigs. Multilocus sequence typing (MLST) was applied in MLST 2.0 (https://cge.cbs.dtu.dk/services/MLST). Kaptive Web (https://github.com/katholt/Kaptive) was used for capsule serotyping analysis of virulence genes on online databases (http://www.mgc.ac.cn/cgi-bin/VFs/v5/main.cgi). Resistance genes were identified using ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/), and PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/) was used to analyze plasmids.
Single-Nucleotide Polymorphism (SNP) Analysis and Plasmid Alignment Analysis
Using the sequence of strain KPN_FJ723042 as a reference, all strain sequences were submitted to CSI Phylogeny (https://cge.cbs.dtu.dk/services/CSIPhylogeny/) to perform SNP analysis. Analysis results were imported into the FigTree 1.4.3 software (http://tree.bio.ed.ac.uk/software/fifigtree/) to generate the maximum likelihood tree. Strains were grouped according to the relationship between near and far. All evolutionary trees were created using iTOLCitation22 (http://itol2.embl.de).
Statistical Analysis
All analyses were performed using SPSS v.26.0 software (SPSS Inc., Chicago, IL, United States). Continuous variables are expressed as the mean ± SD or median with interquartile ranges (IQRs) according to the normality of the data distribution. Categorical variables were compared using the χ2 tests. Univariate logistic regression was performed on the variables to estimate odds ratios (ORs) and their 95% CIs. Multivariate logistic regression was performed to determine the effects on different risk factors when controlling for other factors. P < 0.05 was used to indicate statistical significance.
Results
CRE Isolates
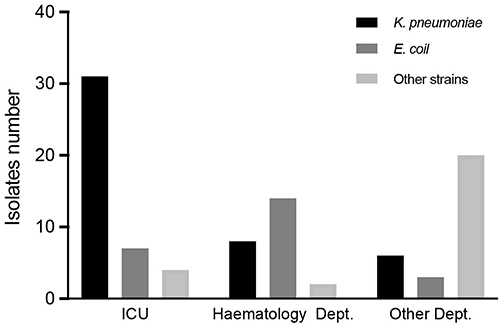
During the study period, we screened CRE strains in 1203 hospitalized patients. Ultimately, 91 CRE strains from 85 patients were included in the study. The data showed an elevated rate of CRE colonization among these hospitalized patients (85/1203, 7.15%). Among these isolates, K. pneumoniae was the most common strain (n=45, 49.45%), followed by Escherichia coli (n=24, 26.37%), Enterobacter cloacae (n=14, 15.38%), Klebsiella aerogenes (n=4, 4.39%), Citrobacter freundii (n=2, 2.19%), and Serratia marcescens (n=2, 2.19%). We identified 42 strains from the ICUs, 20 from the hematology department, and the remaining 29 from other departments ().
Antimicrobial Susceptibility of CRE
According to the CLSI and EUCAST breakpoints, all CRE isolates were resistant to cefazolin, ceftriaxone, cefuroxime, cefoxitin, ceftazidime, cefepime, meropenem, imipenem, amoxicillin/clavulanic and ertapenem, and most CREs were resistant to β-lactam and β-lactam/β-lactamase inhibitor combinations, including piperacillin/tazobactam (96.70%), amikacin (61.54%), levofloxacin (78.02%) and aztreonam (85.71%) (). All the strains were sensitive to polymyxin B and tigecycline.
Table 1 Antimicrobial Susceptibility of Carbapenem-Resistant Enterobacteriaceae Isolated from Fecal Samples
Phenotypic and Molecular Characterization
Ninety-one CRE had detectable carbapenemase genes according to PCR analysis. The 91 carbapenemase-producing CRE were resistant to all three tested carbapenems (imipenem, meropenem, and ertapenem), showing a positive correlation between genotype and phenotype. KPC-2 (53.84%, 49/91) was the most prevalent carbapenemase, followed by NDM-1/5 (42.85%, 39/91), IMP-4 (2.19%, 2/91) and OXA-48 (3.29%, 3/91), including 2 CRE isolates carrying two different carbapenemase genes ().
Table 2 Molecular Characteristics of Carbapenem-Resistant Enterobacteriaceae Isolated from Faecal Samples
Analysis of Risk Factors for CRE Colonization
The results of risk factor analysis for comparing CRKP rectal carriers with non-CRKP rectal carriers are shown in . Variables associated with CRKP rectal carriage development included surgery within 6 months, severe anemia, tracheotomy, and chemotherapy in the past three months. Multivariate logistic regression analysis demonstrated that tracheotomy (OR: 2.80, 95% confidence interval [CI]: (1.11–7.05), p = 0.03) and chemotherapy in the past three months (OR: 0.27, 95% CI: (0.08–0.96), p = 0.04) were independent risk factors for the acquisition of rectal CRE colonization in high-risk patients.
Table 3 Analysis of Risk Factors Between the CRKP Colonization Group and the Non-CRKP Colonization Group
Time-to-Event Analysis
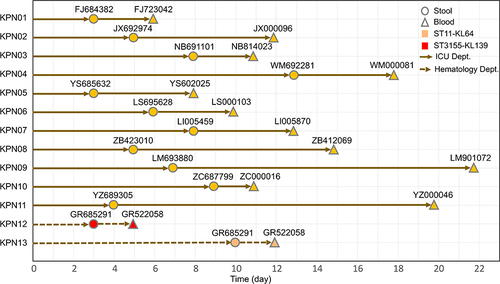
Throughout the study period, a total of 45 patients (55.56%) were detected to have rectal colonization by CRKP. Among the CRKP-colonized patients, 13 (28.89%) developed CRKP BSI after a median time of 7 days (range: 3–13 days), and their risk of developing CRKP-related BSI was much higher than those with colonization by non-CRKP CRE strains. CRKP BSI was mostly observed in patients from the ICU (11/13, 84.61%), followed by the hematology department (2/13,15.38%). In the blood and fecal specimens of these patients, KL64 CRKP isolates were the most common (24/26; 92.31%), followed by KL139 (2/26, 7.70%). According to the MLST typing results, 24 strains (77%, 36/47) colonization and clinically infectious KL64-CRKP strains belonged to the dominant ST11 (92.31%, 24/26), followed by ST3155 (7.70%, 2/26), which belonged to the KL139 serotype ().
Figure 3 Timeline from admission to colonization and subsequent BSI in CRKP colonized patients. The circles represent the day of colonization, while the triangles show the day of BSI. During the study, 13 patients developed BSI from their intestinal colonizing strains, showing a median time of 7 days (range: 3–13 days) from admission to colonization. The median time from CRKP colonization to BSI was 6 days (range, 2–16 days).
Colonization and BSI CRKP Isolate Whole-Genome Sequencing
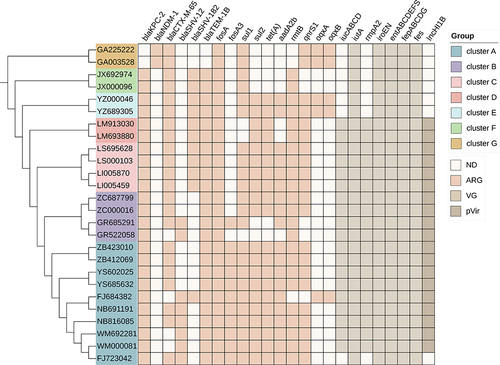
Homology analysis was performed on 26 strains from 13 patients with CRKP BSI. The results showed that clonal-associated colonization and corresponding clinically infectious Klebsiella isolates were found in 84.61% (11/13) of patients. To determine whether the infection was caused by the patient’s own colonizing bacteria, genome-wide sequence analysis was performed on 26 intestinal colonization and BSI CRKP isolated from 13 patients at the same time. The results showed that all were genetically homologous strains, suggesting that horizontal transmission of ST11 might have occurred in the ICU ward across a short distance, and both strains carried the blaKPC-2 gene encoding carbapenem. Among them, the clonal analysis of group A showed that there were 10 ST11 isolates in the group, consisting of colonization strains and clinical isolates from 5 patients in the ICU of internal medicine, and they were all closely related to each other, which indicated that there was a clone of ST11 CRKP in our hospital. Genome analysis of resistance genes, virulence genes, and plasmids showed that all strains carried multiple resistance genes, of which 24 CRKP strains carried both virulence genes and virulence plasmids ().
Figure 4 Core-genome phylogeny of the CRKP isolates, antimicrobial resistance genes, virulence-associated genes and virulence-associated plasmids. The maximum likelihood phylogeny tree was based on single-nucleotide polymorphisms in the core genomes of 26 CRKP isolates. Genome clusters are shaded using different colors. Distribution of virulence-associated genes, antimicrobial resistance genes and virulence-associated plasmid in CRKP strains. Heat maps were generated by aligning the draft genome sequence of each isolate with the sequences deposited in the BIGSdb Klebsiella genome database. The scale bar indicates nucleotide divergence. The origins of the isolates are shown in different colors. Antimicrobial resistance genes, virulence-associated genes and virulence-associated plasmid are shown on the top.
Discussion
CRE rectal colonization was previously identified as an important epidemiological risk factor for subsequent CRE infection;Citation23 however, only a small number of colonized patients eventually develop clinical infection.Citation24 In this study, a total of 85 patients were identified as CRE carriers, showing a detection rate of 7.07%, of whom 24.71% of the carriers were found to have CRE infections. Although CRE infection rates have been reported to vary in individual studies (ranging from 7.6% to 44.4%) in patients with CRE colonization,Citation25 there are also reports suggesting that the overall risk of CRE infection in patients with CRE colonization is 16.5%,Citation26 and individual study infection rates vary, possibly due to differences in individual type, patient population, and clinical setting. Therefore, the overall level of risk remains unclear. Our study provides a comprehensive assessment of infection risk and implications for preventing and treating CRE infections.
Compared to the isolates in a previous study in China,Citation27 the CRE isolates in our study exhibited higher resistance rates to β-lactam antimicrobial agents, including penicillins, carbapenems, cephalosporins, and the monobactam aztreonam. In contrast, the CRE isolates showed a certain level of sensitivity to non-β-lactam antibiotics. A substantial proportion of isolates showed susceptibility to trimethoprim/sulfamethoxazole (70%) and amikacin (36.7%), among others, and although the rate of nonsusceptibility to tigecycline was 15.8%, all the isolates remained susceptible to colistin. Therefore, in some cases, combining colistin and/or tigecycline with these other antimicrobial agents could be considered for treating complex infections caused by CRE.
CRE carriers may develop systemic infections and, in severe cases, might be fatal. Thus, the occurrence of CRE infection should be timely identified to prevent progression to intestinal CRE colonization, which is why this study investigated related clinical predictors and risk factors. Recently published case-control studies suggested that solid organCitation28 stem cell transplantation, mechanical ventilation, fecal incontinence and exposure to carbapenems, vancomycin and metronidazole over the past 30 days were independent factors associated with CRE colonization.Citation29 Comparatively, in this present prospective case-control study, surgery within 6 months, severe anemia, tracheotomy and chemotherapy in the past three months were the reasons for a higher incidence of CRE colonization in these patients, thus demonstrating that the combination of antibiotics is a key factor accelerating the risk of CRE colonization and infection.
Logistic regression analysis showed that tracheotomy and chemotherapy in the past three months were independent risk factors for infection originating from intestinal colonization. The clinical infections in CRKP carriers are consistent with a previous study,Citation30 suggesting that CRKP colonization also requires continual monitoring during patient hospitalization. Tracheotomy is strongly associated with the development of CRE infection in CRE carriers, concordant with another study,Citation31 as it was an independent risk factor, possibly related to the invasive procedures that may disrupt the patient’s natural immune barrier and lead to CRE infection. Moreover, we also found that chemotherapy within the past three months was negatively correlated with CRE colonization after hospitalization. This may be because the chemotherapy process disrupts the adhesion of CRE in the intestine and reduces the probability of CRE colonization in patients, which is similar to the idea proposed by Micozzi et alCitation32 that carriers may have a reduced risk of KPC BSI when an intensive chemotherapy program is appropriately scheduled or implemented. These indicate that different treatment strategies might lead to patients’ disease progressing in different directionsCitation33 but should be further confirmed in colonized patients and infection severity.
As CRKP is commonly observed in hospital settings worldwide, many hospitals have introduced infection control programs to limit infection. Fifteen of the 45 CRKP carriers in this study developed CRKP infection, and 13 were bloodstream infections, which showed a much higher incidence of infection than other CRE carriers. These CRKP carriers developed BSIs of the same strain 2–7 days after detection in the intestine, so CRE is most likely to turn infectious within 2–7 days after colonization. Therefore, it is particularly important to implement proactive screening to reduce the risk of nosocomial transmission caused by CRE colonization or infection by early detecting high-risk patients with CRE infection or colonization and applying appropriate protective measures. It has been reported in literature that BSI-related mortality decreased from 50% to 6% in patients with a high risk of KPC colonization who were actively screened for initial intervention.Citation34 At the same time, according to MLST, of the 26 clonal-associated colonization–infection pairs, ST11 was dominant (92.30%, 24/26) among the 26 CRKP strains (13 clonal-related colonization–infection pairs). Similarly, ST11 was reported to be the most common type of CRKP in China,Citation35–37 and at present, there is a lack of epidemiological information on ST11-KL64 CRKP, although ST11-KL47 CRKP is mostly reported in studies of hospital epidemic transmission in China.Citation35 Notably, research institutes in the Taiwan Province of China detected ST11-KL64 as the most common pod type among CRKP-ST11 (32/40) between 2010 and 2011Citation36. ST11-KL64 produces more biofilm, is more resistant to killing by human neutrophils, and shows a more rapid killing effect in the large wax borer infection model. Moreover, rmpA-rmpA2 coexists on the same plasmid of ST11-KL64 as the regulator of mucoid phenotype gene A (rmpA), which confers more virulence than ST11-KL47 and increases survival in hospital settings.Citation38 Therefore, we believe that ST11-KL64 CRKP might be circulating in our hospital, indicating that bacterial clonality has a significant impact on the clinical development process of patients with intestinal colonization.
In this present study, 24 strains of ST11-CRKP carried KPC-2. ST11 is a monolocus variant of ST258 within the same clonal complex,Citation39 which is considered to be responsible for the global spread of KPC over the past 20 years, with KPC-2 and KPC-3 being the dominant subtypes.Citation40 Therefore, our study concluded that carbapenemase types were closely related to specific STs and that the risk of BSI in KPC-carrying K. pneumoniae rectal carriers was much higher than in NDM-carrying K. pneumoniae carriers. While our molecular results support the association between CRKP colonization and BSI, suggesting that hospitalized patients are infected with their colonized strains, our study highlights the importance of screening for specific carbapenemase types with rectal colonization. Regarding the strains of CRKP in this study, 17 strains of the rmpA2-positive CRKP strain all carried the aerobactin synthetic gene cluster iucABCD-iutA, which may be located in the same plasmid. These CRKP strains all had virulence plasmids that encoded virulence genes, such as the salmonelloxin gene iroEN; thus, these strains can be defined as carbapenem-resistant hypervirulent K. pneumoniae (CR-HvKP).
CR-HvKP has been reported to account for only 3% of infections caused by ST11 CRKP strains across China,Citation41 but the proportion of CR-HvKP in this study was much higher, which might be related to the low sample size in this study. This type of strain can evade the host’s immune response and colonize the intestine, where the probability of colonization and survival is greater. The outbreak of two clonal strains in the ICU is related to CR-HvKP. The CRKP strains in this study carried more resistance genes and higher virulence plasmid loads than other CRE strains, suggesting that they have more opportunities to carry these captured plasmids for a long time and transfer the plasmids to other clinically important gram-negative bacteria,Citation42 a phenomenon that, especially for CRKP carriers, may largely contribute to the spread of resistant strains in hospital settings.
CRKP colonization can persist and spread for years, even among food workers in some developing countries. Fecal carriage rates of multidrug-resistant Enterobacteriaceae are high, signaling the spread of resistant bacteria to hospitals and communities.Citation43 In addition, clonal outbreaks are triggered in long-term care facilities, and patients with new colonization may develop fatal infections.Citation44 In the current coronavirus disease 2019 (COVID-19) era, overuse of antibiotics may increase selection pressures in hospitals and communities and may increase the incidence and transmission of CRE.Citation45
Our study had some limitations. First, the study was conducted in a single center, and the results may not be generalizable to other institutions. Second, the risk factors identified in this study are associated with areas where endemic/persistent CRKP strain outbreaks occur and may not be appropriate for other settings with low KPC prevalence or strains with different resistance mechanisms. Third, this study lacked a detailed assessment of the genomes of each strain, underlying mechanisms, and associated pathways. Based on these preliminary data reported in this study, we aim to perform deeper investigations and compare the genetic homology of CRE colonization isolates from patients with CRE colonization detected from public facilities to obtain additional evidence that could be used to limit transmission by strengthening infection control.
Conclusion
In summary, the rate of CRE rectal colonization was quite high at our institutions, especially in the ICUs, which was found to independently associate with tracheotomy and central venous catheterization and could lead to BSI within 1 week of rectal colonization. Routine clinical screening for rectal carriage of CRE may help reduce the current high rate of hospital-acquired CRE infections. Whole genome sequencing showed that the BSI was caused by genetically homologous strains of the rectal CRE. Horizontal transmission from other patients was also detected, reflecting the epidemic outbreak of ST11-KL64 CRKP clones in our hospital, urging the need for strict infection control measures to prevent this transmission route.
Ethical Approval
The Ethical Committee for the Second Hospital of Anhui Medical University (No. YX2022083) approved the study. All samples were collected as part of routine management/surveillance and were anonymized prior to research use. The research was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi:10.1016/S1473-3099(13)70190-7
- Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2):e01882–17. doi:10.1128/AAC.01882-17
- Jacob JT, Klein E, Laxminarayan R, et al. Vital signs: carbapenem-resistant Enterobacteriaceae. Mmwr Morb Mortal Wkly Rep. 2013;62(9):165–170. doi:10.1186/1471-2458-13-207
- Davies DS Antimicrobial resistance poses ‘catastrophic threat’; 2014. Available from: http://www.facebook.com/EuropeanPharmaceuticalReview. Accessed October 17, 2021.
- Freedberg DE, Zhou MJ, Cohen ME, et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018;44(8):1203–1211. doi:10.1007/s00134-018-5268-8
- Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. doi:10.1128/MMBR.00078-15
- Zhang X, Zhao Y, Wu Q, et al. Zebrafish and galleria mellonella: models to identify the subsequent infection and evaluate the immunological differences in different; Klebsiella pneumoniae intestinal colonization strains. Front Microbiol. 2019;10:2750. doi:10.3389/fmicb.2019.02750
- Xie J, Peters BM, Li B, et al. Clinical features and antimicrobial resistance profiles of important Enterobacteriaceae pathogens in Guangzhou representative of Southern China, 2001–2015. Microb Pathog. 2017;2(107):206–211. doi:10.1016/j.micpath.2017.03.038
- Lee CR, Lee JH, Park KS, et al. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7(895):1–30. doi:10.3389/fmicb.2016.00895
- Peng C, Feng DH, Zhan Y, et al. Molecular epidemiology, microbial virulence, and resistance of carbapenem-resistant Enterobacterales isolates in a teaching hospital in Guangzhou, China. Microb Drug Resist. 2022;28(6):698–709. doi:10.1089/mdr.2021.0156
- Wen S, Feng D, Lu Z, et al. Microbial infection pattern, pathogenic features and resistance mechanism of carbapenem-resistant Gram-negative bacilli during long-term hospitalization. Microb Pathog. 2018;4(117):356–360. doi:10.1016/j.micpath.2018.02.025
- Selden R, Lee S, Wang WL, Bennett JV, Eickhoff TC. Nosocomial Klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med. 1971;74(5):657–664. doi:10.7326/0003-4819-74-5-657
- Munoz-Price LS, Quinn JP. Deconstructing the infection control bundles for the containment of carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2013;26(4):378–387. doi:10.1097/01.qco.0000431853.71500.77
- Jarvis WR, Munn VP, Highsmith AK, Culver DH, Hughes JM. The epidemiology of nosocomial infections caused by Klebsiella pneumoniae. Infect Control. 1985;6(2):68–74. doi:10.1017/s0195941700062639
- CLSI. Performance standards for antimicrobial susceptibility testing. 32nd ed. CLSI Supplement M100. Clinical and Laboratory Standards Institute; 2022.
- Poirel L, Walsh TR, Cuvillier V, et al. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi:10.1016/j.diagmicrobio
- Doyle J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. doi:10.2307/4119796
- Lindgreen S. AdapterRemoval: easy cleaning of next-generation sequencing reads. BMC Res Notes. 2012;2(5):337. doi:10.1186/1756-0500-5-337
- Luo R, Liu B, Xie Y, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1(1):18. doi:10.1186/2047-217X-1-18
- Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021
- Coil D, Jospin G, Darling AE. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics. 2015;31(4):587–589. doi:10.1093/bioinformatics/btu661
- Letunic I, Bork P. Interactive Tree of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39(Web Server issue):W475–W478. doi:10.1093/nar/gkr201
- McConville TH, Sullivan SB, Gomez-Simmonds A, et al. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS One. 2017;12(10):e0186195. doi:10.1371/journal.pone.0186195
- Olivier CN, Blake RK, Steed LL, Salgado CD. Risk of vancomycin-resistant Enterococcus (VRE) bloodstream infection among patients colonized with VRE. Infect Control Hosp Epidemiol. 2008;29(5):404–409. doi:10.1086/587647
- Lübbert C, Becker-Rux D, Rodloff AC, et al. Colonization of liver transplant recipients with KPC-producing Klebsiella pneumoniae is associated with high infection rates and excess mortality: a case-control analysis. Infection. 2014;42(2):309–316. doi:10.1007/s15010-013-0547-3
- Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control. 2016;44(5):539–543. doi:10.1016/j.ajic.2015.12.005
- Zhang Y, Jin L, Ouyang P, et al. Evolution of hypervirulence in carbapenem-resistant Klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J Antimicrob Chemother. 2020;75(2):327–336. doi:10.1093/jac/dkz446
- Prado V, Hernández-Tejero M, Mücke MM, et al. Rectal colonization by resistant bacteria increases the risk of infection by the colonizing strain in critically ill patients with cirrhosis. J Hepatol. 2022;76(5):1079–1089. doi:10.1016/j.jhep.2021.12.042
- Mills JP, Talati NJ, Alby K, Han JH. The epidemiology of carbapenem-resistant Klebsiella pneumoniae colonization and infection among long-term acute care hospital residents. Infect Control Hosp Epidemiol. 2016;37(1):55–60. doi:10.1017/ice.2015.254
- Qin X, Wu S, Hao M, et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis. 2020;221(Suppl 2):S206–S214. doi:10.1093/infdis/jiz622
- Kömürcü B, Tükenmez Tigen E, Toptaş T, et al. Rectal colonization with multidrug-resistant gram-negative bacteria in patients with hematological malignancies: a prospective study. Expert Rev Hematol. 2020;13(8):923–927. doi:10.1080/17474086.2020.1787145
- Micozzi A, Gentile G, Santilli S, et al. Reduced mortality from KPC-K.pneumoniae bloodstream infection in high-risk patients with hematological malignancies colonized by KPC-K.pneumoniae. BMC Infect Dis. 2021;21(1):1079. doi:10.1186/s12879-021-06747-8
- Cano Á, Gutiérrez-Gutiérrez B, Machuca I, et al. Association between rectal colonisation by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae and mortality: a prospective, observational study. J Glob Antimicrob Resist. 2022;29:476–482. doi:10.1016/j.jgar.2021.10.024
- Shu LB, Lu Q, Sun RH, et al. Prevalence and phenotypic characterization of carbapenem-resistant Klebsiella pneumoniae strains recovered from sputum and fecal samples of ICU patients in Zhejiang Province, China. Infect Drug Resist. 2018;12:11–18. doi:10.2147/IDR.S175823
- Liu Y, Liu PP, Wang LH, et al. Capsular polysaccharide types and virulence-related traits of epidemic KPC-producing Klebsiella pneumoniae isolates in a Chinese university hospital. Microb Drug Resist. 2017;23(7):901–907. doi:10.1089/mdr.2016.0222
- Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of carbapenem-resistant enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi:10.1093/cid/ciy660
- Pan YJ, Lin TL, Lin YT, et al. identification of capsular types in carbapenem-resistant Klebsiella pneumoniae strains by wzc sequencing and implications for capsule depolymerase treatment. Antimicrob Agents Chemother. 2015;59(2):1038–1047. doi:10.1128/AAC.03560-14
- Shen P, Xiao T, David S, et al. A novel subclone of carbapenem-resistant Klebsiella pneumoniae St11 emerges with enhanced transmissibility and mortality. SSRN Electronic J. 2018;2. doi:10.2139/ssrn.3238296
- Chen L, Mathema B, Chavda KD, et al. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–696. doi:10.1016/j.tim.2014.09.003
- Migliorini LB, Leaden L, de Sales RO, et al. The Gastrointestinal load of carbapenem-resistant enterobacteriacea is associated with the transition from colonization to infection by Klebsiella pneumoniae isolates harboring the blaKPC gene. Front Cell Infect Microbiol. 2022;12:928578. doi:10.3389/fcimb.2022.928578
- Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. doi:10.1016/S1473-3099(17)30489-9
- Wyres KL, Holt KE. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol. 2018;45:131–139. doi:10.1016/j.mib.2018.04.004
- Amare A, Eshetie S, Kasew D, et al. High prevalence of fecal carriage of Extended-spectrum beta-lactamase and carbapenemase-producing Enterobacteriaceae among food handlers at the University of Gondar, Northwest Ethiopia. PLoS One. 2022;17(3):e0264818. doi:10.1371/journal.pone.0264818
- Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol. 2018;8:4. doi:10.3389/fcimb.2018.00004
- Markovska R, Stankova P, Stoeva T, et al. Fecal carriage and epidemiology of extended-spectrum beta-lactamase/carbapenemases producing enterobacterales isolates in bulgarian hospitals. Antibiotics. 2021;10(6):747. doi:10.3390/antibiotics10060747.