Abstract
Background
Candida spp. are a frequent cause of nosocomial bloodstream infections worldwide.
Objective
To evaluate the use patterns and outcomes associated with intravenous (IV) fluconazole therapy in intensive care units in Spain and Germany.
Patients and methods
The research reported here was a prospective multicenter longitudinal observational study in adult intensive care unit patients receiving IV fluconazole. Demographic, microbiologic, therapy success, length of hospital stay, adverse event, and all-cause mortality data were collected at 14 sites in Spain and five in Germany, from February 2004 to November 2005.
Results
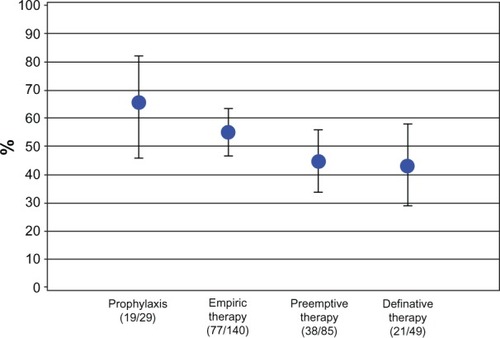
Patients (n = 303) received prophylaxis (n = 29), empiric therapy (n = 140), preemptive therapy (n = 85), or definitive therapy (n = 49). A total of 298 patients (98.4%) were treated with IV fluconazole as first-line therapy. The treating physicians judged therapy successful in 66% of prophylactic, 55% of empiric, 45% of preemptive, and 43% of definitive group patients. In the subgroup of 152 patients with proven and specified Candida infection only, 32% suffered from Candida specified as potentially resistant to IV fluconazole. The overall mortality rate was 42%.
Conclusion
Our study informs treatment decision makers that approximately 32% of the patients with microbiological results available suffered from Candida specified as potentially resistant to IV fluconazole, highlighting the importance of appropriate therapy.
Introduction
Candida spp. are a frequent cause of nosocomial bloodstream infections worldwide.Citation1–Citation7Candida albicans is the most prevalent species found in blood cultures, although an increasing proportion of Candida bloodstream isolates due to non-albicans Candida have also been reported in many countries.Citation8,Citation9 The most frequent non-albicans Candida spp. reported in Spain and Germany have been found to be C. parapsilosis and C. glabrata, respectively.Citation6,Citation10
Despite the frequency of occurrence of invasive candidiasis and the high associated mortality rates, this condition remains difficult to diagnose because of the prevalence of colonization without accompanying infection, nonspecific symptoms, and variable presentation. Diagnosis is therefore increasingly based on surrogate markers such as persistent pyrexia despite broad-spectrum antibiotic use, raised serum C-reactive protein, and the presence of other individual risk factors.Citation1,Citation4,Citation11
Once a diagnosis is made, azoles, polyenes, or echinocan-dins are typically selected for treatment. Many agents have efficacy- or safety-related limitations. Drug resistance and lack of coverage of non-albicans Candida is an issue with fluconazole.Citation8,Citation12,Citation13 Amphotericin B products are associated with renal and other toxicities,Citation14,Citation15 while drug interactions are common with itraconazole and voriconazole.Citation3,Citation16 Once an agent is selected, it is necessary to determine whether the patient requires antifungal prophylaxis, empiric therapy, preemptive therapy, or definitive (sometimes referred to as “targeted”) antifungal therapy.Citation4
Definitive evidence supporting the use of these strategies in the intensive care unit (ICU) is lacking. While a few studies have shown that fluconazole therapy may be beneficial when used in certain critically ill surgical ICU patients,Citation1,Citation17–Citation19 treatment failure was observed more often in studies that also included C. krusei infections.Citation20,Citation21 Moreover, some studies indicate that prophylactic and empiric use of fluconazole may shift the flora towards non-C. albicans with decreased susceptibility to fluconazole.Citation22 In a recently published French study, in vitro susceptibility to fluconazole was higher in patients naive to azole agents (84%) than in patients previously exposed to azole agents (70%).Citation9
The morbidity, mortality, and health care costs associated with invasive fungal infections warrant further exploration in the ICU patient population to determine the effectiveness of prophylaxis as well as empiric, preemptive, and definitive therapies. To begin addressing these issues, we conducted a prospective study to evaluate the utilization patterns and outcomes associated with intravenous (IV) fluconazole therapy within hospital ICUs in Spain and Germany. The epidemiological results as well as clinical and therapeutic aspects of the study are presented in this paper.
Patients and methods
Study design and patient population
This prospective multicenter longitudinal real-world observational study was designed to collect demographic, all-cause mortality, therapy response, and length of hospital stay data on adult ICU patients receiving IV azole therapy for the treatment of fungal infections. The study was conducted at 19 sites, 14 in Spain and five in Germany, from February 2004 to November 2005. Study patients were observed until discharge from the hospital or death, so that the data collection period was dependent on the length of hospital stay. The study was submitted to the responsible ethics committees in accordance with local regulations, and a positive decision (approval) was made: in Germany, by the Ethical Review Board of the Bavarian State Chamber of Physicians, Munich, Germany (Bayerische Landesärztekammer Ethik Kommission Nummer 03099) and, in Spain, by the Ethical Review Board of the of the Bellvitge Hospital, Barcelona, Spain (Comité Ético De Investigación Clínico ref 225/03). Data collection was conducted in accordance with the ethical standards outlined in the Declaration of HelsinkiCitation23 and Guideline for Good Clinical Practice.Citation24
Eligible patients were those who were admitted to the ICU or surgical ICU during the study period, initiated an azole intravenously during ICU/surgical ICU admission, were between 18 and 100 years old, had signed informed consent, and were not on an investigational azole. Attending physicians were responsible for selecting the antifungal therapy.
Study procedures
Efficacy and safety assessments
Internet-based electronic case report forms were used to collect patient data. Demographic characteristics included age, weight, sex, race, and medical history. Antifungal treatment characteristics (prophylaxis; empiric, preemptive, and/or defnitive therapy),Citation4 drugs received, and duration of therapy were recorded. Information on comorbidities and predisposing risk factors was collected. Severity of illness measures, including Acute Physiology and Chronic Health Evaluation (APACHE) II scores, and Sequential Organ Failure Assessment (SOFA) scores, were calculated for all patients at the start of IV fluconazole therapy. Microbiological information included dates, type, site of cultures (primarily sterile or primarily non-sterile), and identification of isolates. Location and duration of hospital stay from initiation of IV fluconazole treatment to hospital discharge, hospital discharge diagnosis, and clinical outcomes and mortality were also recorded. Drug interactions and adverse events were recorded for all concomitant medications administered during the ICU stay while the patient was receiving IV fluconazole.
During a hospital stay, antifungal therapy may often change for a patient. Therefore, the number of treatment cycles within a hospital stay with fluconazole and non-fuconazole was determined. A “treatment cycle” was defined as a period of consecutive use of a particular antifungal drug, with no further specification regarding duration or dose, since this was an observational study.
During the course of treatment, cultures from primarily sterile and primarily non-sterile sites were collected for isolate identification from each patient at the time the treating physician decided to perform microbiological testing as part of routine practice. Fungal cultures were incubated and isolates identified according to standardized procedures at the individual site.
Definitions of therapeutic response
All prophylaxis and therapy definitions were made prospectively during the initial study design according to the Revised 1994 British Society for Antimicrobial Chemotherapy Working Party definitions, as described by Flanagan and Barnes.Citation4 The treating physician was responsible for assigning individuals to one of these categories based on clinical and microbiological information. Predefined criteria for successful therapy according to treatment arm are depicted in . Prophylaxis, preemptive, and empiric therapy were considered unsuccessful if one or more of the criteria of successful treatment were not met. Definitive therapy was considered unsuccessful if there was no improvement in attributable symptoms and signs, and no improvement in attributable abnormalities detected by radiography, bronchoscopy, endoscopy, or other procedures. Successful empiric therapy also required absence of breakthrough fungal infections during the observation period – that is, the time of hospitalization. “Breakthrough infection” was defined as any identification of an isolate at least 5 days after initialization of IV fluconazole treatment in the prophylaxis and empiric therapy groups.
Figure 1 Predefined definitions of successful therapy according to individual treatment arm.
Abbreviation: IV, intravenous.

Finally, an assessment was made for patients with mycology results available. Based on recent literature,Citation9,Citation12,Citation13 patients with Candida infection only were classified as potentially sensitive versus potentially resistant to IV fluconazole treatment by a panel of physicians and microbiologists in case mycology results had been obtained at the individual sites. Candida spp. were classified as potentially sensitive to fluconazole treatment (C. albicans, C. parapsilosis, and C. guillermondii) or potentially resistant to fluconazole treatment (C. glabrata, C. krusei, and C. tropicalis). Patients suffering from both species potentially sensitive and potentially resistant to fluconazole treatment were counted in the latter group. Patients having only nonspecified Candida spp. or non-Candida coinfection were excluded from these analyses.
Statistical analysis
An exploratory data analysis was conducted to identify predictors of successful fluconazole therapy by using a significance level of 5%. Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated for the association of the individual factor with successful therapy. The following characteristics were assessed based on the judgment of the treating physician: patient age > 64 years, female sex, patient weight ≥ 70 kg, APACHE II score, neutropenic status on admission to ICU, mechanical ventilation at initiation of prophylaxis/therapy, reason for fluconazole therapy, number of IV fluconazole treatment cycles, number of non- fluconazole treatment cycles, number of cultures from primarily sterile sites, number of cultures from primarily non-sterile sites, C. albicans isolate, non-albicans Candida isolate, medical conditions (congenital disease, diabetes mellitus, endocrine disorders, ear nose throat disease and dental disease, eye problems and cataracts, gastrointestinal tract disease, genitourinary disease, heart disease, hematological disease, hypertension, liver disease, lung disease, neoplastic disease, neurologic and psychiatric disease, rheumatologic/skeletal disease, skin disease, vascular disease), length of hospital stay, drug–drug interactions and adverse events related to fluconazole therapy, risk factors (active malignancy, acute renal failure, human immunodeficiency virus, recent broad-spectrum antibiotic use, recent use of central venous catheter, diabetes mellitus, (continuous) renal replacement therapy including hemodialysis, immunosuppressive medication, neutropenia (absolute neutrophil count < 500 cells/μL), recent parenteral nutrition (hyperalimentation), prior fungal colonization, major surgery (within 30 days), trauma, solid organ transplantation, and overall number of risk factors. Model predictors were determined based on literature review and expert clinical opinion. A panel of physicians, pharmacists, and statisticians prioritized possible predictors of successful therapy based on relevant literature.
Risk factors were preselected for the next analysis step. Variables included in the model were significant at P ≤ 0.20 in univariate models. A backward variable selection method, with a selection level of 5%, was used to identify the list of predictors for successful therapy. ORs with 95% CIs were calculated.
Results
Patient accounting and demographics
Patients (n = 325) were enrolled in one of four treatment arms at the discretion of the individual treating physician: prophylaxis (n = 29; 10%), empiric therapy (n = 140; 46%), preemptive therapy (n = 85; 28%), or definitive therapy (n = 49; 16%). Three patients were excluded due to lack of treatment information. Subsequent analyses focused only on patients who received IV fluconazole during their hospitalization. This resulted in the elimination of 19 additional patients (seven from Germany and twelve from Spain) who received other azoles. Thus, data for 303 patients (112 from Germany and 191 from Spain) were analyzed.
The final cohort of 303 patients on IV fluconazole therapy had a mean age of 60 ± 16 years. The mean APACHE II score was 19 ± 10, with the widest range of scores observed in patients in the definitive therapy group (21 ± 15; ). SOFA scores ranged from four to 14, with a mean of nine. Seven percent of patients had neutropenic status at ICU admission (<500 cells/μL) and 82% were on mechanical ventilation when they entered the study. Major risk factors for developing fungal infections included recent use of broad-spectrum antibiotics (95%), recent use of a central venous catheter (97%), the status of the patient’s recent parenteral nutrition (67%), major surgery within 30 days (69%), and acute renal failure (47%).
Table 1 General patient characteristics
Mycology
Pathogens at treatment initiation of IV fluconazole therapy or under treatment with IV fluconazole were available from 251 patients. In 131 of these 251 patients, only one species could be identified. Colonization with two species was present in 90 patients, with three species in 24 patients, and with four species in six patients. The number of patients suffering from C. albicans-only infection was 87 patients (35%), whereas 63 patients (25%) did not suffer from C. albicans at all. Thirteen patients (5%) were infected with a combination of Candida with non-Candida spp., which were mainly Aspergillus (nine patients, 4%).
The microbiology results of the 407 isolates are shown in . To sum, Candida was the most common isolate (393/407 isolates, 96.5%), with C. albicans in 190 isolates (46.6%), non-albicans Candida in 92 isolates (22.6%), and nonspecified Candida in 111 isolates (27.3%). Aspergillus was the most prominent non-Candida isolate (n = 10; 2.5%).
Table 2 Pathogens (n = 407) isolated at initiation or on intravenous fluconazole treatment in 251 patients with mycology results available
Fluconazole therapy
Patients started antifungal therapy within 20.1 ± 16.8 days of being admitted to hospital and had their initial fluconazole therapy in 15.3 ± 15.1 days of being admitted to the ICU for the first time (). For 263/303 patients with at least one documented treatment dose available, the overall mean (median; minimum [min]/maximum [max]) loading dose was 648 mg/day (400 mg; 100 mg/1200 mg), and the highest follow-up prophylaxis/treatment dose was 543 mg/day (400 mg; min 50 mg/max 1600 mg). The mean loading and follow-up dose were (n = 17/29) 600 mg (400 mg; min 200 mg/max 1200 mg) and 438 mg (400 mg; 50 mg/800 mg) for prophylaxis; for empiric therapy (n = 113/140), 650 mg (400 mg; min 100 mg/max 1200 mg) and 565 mg (400 mg; min 100 mg/max 1600 mg); for preemptive therapy (n = 84/85), 661 mg (400 mg; min 200 mg/max 1200 mg) and 537 mg (min 200 mg/max 1200 mg); and for definitive therapy (n = 49/49), 637 mg (400 mg; min 200 mg/max 1200 mg) and 539 mg (400 mg; min 200 mg/max 800 mg). A total of 298 patients (98%) initiated IV fluconazole as first-line therapy; 111 (99%) were from Germany and 187 (98%) were from Spain. The remaining five patients who received fluconazole as second-line therapy had received oral fluconazole (n = 2), amphotericin B (n = 1), liposomal amphotericin B (n = 1), and caspofungin (n = 1) as first-line therapy. All patients in the prophylaxis and empiric therapy groups had IV fluconazole as first-line therapy, while 99% of patients in the preemptive therapy group and 92% of patients in the definitive therapy group had IV fluconazole as first-line therapy.
Table 3 Antifungal therapy characteristics by treatment group
The mean length of hospital stay for patients from initiation of IV fluconazole therapy to ICU and hospital discharge was 12.7 ± SD 16.1 days and 37.9 ± SD 46.9 days, respectively.
There were 124 adverse events related to fluconazole therapy in 31 of 303 patients (10.2%; of these, 22 were clinical [18%] and 102 were laboratory [82%]). In 17 patients, fluconazole interacted with concomitant medications.
The overall mortality rate for this study was 42% (n = 127).
Outcomes of fluconazole therapy by type of therapy
Based on the judgment of the individual physician, prophylaxis was successful in 19 of 29 patients (66%, 95% CI 45%–82%) and empiric therapy was successful in 77 of 140 (55%, 95% CI46%–63%) patients. In the preemptive therapy group, complete response was present in 38 of 85 patients (45%, 95% CI 33%–59%). Complete response to definitive therapy was evident in 21 of 49 patients (43%, 95% CI 42%–58%; ). Overall, the success rate was 51% (78/152, 95% CI 43%–60%; ).
Table 4 Treatment response by sensitivity to fluconazole treatment in 152 patients with proven Candida infection only
In the prophylaxis and empiric therapy groups, 14/29 and 101/140 patients, respectively, had at least one mycology result available. Breakthrough infections, clinically and/or microbiologically identified after at least 5 days of IV fluconazole therapy were present in ten patients (71% of the 14 patients with mycology results available and 34% of all 29 patients) in the prophylaxis group and in 38 patients (60% of the 63 patients with mycology results available and 27% of all 140 patients) in the empiric therapy group. In patients with proven Candida infection, the proportion of patients suffering from an infection of C. glabrata, C. krusei and/or C. tropicalis – species regarded as potentially resistant to IV fluconazole – was 32% (49/152). Overall, the success rate by sensitivity of Candida spp. to fluconazole was 58/103 for potentially sensitive Candida spp. (C. albicans, C. tropicalis, and C. guillermondii; 56%) and 20/49 for potentially resistant Candida spp. (C. glabrata, C. krusei, and C. tropicalis; 41%; ).
Univariate analysis of association of successful therapy
An explorative data analysis was conducted. Crude ORs with corresponding 95% CIs were calculated. Statistically significant associations (ORs, with 95% CIs not including 1.0) for the entire cohort were found for 11/48 characteristics examined. Factors associated with no treatment response, or indicating that treatment response was less likely, were found for: patient age ≥ 65 years (OR 0.46), number of non-IV fluconazole treatment cycles (OR 0.42), colonization with Candida not specified as C. albicans (OR 0.40), CRRT (OR 0.36), potential drug–drug interactions (OR 0.55), and heart disease (OR 0.57). A high OR, indicating that treatment success is more likely, was found for recent use of central venous catheter (OR 8.60).
Multivariate analysis
Risk factors were preselected for the next analysis. Only the empiric therapy model is presented here because only this arm recruited a sufficient number of patients for multivariate analyses (). The other treatment strategies (prophylaxis, preemptive, and definitive therapies) had sample sizes that were too small to afford multivariate analyses. A multivariate logistic regression model was created to test for the significant predictors of empiric therapy success. In the multivariate logistic model for successful treatment with empiric therapy, the significant predictors were colonization with Candida not specified as C. albicans (OR 0.21, 95% CI 0.08–0.54) and CRRT (OR 0.24, 95% CI 0.08–0.70), which were inversely associated with successful empiric therapy, and prior fungal colonization (OR 9.36, 95% CI 1.50–58.19), which was positively associated with successful empiric therapy.
Table 5 Multivariate logistic regression for predictors of successful empiric therapy
Discussion
This is the largest prospective study to evaluate the real-world treatment patterns related to invasive fungal infection in the ICU setting in Spain and Germany. Many previous studies identifying potential risk factors for developing candidemia have been limited by population, which frequently involved either patients with hematologic or solid organ malignancies, and by data obtained from a single institution or over a long period of time to ensure an adequate number of cases.Citation25–Citation27 Moreover, few studies were prospective in design and few focused on ICU populations.Citation9
Fluconazole prophylaxis can reduce the incidence of Candida infection and colonization in selected critically ill patients, in both the medical and the surgical ICU settings.Citation2,Citation7,Citation17,Citation28,Citation29 The present study observed a complete response to IV fluconazole prophylaxis in 66% of patients. Studies have reported that early treatment with fluconazole has a favorable effect on the clinical course of high-risk patients and late antifungal treatment is associated with a poor prognosis.Citation11,Citation27,Citation30,Citation31 Complete response rate for fluconazole in our study was comparable to studies that included all Candida spp.Citation20 but lower compared with those in which, for example, C. krusei was excluded.Citation21 However, we also observed that therapeutic success was strongly associated with type of fluconazole therapy, with empiric, preemptive, and definitive therapies being less successful and associated with higher mortality rates. It should be mentioned that the number of prophylaxis patients in our study was lower than in the treatment groups but patients were younger and differed with regard to the risk factors of acquired immu-nodeficiency syndrome/human immunodeficiency virus, use of immunosuppressive medications, and major surgery, which were relatively higher in number in the prophylaxis group compared with in the treatment groups. In addition, as fluconazole prophylaxis does not resolve resistant and non-albicans Candida infections, this may contribute to the low response rates in our cohort. At least one isolate potentially resistant to IV fluconazole was identified in 86/407 isolates (21%). In patients with at least one isolate identified, a breakthrough infection was found in 34% of all patients receiving prophylaxis with IV fluconazole and in 27% of all patients receiving empiric therapy. Very recently, in a French group studied over similar period, non-albicans Candida spp. (ie, C. glabrata, C. parapsilosis, C. krusei, and C. tropicalis) comprised almost half of the Candida isolates and reduced susceptibility to fluconazole was observed in 17.1% of Candida isolates.Citation9
Interestingly, in 13 of 251 patients (5%), non-Candida fungal infection was diagnosed at least once, mainly Aspergillus. This is in accordance with recently published data reporting a rate of 6.7% for Aspergillus in the ICU.Citation32 This certainly warrants further investigation.
It has been suggested that early therapy be initiated in high-risk patients due to the high mortality, prevalence of invasive candidiasis, and poor reliability of available diagnostic methods.Citation29,Citation33–Citation35 The present study shows that empiric antifungal treatment is common in the ICU setting in Spain and Germany. While an expert panel of the Infectious Disease Society of America no longer favors first-line fluconazole treatment in patients with moderately severe to severe illness or patients who have had recent azole exposure,Citation34 European recommendations, which were valid at study initiation, focused on the use of fluconazole.Citation29 The British Society for Antimicrobial Chemotherapy Working Party has recommended empiric therapy for surgical and ICU patients thought to have deep candidiasis but for whom microbiologic, histological, or serologic confirmation cannot be obtained.Citation35 This is also addressed in the most recent guidelines of the European Society of Clinical Microbiology and Infectious Diseases, which in addition downgraded fluconazole recommendation in non-neutropenic patients from AI to CIII.Citation36 In our study, CRRT and colonization with Candida not specified as C. albicans were the only significant negative predictors of successful therapy in the multivariate empiric model, the only arm with sufficient sample size to conduct multivariate analysis. The risk factor “prior fungal colonization” was associated with successful therapy. This might be regarded as a positive bias in our study, as the treating physician had been alerted that this was a possible reason for clinical signs of unknown origin. Finally, recent use of central venous catheter could not be confirmed as a positive predictor of successful therapy outcome by multivariate analysis.
Many studies have reported a steady increase in the number and prevalence of non-albicans Candida, which account for 40%–60% of the species reported as causes of invasive disease.Citation6,Citation10,Citation37–Citation39 Some of these species have reduced susceptibility or intrinsic resistance to fluconazole, and the increasing use of azoles is thought to account for these changes.Citation1,Citation31 A monocentric study from Spain in 226 candi-demia episodes conducted from 2004 to 2009 found that previous use of fluconazole is an independent risk factor for fluconazole-resistant candidemia.Citation40 In the Spanish study, predictive factors for isolation of C. glabrata or C. krusei were neutropenia, chronic renal disease, and solid organ transplantation. Interestingly, the authors found a relationship between C. krusei and immunocompromised patients, as well as C. glabrata and elderly patients with underlying medical diseases.Citation40 A study of the evolving trends of candidiasis in an Italian ICU from the 1980s to 2000 found that the rate of invasive Candida infections and colonization appeared stable, but there were an increased number of mixed colonizations (39% versus 6%), a reduction of colonization by C. albicans (78% versus 93%), and increased C. glabrata involvement (35%).Citation10 In the study reported here, we report mono-colonization with C. albicans in only 87/251 patients (35%) with mycology results available. Prophylactic use of fluconazole can shift the flora in the ICU away from C. albicans toward different fungal organisms.Citation41 However, the use of early therapy may explain why overall candidal infection rates remained stable in both Spain and Germany.Citation6,Citation10 Our data confirm the emergence of non-albicans Candida and moulds, pathogens potentially resistant to fluconazole, in the ICU.
Treatment failure was high in both Candida spp. potentially sensitive to fluconazole (44% of cases) and Candida spp. potentially resistant to fluconazole (59% of cases). These real-world outcomes in a heterogeneous population support findings from randomized clinical trials that also included C. krusei infections, reporting failure rates of 31%–64% in patients treated with fluconazole.Citation20,Citation21
Our study has several limitations, including the small sample size of most of the study arms. In addition, causality cannot be demonstrated in an observational study and the assessments were based on evaluation by the treating physician and local diagnostic and treatment standards that were not reviewed by a panel. Information regarding the rationale for the use of fluconazole is limited and there are no data on detecting fluconazole-resistant yeast. Moreover, data on the base line of invasive fungal infection and colonization of each center were not collected and thus could not be considered as predictors of successful therapy. Based on current guidelines,Citation34,Citation36 some of the patients might not have been suitable for fluconazole treatment. Causes of the deaths were not recorded – although no death was considered related to fluconazole therapy – and the follow-up period after the end of fluconazole therapy was relatively short.
Conclusion
The prevalence of pathogens potentially resistant to fluconazole is evident in the intensive care setting in Germany and Spain. With empiric therapy being the most prevalent therapeutic strategy, more consideration needs to be given to selecting the appropriate agent for ICU patients. The importance of appropriate therapy is highlighted due to the fact that approximately 32% of the patients in our study with microbiological results available suffered from Candida specified as potentially resistant to fluconazole. Moreover, according to our data, fluconazole use for proven systemic albicans and non-albicans Candida infections was associated with treatment failure in about 50% of cases overall. Future studies may further help to elucidate whether early initiation of antifungal therapy with a broad-spectrum agent improves treatment outcomes in ICU populations.
Authors’ contributions
Heimo Wissing, Jose Ballus, Gonzalo Nocea, and Karl J Krobot made substantial contributions to conception and design of the study. Heimo Wissing, Jose Ballus, and Tobias M Bingold made substantial contributions to acquisition of data. Heimo Wissing, Gonzalo Nocea, Peter Kaskel, Ritesh N Kumar, Karl J Krobot, and Panagiotis Mavros made substantial contributions to analysis and interpretation of data. Heimo Wissing, Tobias M Bingold, Peter Kaskel, Ritesh N Kumar, and Karl J Krobot were involved in drafting the manuscript and in revising the manuscript critically for important intellectual content. All authors gave final approval of the version to be published.
Study group members
In addition to the authors of the present study, the following investigators in each country participated in the study.
Spain
M Palomar, A Socias, A Campos (Hospital Univeritario Vall d’Hebron, Barcelona); J Ballus, MR Cañizares Mediano, L-T Sánchez Salido, A Cabrejas Ayuso (Hospital Universitario de Bellvitge, Barcelona); A Bonet, N López de Albina (Hospital Universitario de Girona Doctor Josep Trueta, Gerona); A Martínez Pellus, M Bru Cartagena, D Martínez Bano, M Royo-Villanova, S Sánchez Cámara (Hospital Universitario Virgen de la Arrixaca, Murcia); J Blanquer Olivas, J Ferreres Franco, N Carbonell Monleón (Hospital Universitario Clínico de Valencia, Valencia); C Blanco, JM Raurich Puigdevall, JI Ayestarán (Hospital Universitario Son Dureta, Palma de Mallorca); P Marco Garde, A Murguialdai (Hospital Donostia, San Sebastián); L Alvarez Rocha, R Galeiras, P Rascado (Complejo Hospitalario Universitario Juan Canalejo, La Coruña); D Parra, V Español (Hospital Universitario Central de Asturias, Oviedo, Asturias); J Solé Violan, JM Ferrer, J Cabrera, O Pérez (Hospital Universitario de Gran Canaria Doctor Negrín, Las Palmas de Gran Canaria, Gran Canaria); V Caruezo Rodríguez, V Ginesta, J Álvarez, M Bouzada, MV Pombo, S del Río, J Oliveira, E San Luis (Complejo Hospitalario Universitario de Santiago, Santiago de Compostela, La Coruña); JL Teja Barbero, A Castellanos, JM Santidrián, T Obeso (Hospital Universitario Marqués de Valdecilla, Santander, Cantabria); G González Díaz, A Renedo Villarroya (Hospital General Universitario Morales Meseguer, Murcia); and MA Chiveli, P Herrera Melian (Hospital Universitario La Fe de Valencia, Valencia).
Germany
F Hinder (University Hospital of Munster); F Logemann (University Hospital of Hannover); M Thörner (University Hospital of Frankfurt/Main); and M Rist (Academic Hospital of Dachau).
Acknowledgments
This paper was presented in part at the Intensive Care And Emergency Medicine 27th International Symposium, Brussels, Belgium, March 27, 2007 (P113) and Sepsis Update 2009, Weimar, Germany, September 11, 2009 (O005).
We are grateful to Prof Dr Peter Rath, of the Institute of Medical Microbiology, University of Essen, Germany, for his valuable suggestions. We thank Syreon Corporation for managing the study and Wendy Horn for assisting in manuscript development. We are indebted to all participating study sites in Spain and Germany that contributed patients to the study.
Disclosure
Financing for this study came from Merck & Co, Whitehouse Station, New Jersey, USA.
Gonzalo Nocea, Karl J Krobot, Peter Kaskel, Ritesh N Kumar, and Panagiotis Mavros are employed by Merck and Co. Jose Ballus has received investigator payments from Merck, Sharp and Dohme, Madrid, Spain. The Department of Anesthesiology, Intensive Care and Pain Therapy, University Hospital of Frankfurt/Main, Germany (where this study’s primary investigator, Heimo Wissing, is employed) has received research funding and travel grants from MSD SHARP & DOHME GMBH, Haar, Germany.
References
- Ostrosky-ZeichnerLPappasPGInvasive candidiasis in the intensive care unitCrit Care Med200634385786316505666
- KettDHAzoulayEEcheverriaPMVincentJLExtended Prevalence of Infection in ICU Study (EPIC II) Group of InvestigatorsCandida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit studyCrit Care Med201139466567021169817
- LipsettPASurgical critical care: fungal infections in surgical patientsCrit Care Med34Suppl 9S215S22416917426
- FlanaganPGBarnesRAFungal infection in the intensive care unitJ Hosp Infect19983831631779561467
- Alvarez-LermaFNolla-SalasJLeónCEPCAN Study GroupCandiduria in critically ill patients admitted to intensive care medical unitsIntensive Care Med20032971069107612756441
- Borg-von ZepelinMKunzLRüchelRReichardUWeigMGrossUEpidemiology and antifungal susceptibilities of Candida spp. to six antifungal agents: results from a surveillance study on fungaemia in Germany from July 2004 to August 2005J Antimicrob Chemother200760242442817562683
- PfallerMADiekemaDJEpidemiology of invasive candidiasis: a persistent public health problemClin Microbiol Rev200720113316317223626
- BassettiMRighiECostaAEpidemiological trends in nosoco-mial candidemia in intensive careBMC Infect Dis200662116472387
- LeroyOGangneuxJPMontraversPAmarCand Study GroupEpidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006)Crit Care Med20093751612161819325476
- TortoranoAMCaspaniLRigoniALBiraghiESicignanoAVivianiMACandidosis in the intensive care unit: a 20-year surveyJ Hosp Infect200457181315142710
- GareyKWRegeMPaiMPTime to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional studyClin Infect Dis2006431253116758414
- CharlierCHartELefortAFluconazole for the management of invasive candidiasis: where do we stand after 15 years?J Antimicrob Chemother200657338441016449304
- LichtensternCGeissHKBöttingerBWWeigandMAAntimykotische Therapie in der Intensivmedizin [Antimycotic therapy in the intensive care unit]Intensivmed.up2date200732140 German
- UllmannAJSanzMATramarinALongitudinal Evaluation of Antifungal Drugs (LEAD I) InvestigatorsProspective study of ampho-tericin B formulations in immunocompromised patients in 4 European countriesClin Infect Dis2006434e29e3816838223
- FischerMAWinkelmayerWCRubinRHAvornJThe hepatotoxicity of antifungal medications in bone marrow transplant recipientsClin Infect Dis200541330130716007524
- KauffmanCACarverPLUse of azoles for systemic antifungal therapyAdv Pharmacol1997391431899160115
- EggimannPFrancioliPBilleJFluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patientsCrit Care Med19992761066107210397206
- GarbinoJLewDPRomandJAHugonnetSAuckenthalerRPittetDPrevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontaminationIntensive Care Med200228121708171712447512
- PelzRKHendrixCWSwobodaSMDouble-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patientsAnn Surg2001233454254811303137
- SchusterMGEdwardsJEJrSobelJDEmpirical fluconazole versus placebo for intensive care unit patients: a randomized trialAnn Intern Med20081492839018626047
- RexJHPappasPGKarchmerAWNational Institute of Allergy and Infectious Diseases Mycoses Study GroupA randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjectsClin Infect Dis200336101221122812746765
- MontraversPJabbourKClinical consequences of resistant Candida infections in intensive careInt J Antimicrob Agents20062711616343859
- World Medical Association (WMA)WMA Declaration of Helsinki: Ethical Principles for Medical Research Involving Human SubjectsFerney-VoltaireWMA2008 Available from: http://www.wma.net/en/30publications/10policies/b3/17c.pdfAccessed December 2, 2012
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)Guideline for Good Clinical Practice. E6(R1). June 10GenevaICH1996 Available from: http://private.ich.org/LOB/media/MEDIA482.pdfAccessed December 2, 2012
- VazquezJASanchezVDmuchowskiCDembryLMSobelJDZervosMJNosocomial acquisition of Candida albicans: an epidemiologic studyJ Infect Dis199316811952018515108
- BorzottaAPBeardsleyKCandida infections in critically ill trauma patients: a retrospective case-control studyArch Surg1999134665766510367877
- De WaeleJJVogelaersDBlotSColardynFFungal infections in patients with severe acute pancreatitis and the use of prophylactic therapyClin Infect Dis200337220821312856213
- RichardsMJEdwardsJRCulverDHGaynesRPNosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance SystemCrit Care Med199927588789210362409
- VincentJLAnaissieEBruiningHEpidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive careIntensive Care Med19982432062169565801
- Nolla-SalasJSitges-SerraALeón-GilCCandidemia in non-neutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICUIntensive Care Med199723123309037636
- ShorrAFChungKJacksonWLWatermanPEKollefMHFluconazole prophylaxis in critically ill surgical patients: a meta-analysisCrit Care Med20053391928193516148461
- MeerssemanWVan WijngaerdenEInvasive aspergillosis in the ICU: an emerging diseaseIntensive Care Med200733101679168117646965
- SobelJDRexJHInvasive candidiasis: turning risk into a practical prevention policy?Clin Infect Dis20013318718910.1086/32181211418878
- PappasPGKauffmanCAAndesDInfectious Diseases Society of AmericaClinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of AmericaClin Infect Dis200948550353519191635
- DenningDWManagement of deep Candida infection in surgical and intensive care unit patientsIntensive Care Med19942075225287995872
- CornelyOABassettiMCalandraTESCMID Fungal Infection Study GroupESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patientsClin Microbiol Infect201218Suppl 7193723137135
- KullbergBJOude LashofAMEpidemiology of opportunistic invasive mycosesEur J Med Res20027518319112069910
- PappasPGRexJHLeeJNIAID Mycoses Study GroupA prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patientsClin Infect Dis200337563464312942393
- PfallerMAJonesRNDoernGVInternational surveillance of blood stream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. SENTRY Participant Group (Europe)Diagn Microbiol Infect Dis1999351192510529877
- Garnacho-MonteroJDíaz-MartínAGarcía-CabreraERisk factors for fluconazole-resistant candidemiaAntimicrob Agents Chemother20105483149315420498325
- KamLWLinJDManagement of systemic candidal infections in the intensive care unitAm Syst Health Care Pharm20025913341