Abstract
Objective
Carbapenem-resistant Enterobacteriaceae (CRE) have become an increasingly common cause of healthcare-related infections and present a serious challenge to clinical treatment. This study examined the phenotypic, genotypic characterization, clinical, and microbiological data of CRE in the Huizhou Municipal Central Hospital.
Methods
We conducted a phenotypic susceptibility evaluation and whole genome sequence analysis for 52 CRE strains isolated from 37 patients and 2 medical device-related samples during 2013–2017 to characterize risk factors, antimicrobial resistance profiles, dominant clones and hospital transmission.
Results
Long-term hospitalization, treatment time with antibiotics and use of invasive devices were linked to the risk of CRE infection. The carbapenem resistance genes (CRGs) we found included blaNDM (82.7%), blaIMP (19.2%) and blaKPC (3.8%), Escherichia coli (44.2%) and Klebsiella pneumoniae (44.2%) were the dominant species we identified, and the type of CRG carried by isolates was highly correlated with species. The coexistence of CRGs with a variety of other antibiotic resistance genes leads to an increased prevalence of high resistance levels for CRE to β-lactams and other antibiotic classes such as aminoglycosides and fluoroquinolones. These isolates were sensitive only to colistin and tigecycline. In addition to this, we observed significantly genomic diversity of CRE isolates in this hospital. Importantly, we found that long-term transmission of multiple CRE clones had occurred at this hospital between various wards.
Conclusion
Evaluating and improving the current infection control strategies may be necessary, and reducing nosocomial transmission remains the primary control element for CRE infections in China.
Introduction
Enterobacteriaceae are frequent pathogens of community-acquired and nosocomial infections which can result in sepsis, bacteremia and pneumonia.Citation1 Over the last decades, the excessive and irrational use of antibiotic agents has resulted in rapid and widespread dissemination of multi-drug resistant (MDR) strains in this family. This has resulted in a reliance on carbapenems as the mainstay of therapy for serious infections caused by MDR Enterobacteriaceae and especially for strains expressing extended-spectrum β-lactamases (ESBLs).Citation2,Citation3 This reliance has driven carbapenem resistance, and CRE have emerged and included common bacterial species Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae.Citation4 The rapid emergence and spread of CRE poses a serious threat to global public health and is a great challenge for clinicians.Citation5
The rapid spread of carbapenem resistance in Enterobacteriaceae has been attributed to the acquisition of carbapenemases that include blaNDM, blaKPC, blaVIM, blaOXA and blaIMP which hydrolyze carbapenems and other β-lactams to varying degrees.Citation6–8 CRE can be maintained in a population by both the horizontal transmission of carbapenemase genes (CRGs) associated with mobile elements or via clonal proliferation.Citation9 CRE have considerable epidemic potential, and outbreaks of hospital-associated CRE infections are not rare.Citation10 Interestingly, the genetic diversity in CRE isolates from different types of infections in China is high. For instance, common clones of K. pneumoniae include the high-risk ST11 strains and ST17, ST48, ST412 and ST65,Citation11 while ST167 and ST410 are dominant in E. coli isolates.Citation12 CRE typically carry a variety of antibiotic resistance genes and are insensitive to almost all β-lactams including cephalosporins and carbapenems, quinolones, aminoglycosides and sulfonamides leaving few effective treatment options.Citation13,Citation14 Invasive infections caused by CRE are often associated with significant morbidity and mortality, especially in patients who are admitted to hospital for long periods, are critically ill or were exposed to invasive devices such as catheters and central venous ports.Citation15 The direct consequence is reduced therapeutic effects of antibiotics, a shortened developmental cycle for new drugs, increased treatment costs and recuperation time for patients and the direct threat to human health.
There is currently abundant information regarding CRE in China, but due to the natural environmental and economic differences from region to region, implementation of disinfection measures, the varieties and frequency of antibiotic use are not consistent between hospitals. This has resulted in a non-uniform distribution of CRE occurrence. In the current study, we concentrated a retrospective cohort of patients admitted to Huizhou Municipal Central Hospital over a 5-years period who were colonized or infected with CRE. The aim was to describe the clinical features of CRE patients, define the range of CRE species, identify the dominant CRGs and elucidate patterns of clonal or horizontal transmission using whole genome sequencing (WGS) to provide a laboratory basis for scientific selection of antimicrobial agents and control of infection and outbreak of antibiotic resistant strains.
Methods
Ethics Statement
All samples and information of patients were collected in this study under authorization from Huizhou Municipal Central Hospital.
Bacterial Strains
We examined 52 clinical Enterobacteriaceae isolates that possessed reduced sensitivity to meropenems were tested using the Vitek 2 system (bioMérieux SA, Marcy-l’Etoile, France) in the Bacteria Testing Center of Huizhou Municipal Central Hospital during 2013 to 2017. These strains were primarily collected from qualified pathogenic specimens in emergency and inpatient wards and included sputum, urine, blood, wound and other samples. All isolates were re-identified using the MALDI-TOF 80 MS Axima system (Shimadzu-Biotech, Kyoto, Japan) and were stored at −80°C for minimum inhibitory concentration (MIC) testing and investigation of resistance mechanisms.
Collection of Clinical Date
Clinical information of CRE patients was collected from the electronic medical record and included CRE testing at admission, admission clinical services, length of stay and the body site of the initial CRE specimen. Other significant risk factors for infection also were recorded including surgical procedures, endoscopy and antibiotic exposure. We recorded any antibiotic treatment and its duration in CRE infected patients, as well as the outcome (death or survival) up to hospital discharge. According to the bacterial identification results, according to the ratio of >1:2, the control group (patients with CSE, that is, Enterobacteriaceae bacteria sensitive to imipenem and ertapenem) was selected, and at least two of the hospital stay, age (±5 years), gender, and specimen type were consistent.
MIC Testing
Antimicrobial susceptibility was evaluated by the agar dilution except for colistin and tigecycline that used the broth dilution method at according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (M100-S28, 2018), and the results were interpreted according to CLSI categories and minimum inhibitory concentration (MIC) breakpoints.Citation16 For colistin and tigecycline, the resistance breakpoints were determined according to EUCAST recommendations interpreted with >2 and >0.25 mg/liter, respectively. Quality control of the procedure was conducted using the susceptible E. coli ATCC 25922 as a reference.
Whole Genome Sequencing
Total genomic DNA of 52 isolates was extracted using a Genomic DNA Purification Kit (Tiangen, Beijing, China) as per the manufacturer’s instructions. WGS was performed with the Illumina Miseq System (Illumina, San Diego, CA), and the paired-end Illumina reads were assembled by SPAdes v3.6.2.Citation17
Sequence Types (ST), Antibiotic Resistance Gene and Plasmid Profiles
The initial annotation of draft genomes of all strains was performed using RAST (Rapid Annotation using Subsystem Technology)Citation18 The CGE platform (http://www.genomicepidemiology.org/) was used for analyses of multilocus sequence typing (MLST-2.0), acquired resistance genes (ResFinder 4.1, all antibiotic resistance databases were selected with a cut-off value of 95% identity and 80% minimum coverage) and plasmid incompatibility groups (PlasmidFinder-2.1 version, using the Enterobacteriaceae database with parameters of minimum 95% identity and 85% query coverage).
Phylogenetic Analyses
Parsnp (v1.2)Citation19 was used to create a phylogeny using the draft assemblies for all CRE isolates of the three bacterial species that were identified: E. coli, K. pneumoniae and E. cloacae. Single isolates from each group were randomly selected as a reference and visualized using the online tool iTOL v5.Citation19 The population structure of each phylogenetic tree was defined using hierBAPS v6.0.Citation20 The relationship matrices were built both manually and using the Neighbor-Joining function (Hamming distance, Saitou– Neicriterion) in Phyloviz v2.0.Citation21
Statistical Analysis
Single-factor analysis: Categorical variables were compared using the chi-square test; Continuous variables were compared by t-test or nonparametric test according to their distribution. Multi-variate analysis: significant variables with P value of <0.05 were then selected into a logistic regression model for multi-variate analysis to evaluate independent risk factors of CRE infection. All statistical analysis was performed using SPSS software version 26.0, and the level of significance was set at p < 0.05 for all tests.
Data Availability
All genome assemblies of 52 isolates were deposited in GenBank and are registered with BioProject: PRJNA832055.
Results
Clinical Characteristics of Patients with CRE Isolates
We analyzed 52 CRE isolates from 37 patients in Huizhou Municipal Central Hospital, in Guangdong Province, China, between 2013 and 2017 (). Within this group, out of the 52 samples, the great majority were E coli (23/52; 44.2%) and K. pneumoniae strains (23/52; 44.2%), with six strains belonging to the E. cloacae species (6/52; 11.6%), which were all separated in 2013. The most common primary sites of isolation were sputum (32.2%), wound (26.9%), urine (21.1%), blood (13.5%) and central venous catheter (5.8%) (). The clinical features of these patients indicated that most were male (26/37, 70.27%) and the average age was 53.12 years but included two infants younger <6 months of age. These patients were admitted in more than 10 wards, and the most frequent infections were lower respiratory (n = 9, 24.32%), catheter and ventilator-associated (n = 7, 18.92%) and burn wound (n = 7, 18.92%) infections. Hospital admission times >1 month were common (22/39, 56.4%) and associated CRE risk factors for infection during hospitalization included catheter use (30/37, 76.9%, p < 0.5), ventilator use (26/37, 66.7%, p < 0.5), intravascular catheter use (22/37, 59.46%, p < 0.5), surgery (15/37, 40.54%, p < 0.5), tracheotomy (12/37, 32.43%, p < 0.5) and previous exposure to antibiotic agents most commonly meropenem, vancomycin, levofloxacin or tigecycline, often in combination (27/37, 73.0%, p < 0.5) and 81.80% of the patient group survived to hospital discharge () ().
Table 1 Comparison of Clinical Characteristics of Patients Harboring CRE and CSE Isolates
Figure 1 Timelines of 52 CRE isolates containing carbapenemases from different wards during 2013 to 2017.
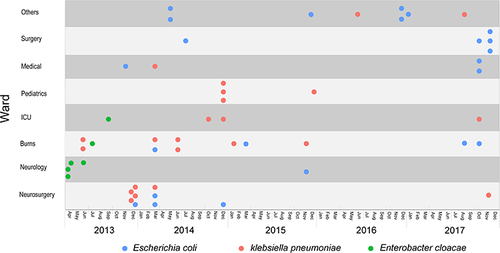
Figure 2 Core genome phylogenetic tree for all CRE isolates according to ARG and plasmid type. (A), E. coli (B), K. pneumoniae and (C), E. cloacae. Colors illustrated lineages, years, source, wards, ST types and carbapenemases, respectively. Red-filled squares indicate possession of the indicated ARG and blue-filled squares indicated plasmid Inc type.
Evaluation of Antimicrobial Susceptibility of CRE Isolates
These CRE isolates showed high resistance (>90%) to all beta-lactams, including ampicillin, cefotaxime, ceftriaxone and meropenem. Resistance to other antibiotics classes was also frequent with 100% resistant to sulfamethoxazole/trimethoprim, 92.3% to ciprofloxacin, 84.6% to florfenicol, 76.9% to gentamicin, 69.2% to fosfomycin. The sensitivity to tigecycline and colistin were low at 1.9% and 13.5%, respectively. Notably, 10% of isolates were resistant to all antibiotics used in this study except tigecycline and colistin ().
Table 2 Results of Multivariate Analysis of CRE Patients
Table 3 Antimicrobial Susceptibility of Patient CRE Isolates
Population Structure of CRE Isolates
We analyzed the genomes of 52 CRE isolates that were sequenced using the Illumina HiSeq platform. Multilocus sequence typing (MLST) analysis revealed 10 distinct STs in K. pneumoniae that included ST3073 (5/23), ST147 (4/23), and ST11 (4/23), ST17 (3/23), ST34 (2/23), and the remaining were single STs that included ST1040, ST392, ST48, ST45 and ST1. In E. coli, ST167 accounted for most infections (13/23), followed by ST410 (4/23), ST10 (2/23), ST3489 (2/23) ST6838 (1/23) and ST38 (1/23). Two STs were identified in E. cloacae and included ST93 (4/6) and ST88 (2/6) ().
In addition, we further analyzed population structure by constructing phylogenetic trees based on the core genomes of these isolates. Bayesian analysis revealed 4 different lineages among the 23 E. coli isolates. The major lineage was lineage D (16/23, 69.6%) and included 13 isolates belonging to ST167 sharing <155 SNPs (0.15%) of 103,882 total SNPs (). The 23 K. pneumoniae isolates were classified into 5 lineages with the majority belonging to lineage B (8/23, 34.8%) (). The 6 E. cloacae were divided into two lineages, lineage A included 4 strains of ST93 and lineage B included 2 strains of ST88 ().
Plasmid Replicon and Antimicrobial Resistance Genes (ARGs) in CRE Isolates
A total of 15 plasmids replicon types were detected among the 52 CRE isolates, and the number of plasmid replicons were found to vary from 2 to 6 in a single isolate. The IncFIB plasmid replicon was the most prevalent among these strains (41/52, 78.8%), followed by IncFII (39/52, 75.0%) and IncX3 (25/52, 48.1%) ().
We also examined genes associated with carbapenem resistance as well as the MDR patterns observed in MIC tests. ARG analysis indicated acquired CRGs in 52 isolates and these predominantly harbored blaNDM-5 (42.3%), blaNDM-1 (36.5%), blaIMP-4 (19.2%), blaKPC-2 (3.8%), blaNDM-9 (3.8%) as well as dual-carbapenemases with the combination of blaKPC,blaNDM or blaIMP (5.8%). The CRG type carried by isolates was highly correlated with species and all blaNDM-5 were carried by E. coli, blaNDM-1,blaNDM-9 and blaKPC-2 were primarily carried by K. pneumoniae and blaIMP-4 was harbored by E. cloacae and K. pneumoniae. In addition, 92.3% isolates carried ESBL genes and blaTEM (73.1%) and blaCTX (59.6%) predominated followed by blaSHV (34.6%) and blaOXA (17.3%). Additionally, we also identified 19 ARGs that were not β-lactamases and these conferred resistance to numerous antibiotic classes of antibiotics including gentamicin, amikacin, florfenicol, tigecycline ciprofloxacin, fosfomycin and sulfamethoxazole/trimethoprim. There were 9 highly prevalent ARGs with detection rates >50% and included aac, aad, aph, dfrA, floR, mph, qacE, sul, tet ().
CRE Isolates Dominant Clones and Potential Hospital Transmission
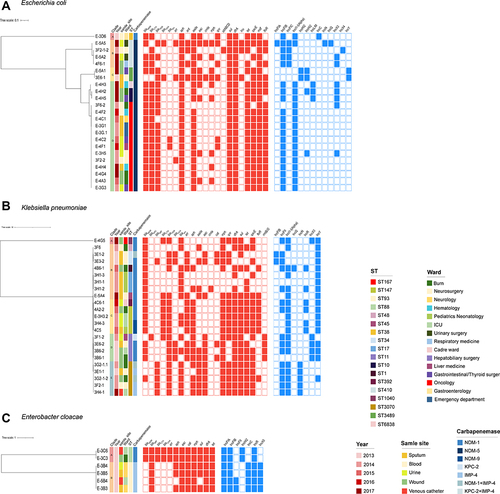
A number of ST167 E. coli were recovered from multiple patients in 2014–2017 indicating possible clonal relationships and ongoing transmission within this hospital. To investigate this, we analyzed SNPs in these ST167 isolates and this revealed 3 distinct groups. The first group of 11 isolates differed by <4 core SNPs overall and 5 were identical (SNP = 0). This group of similar strains were found within 8 wards over a period of 3 years indicating probable persistent transmission in multiple wards of the hospital for many years. The second and third groups contained only single isolates, and these shared 52 and 155 SNPs with the closest isolate in the first group, respectively. These SNP distances suggested that direct transmission between these groups of isolates was unlikely and that transmission from an intermediate source (environmental or unsampled patients/staff) was the more probable explanation.
To identify other possible transmission events, we also analysed an additional 6 ST groups containing two or more isolates from different patients using the same SNP comparison method. We also identify probable transmission events for ST3073, ST147, ST410, ST11, ST93 and ST88. The ST3073 group had a cluster of 5 close isolates (shared ≤13 SNP), two of which from a same patient and three were identical. The clustering of closely related isolates mainly in three wards over a period of 6 months (2013.12–2014.06) and a distantly related isolate collected from the same ward in 2017 (shared 13 SNP). Notably, one of the three same isolates was recovered from the central venous catheter used by the patient. Two separate clusters were detected, respectively, in the ST147, ST410 and ST11 group. Each group consisted of a cluster of three closely related strains and a single strain, which were collected from three wards separately, and time spans up to four years. This likely indicated the short-term transmission between the two patients with identical isolates, with additional long-term transmission or a common reservoir in the environment of this hospital for at least 4 years.
Two unrelated groups at the core SNP level were found in E. cloacae (shared 35,849 SNPs) and ST93 and ST88 members. Four isolates of the ST93 group were on average 3.5 SNPs away from the next closest isolate (range = 1–5 SNPs), while two isolates in the ST88 group had 16 SNP distance, all collected within 2 months of each other times and probably indicative of short-term transmission ().
Figure 3 Core SNP differences between isolates grouped by ST. Isolates listed close to each other were identical at the core genome level. Black lines, the number of SNPs differences between isolates. Color of font and background represent the wards and time that the isolate was collected, respectively.
Analysis of CRG Contexts in CRE Isolates
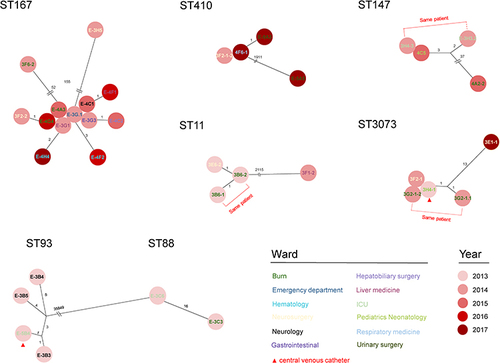
The genomic environments of CRGs (blaNDM,blaIMP and blaKPC) were determined using BLAST alignments for CRG-carrying contigs. We found 10 different genetic contexts (Types I–X) surrounding the blaNDM gene. Types I, II, III and IV were present in the K. pneumoniae strains surrounding copies of blaNDM except type I that included 2 blaNDM-9, the others were blaNDM-1. The type I genetic environment was identical to the blaNDM region in IncX3 type plasmid pA575-NDM (MH917283.1). Type II was similar to type I except for the truncation of ISAba125. Type III and type IV were partially or completely lacking the region downstream immediately flanking blaNDM compared to type I. The type V was the same as that in the IncX3 plasmid pEC135 (MH3474884.1). Type VI lacks partial areas following r IS5 compared to Type V. Types VII, VIII, IX and X existed in E. coli and included a copy of blaNDM-5. Type VII was the most prevalent blaNDM genetic context in this study, and an ARG region including sul and aadA was present downstream of blaNDM, and this was identical to a blaNDM-carrying IncFII type plasmid of E. coli from Italy. Type VIII was identical to the blaNDM downstream environment of type VII, but a truncated ISAba125 element was inserted upstream. Type IX was identical to p0128 (MZ606384.1) and was an IncC type plasmid from K. pneumoniae from Italy. Type X genetic context ΔIS26-ΔISAba125-blaNDM-bleMBL-trpF-DsbC-IS26-ΔTnAs3 was novel in the GenBank database ().
Figure 4 Genetic environments of Carbapenem resistance genes. (A) blaNDM (B), blaIMP and (C) blaKPC. Arrows, direction of transcription and genes are grouped by colour. Regions of ≥99.0% nucleotide sequence identity are shaded grey. Δ, truncated gene.
A total of six genetic contexts (Types I–VI) were found in 10/52 blaIMP-carrying CRE. The type I and V genetic environments were novel in the GenBank database. Type I was composed of insertion of Intl1-ant-blaIMP-Itra-qacG into the IncF type plasmid p49210 (CP089028). Type II shared a similar genomic environment with Type I except for a IS26 and ab mccE insertion downstream. Type V possessed an Itra gene deletion between blaIMP and qacG compared to type I. Type III and type IV blaIMP contexts were present in an IncN type plasmid pIMP4_IncN (CP050160.1) from E. coli in Hong Kong ().
The gene environment of the only isolate carrying blaKPC displayed >99% identity to plasmid p12478-KPC except for the truncation of IS26 upstream and downstream ().
Discussion
CRE are a serious challenge in hospital settings due to their high levels of resistance to antibiotics and high frequency of treatment failure.Citation22 The mortality caused by CRE infections was are generally high in the range of 30 to 80%.Citation23,Citation24 However, the mortality in the current study was only 13%, which may be related to unknown outcome of some patients due to treatment failure. A variety of factors, including underlying disease, nutritional status and medical treatment, may explain the discrepancy in mortality among different studies.Citation25 The serious underlying disease, long-term use of antibiotics, immune status alteration, use of invasive devices and prolonged hospitalization are all extremely significant risk factors for CRE infection.Citation26–28 Of the 37 patients in our study, the numbers of subjects who treated with antibiotics >7 days and who used invasive devices including catheter, ventilator, tracheotomy, and intravascular catheter were 73.0%, 76.9%, 66.7%, 32.4% and 56.4%, respectively. There was a significant difference compared with the control group, among the tracheotomy and ventilator use were considered to be an independent risk factor for CRE infection (P < 0.05). There were no additional significant differences in ratios of sex and age between CRE infection patients and non-infection patients. But the average hospitalization time of CRE infection patients was 90.87 days and far more than that of ordinary patients (36.64 days). Those factors may contribute to CRE continuously spread within the hospital. According to the distribution of CRE specimens, CRE strains were mainly isolated from respiratory tract, urinary tract and wound in our hospital. Just by reviewing the medical records, the CRE detected in the respiratory tract, urinary tract and wound cannot be ruled out as colonizing bacteria. If the patient is diagnosed with respiratory tract or urinary tract infection, it must be combined with the patient’s clinical manifestations, whether to use invasive operations (ventilator, catheter), and the condition of antibiotics use must be comprehensively judged. The majority of CRE detected in wounds come from the wounds of burn patients and surgical patients, the patient’s wound condition, wound secretion smear also must be considered. Therefore, in the prevention and control of CRE, we should focus on patients with invasive operations (ventilator, catheter) and reduce the incidence of nosocomial infection by improving the quality of care and aseptic operation techniques, shortening the indwelling time of catheters, strengthening the cleaning and disinfection of the environment and the surface of objects, and rational use of antibiotics.
In our study, we found that the majority of CRE patients in this hospital carried blaNDM, which was consistent with its high prevalence in China.Citation29 This prevalence was followed by blaIMP-4 that was the most prevalent blaIMP variants since it was first identified from Hong Kong in the mid-1990sCitation30 and recently in Australia and China.Citation31,Citation32 These CRGs usually coexist with other ARGs and this poses a great challenge to the treatment of CRE infections. This emphasized the need to use an individualized therapy based on in vitro antimicrobial susceptibility profiles, molecular type, infection severity and patient health status.Citation33 Our study described the antimicrobial susceptibility profiles and molecular epidemiological characteristics of CRE in this hospital, which provided information for choice of clinical treatment. For instance, these CRE isolates showed high resistance to almost all antibiotics evaluated including the β-lactam classes, aminoglycosides and fluoroquinolones and displayed sensitivity only to tigecycline and colistin. The use of tigecycline in endovascular infections such as bacteremia is controversial due to its lack of providing sufficient serum concentrations.Citation34 The specific drug and colistin combinations may be a useful treatment option for CRE infections.
CRE outbreaks in healthcare facilities are common, and this prevalence could eventually result in hospital or community spillover events.Citation35 CRE epidemiological surveillance has become a priority in controlling the infection and outbreak of these drug-resistant strains. WGS had provided useful data for epidemiological surveillance and nosocomial outbreak investigations due to its high discriminatory power and reduced cost.Citation36,Citation37 In this study, we explored the epidemiology of CRE in a Single Hospital of China using genomic analysis combined with clinical history characteristics. In this study, with the exception of partial clinical strains of CRE clustered in the burn ward, the majority of the remaining strains were found in different wards suggesting healthcare-associated clonal transmission of some strains was likely. In support of this, we determined multiple clonal group transmission events that involved ST167, ST410, ST147, ST11, ST3073 and ST93 by analyzing cgMLST and SNP distance. We presumed that the number of core SNP differences between isolates agreed with the SNP threshold defined in a Europe CPE dataset that defined ≤21 SNPs for a highly likely or probable local transmission.Citation38 For example, all the 6 strains of E. cloacae in this study were isolated in 2013 from the department of neurology, the department of burns and the ICU, and they have a close genetic relationship. This likely indicated the short-term clonal transmission of these strains in these departments. Importantly, two of them were isolated from venous catheter, which highlights the importance of performing invasive procedures in a standardized manner. ST167 and ST410 of E. coli were dominant clonal strains that are not only widely disseminated in China but also primary blaNDM-carrying strains and are considered to be of more clinically relevant in a recent multicenter study and are predicted to become a significant problem in clinical settings in China.Citation39 Our data confirmed this prediction. The ST11 derived from the clonal expansion of K. pneumoniae ST258 has become prevalent in many parts of the world since it was first identified in America.Citation40 ST258is the most abundant K. pneumoniae type currently in China and is similar to ST147.Citation12 Combined with the small nosocomial infections of ST11 and ST147 we found in this study, ST11 and ST147 should be the focus of hospital infection control measures and clinical studies. In contrast, reports of K. pneumoniae ST3073 are rare and only a single report from China. One possible cause of the unexplained acquisitions is the presence of undetected colonised hospital patients who were involved in the transmission of blaIMP-4-carrying isolates.
Except for clonal spread, the ARGs carried by CRE and especially carbapenemase genes can shuttle between resistant and susceptible strains by the acquisition of horizontally transmitted accessory genes located on transposons and plasmids. This is also the primary reason for the increase in nosocomial and community infections caused by CRE.Citation41 The dominant types of plasmids carrying blaNDM and blaIMP were IncX3, IncFII and IncN in China.Citation42,Citation43 We detected numerous plasmid replicons in our study and IncX3 and IncFII type predominated although several IncN types were also detected. We also investigated the genetic environments for blaNDM,blaIMP and blaKPC and found abundant mobile genetic elements consist in around CRGs. The majority of genetic environments were similar to genetic contexts previously reported, although they were diverse, and there were no obvious differences between species. These results indicated that ARGs can stably exist among different strains through horizontal transmission, posing a greater threat to the prevention and control of hospital infections.
Our findings illustrate the small transmission of CRE in our hospital, exists in the ward, between the ward and the ward, and this emphasizes the need for aggressive implementation of additional infection control strategies, especially in the environment of the COVID-19 pandemic. Many outbreak events involving CRE nosocomial transmission have been reported in COVID-19 pandemic due to the susceptibility of patients with severe COVID-19 and alterations to usual hospital workflow practices.Citation44 Therefore, pandemic response policies should emphasize the importance of maintaining nosocomial infection control practices to minimize collateral damage caused by disruptions in hospital workflow. It is conducive to the rehabilitation of patients with CRE infection by standardizing invasive operations, rational use of antibacterial drugs, and providing patients with quality care. Through the effective implementation of a series of contact prevention measures and active monitoring, the spread of CRE in hospitals can be effectively reduced.
As a retrospective study, this study has some limitations. First, it was conducted in a single hospital in Huizhou with a small sample size that may not represent the entire population. In addition, the analysis of existing data failed to provide a comprehensive view because insufficient environmental samples and patient foreign residence histories were unknown. These limitations could be resolved in future studies of CRE.
Conclusions
We described the phenotypic and genotypic characteristics of CRE strains obtained from a single hospital over the course of 5 years. The large representation of resistance phenotypes displayed by these strains left few treatment options. Importantly, the multiple CRE lineages we found would prolong nosocomial transmission. Alarmingly, 56% of isolates were associated with intra-hospital transmission, and consequently, containment strategies are needed to mitigate rapidly spreading CRE in healthcare settings.
Sequence Information
All genome assemblies of 52 isolates were deposited in GenBank and are registered with BioProject: PRJNA832055.
Ethical Approval
The ethical protocol was approved by the Ethics Committee of Huizhou Municipal Central Hospital. Informed written consent was obtained from the parents or legal guardian of the patient before inclusion in the study.
Disclosure
The authors have any conflict of interest.
Additional information
Funding
References
- Bell JM, Lubian AF, Partridge S, et al. Australian Group on Antimicrobial Resistance (AGAR) Australian Gram-negative Sepsis Outcome Programme (GNSOP) annual report 2019. Commun Dis Intell. 2020;44. doi:10.33321/cdi.2020.44.80
- Gajdács M, Albericio F. Antibiotic resistance: from the bench to patients. Antibiotics. 2019;8:8–11. doi:10.3390/antibiotics8030129
- Tamma PD, Han JH, Rock C, et al. Carbapenem therapy is associated with improved survival compared to piperacillin-tazobactam for patients with ESBL Bacteremia, for the Antibacterial Resistance Leadership Group. at University of Waterloo on January 21. Clin Infect Dis. 2015;60(9):1–21. doi:10.1093/cid/ciu714
- Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–67. doi:10.1093/cid/cir202
- Schwaber MJ, Carmeli Y. National Center for Infection Control, Ministry of Health, 6 Weizmann St. Israel ([email protected]) (Reprinted). JAMA. 2008;64239:2911.
- Walther-Rasmussen J, Høiby N. OXA-type carbapenemases. J Antimicrob Chemother. 2006;57:373–383. doi:10.1093/jac/dki482
- Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi:10.1016/S1473-3099(10)70143-2
- Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18:263–272. doi:10.1016/j.molmed.2012.03.003
- Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31:1–61. doi:10.1128/CMR.00088-17
- Leitner E, Zarfel G, Luxner J, et al. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother. 2015;59:714–716. doi:10.1128/AAC.04306-14
- Xiao SZ, Wang S, Wu WM, et al. The resistance phenotype and molecular epidemiology of Klebsiella pneumoniae in bloodstream infections in Shanghai, China, 2012–2015. Front Microbiol. 2017;8:1–8. doi:10.3389/fmicb.2017.00250
- Wang Q, Wang X, Wang J, et al. Phenotypic and Genotypic Characterization of Carbapenem-resistant Enterobacteriaceae: data from a Longitudinal Large-scale CRE Study in China (2012–2016). Clin Infect Dis. 2018;67:S196–S205. doi:10.1093/cid/ciy660
- Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20:821–830. doi:10.1111/1469-0691.12719
- Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis. 2018;66:1290–1297. doi:10.1093/cid/cix893
- Miriagou V, Cornaglia G, Edelstein M, Galani I, Giske CG, Gniadkowski M. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin Microbiol Infect. 2010;16(2):112–122. doi:10.1111/j.1469-0691.2009.03116.x
- Dolinsky AL. A consumer complaint framework with resulting strategies. J Serv Market. 1994;8(3):27–39. doi:10.1108/08876049410065598
- Sequencing S. The school of natural sciences presents applied math seminar: sPAdes: a new genome assembly algorithm 2013; 2013.
- Aziz RK, Bartels D, Best A, et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:1–15. doi:10.1186/1471-2164-9-75
- Treangen TJ, Ondov BD, Koren S, Phillippy AM. The harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genom Biol. 2014;15:1–15. doi:10.1186/s13059-014-0524-x
- Cheng L, Connor TR, Sirén J, Aanensen DM, Corander J. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol Biol Evolut. 2013;30:1224–1228. doi:10.1093/molbev/mst028
- Nascimento M, Sousa A, Ramirez M, Francisco AP, Carriço JA, Vaz C. PHYLOViZ 2.0: providing scalable data integration and visualization for multiple phylogenetic inference methods. Bioinformatics. 2017;33:128–129. doi:10.1093/bioinformatics/btw582
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55:4943–4960. doi:10.1128/AAC.00296-11
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hospital Epidemiol. 2009;30:972–976. doi:10.1086/605922
- Tamma PD, Goodman KE, Harris AD, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis. 2017;64:257–264. doi:10.1093/cid/ciw741
- Yu J, Tan K, Rong Z, et al. Nosocomial outbreak of KPC-2- and NDM-1-producing Klebsiella pneumoniae in a neonatal ward: a retrospective study. BMC Infect Dis. 2016;16:1–6. doi:10.1186/s12879-016-1870-y
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hospital Epidemiol. 2008;29:1099–1106. doi:10.1086/592412
- Akturk H, Sutcu M, Somer A, et al. Carbapenem-resistant Klebsiella pneumoniae colonization in pediatric and neonatal intensive care units: risk factors for progression to infection. Brazilian J Infect Dis. 2016;20:134–140. doi:10.1016/j.bjid.2015.12.004
- Zhang Y, Guo LY, Song WQ, Wang Y, Dong F, Liu G. Risk factors for carbapenem-resistant K. pneumoniae bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis. 2018;18:1–10. doi:10.1186/s12879-018-3160-3
- Khan AU, Maryam L, Zarrilli R. Structure, genetics and worldwide spread of New Delhi Metallo-β-lactamase (NDM): a threat to public health. BMC Microbiol. 2017;17:1–12.
- Chu YW, Afzal-Shah M, Houang ETS, et al. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. Collected in Hong Kong between 1994 and 1998. Antimicrob Agents Chemother. 2001;45:710–714. doi:10.1128/AAC.45.3.710-714.2001
- Sidjabat HE, Townell N, Nimmo GR, et al. Dominance of IMP-4-producing Enterobacter cloacae among carbapenemase-producing Enterobacteriaceae in Australia. Antimicrob Agents Chemother. 2015;59:4059–4066. doi:10.1128/AAC.04378-14
- Liu W, Dong H, Yan T, et al. Molecular characterization of blaIMP–4-carrying enterobacterales in Henan Province of China. Front Microbiol. 2021;12:1–8.
- Rodriguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of Infections caused by extended-spectrum-beta. Clin Microbiol Rev. 2018;31:1–42. doi:10.1128/CMR.00079-17
- Cienfuegos-gallet AV, Zhou Y, Ai W, Kreiswirth BN, Yu F, Chen L. Multicenter genomic analysis of carbapenem-resistant Klebsiella pneumoniae from Bacteremia in China. Microbiol Spectr. 2022;10(2):e02290–21.
- Roberts LW, Catchpoole E, Jennison AV, et al. Genomic analysis of carbapenemase-producing Enterobacteriaceae in Queensland reveals widespread transmission of blaimp-4 on an incHI2 plasmid. Microbial Genom. 2020;6. doi:10.1099/mgen.0.000321
- Dallman TJ, Byrne L, Ashton PM, et al. Whole-genome sequencing for national surveillance of Shiga Toxin-Producing Escherichia coli O157. Clin Infect Dise. 2015;61:305–312. doi:10.1093/cid/civ318
- Moura A, Criscuolo A, Pouseele H, et al. epidemiological surveillance of Listeria monocytogenes. Nature Microbiol. 2016;2:1–10.
- David S, Reuter S, Harris SR, et al. Europe PMC Funders Group Europe PMC Funders Author Manuscripts Europe PMC Funders Author Manuscripts Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nature. 2020;4:1919–1929.
- Zhang R, Liu L, Zhou H, et al. Nationwide Surveillance of Clinical Carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine. 2017;19:98–106. doi:10.1016/j.ebiom.2017.04.032
- Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother. 2009;53:3365–3370. doi:10.1128/AAC.00126-09
- Acman M, Wang R, van Dorp L, et al. Role of mobile genetic elements in the global dissemination of the carbapenem resistance gene blaNDM. Nature Commun. 2022;13:1–13. doi:10.1038/s41467-022-28819-2
- Wang Y, Tong MK, Chow KH, et al. Occurrence of highly conjugative IncX3 epidemic plasmid carrying blaNDM in Enterobacteriaceae isolates in geographically widespread areas. Front Microbiol. 2018;9:1–8. doi:10.3389/fmicb.2018.00001
- Wang Y, Lo WU, Lai RWM, et al. IncN ST7 epidemic plasmid carrying blaIMP-4 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. J Antimicrob Chemother. 2017;72:99–103. doi:10.1093/jac/dkw353
- Fernández-García OA, González-Lara MF, Villanueva-Reza M, et al. Outbreak of NDM-1-producing Escherichia coli in a coronavirus disease 2019 intensive care unit in a Mexican Tertiary Care Center. Microbiol Spectr. 2022;10:1–6. doi:10.1128/spectrum.02015-21
