Abstract
We carried out a comprehensive overview of inhibitory effects of selected antibiotics on planktonic and biofilm cells of Staphylococcus aureus (ATCC 29213) and Pseudomonas aeruginosa (ATCC 27853) strains. The possible involvement of protease activity and the lipopolysaccharide (LPS) profile of P. aeruginosa were also analyzed. Biofilm cells of both strains were more resistant to antibiotics than their planktonic counterparts. Protease activity was increased in both strains in the biofilm forms. Challenge with sublethal doses of antibiotics also increased proteolytic activity of biofilm cells. Additionally, the LPS profile of P. aeruginosa showed pattern alterations of the biofilm that can contribute to biofilm resistance and survival. These observations provide evidence for the involvement of bacterial proteolytic activity and LPS profile in the resistance of biofilm bacteria to antibiotics compared to their planktonic counterparts.
Many pathogenic and commensal bacteria are capable of transitioning between lifestyles in the environment and the human host.Citation1 These bacteria must be able to adapt to sudden shifts in availability of nutrients and to primary and secondary host immune defenses.Citation2 One particularly important and clinically relevant example of bacterial adaptation is the ability to grow as biofilms.Citation3–Citation5
Biofilms, a surface-associated bacterial community, are complex and ordered bacterial societies that are capable of growing in connection with different biological or inert surfaces.Citation1 The major clinical consequence of different disease-causing bacteria correlates with the problems of therapeutic killing of attached cells.Citation6 Biofilms are commonly associated with many health problems, such as endocarditis, otitis media, periodontitis, prostatitis, and urinary tract infections.Citation7–Citation10 Several bacteria, such as Escherichia coli, Staphylococcus aureus, Haemophilus influenza, and Pseudomonas aeruginosa, can form biofilms in the body tissues, leading to different infections.Citation10–Citation12 It has been estimated that biofilms account for two-thirds of the bacterial infections that physicians encounter, particularly in immunocompromised patients.Citation13
Antibiotics have been used to treat patients with infectious diseases. They target important bacterial structures and cellular pathways, such as the cell wall, DNA, RNA, protein synthesis machinery, and bacterial metabolism.Citation14 However, uncontrolled or long-term use of antibiotics results in the adaptation and development of resistance leading to treatment failure, prolonged or additional hospitalization, increased costs of care, and increased mortality.Citation11,Citation15 The mechanism of resistance of microbial biofilms to antibiotics is not clear. However, it seems to be multifactorial and may vary from one organism to another.Citation16 In this study we investigated the possible involvement of proteolytic activity and lipopolysaccharides (LPSs) in increased resistance to antibiotics during the biofilm state.
Materials and methods
Bacterial strains and culture
Pseudomonas aeruginosa (ATCC 27853) and S. aureus (ATCC 29213) strains were obtained from the American type culture collection and cultivated on Mueller Hinton agar (Becton Dickinson and Company, Cockeysville, MD, USA) for 24 hours at 37°C under standardized aseptic conditions.
Antimicrobial agents
The following antimicrobial agents were used for susceptibility testing against S. aureus: cefaclor (cephalosporins) at a concentration of (32 μg/mL), amoxicillin (aminoglycosides; 32 μg/mL), cotrimoxazole (sulfonamides/folic acid antagonists; 32 μg/mL), and ciprofloxacin (fluoroquinolones; 0.125 μg/mL). We used amikacin (aminoglycosides, 0.25 μg/mL) and cotrimoxazole (32 μg/mL), ciprofloxacin (0.0625 μg/mL), and ceftazidime (32 μg/mL) (cephalosporins) for susceptibility testing against P. aeruginosa. All antibiotics were used as raw material, and purchased from Sigma-Aldrich, MI, USA.
Bacterial culture
Staphylococcus aureus and P. aeruginosa biofilms were developed as previously describedCitation17 under standardized aseptic conditions. Briefly, 100 μL of bacterial suspension from each strain was cultivated in polypropylene tubes containing 2 mL of trypticase soy broth (TSB) supplemented with 1% glucose (Becton Dickinson and Company, Cockeysville, MD, USA) for 48 hours at 37°C. Culture media was refreshed after 24 hours of incubation. After 48 hours of incubation, biofilm cells were harvested by discarding the culture media and washing the tubes three times with phosphate buffer saline (PBS; pH 7.2) to remove nonadherent bacteria; the adhered cells were then harvested by vortex and centrifugation. The pellet was suspended in PBS (pH 7.2) to achieve the desired turbidity (comparable to a McFarland turbidity standard of 0.5). Screening for biofilm formation was achieved as previously described.Citation18 Briefly, after being emptied from their content, culture tubes were stained with trypan blue or safranin. Biofilms were judged by the appearance of a visible film lining the walls of the tube. Observations were carried out by three independent observers. Biofilms were scored as absent (score 0), weak (score 1), moderate (score 2), or strong (score 3). Average scores were used.
Determination of minimum inhibitory concentrations (MICs) of antibiotics for planktonic and biofilm cells
The MIC values of both S. aureus and P. aeruginosa planktonic and biofilm cells were tested against selected antibiotics. MICs were determined by using the broth macrodilution method.Citation19 Briefly, 100 μL of adjusted bacterial suspensions equivalent to a 0.5 McFarland standard were added to a twofold serial dilution of selected antibiotics diluted in Mueller Hinton broth. The results were observed after 24 hours of incubation at 37°C. The lowest concentration of antibiotic needed to inhibit microbial growth compared to the control culture was defined as the MIC. Tests were performed in triplicate for each antibiotic.
Influence of sub-MICs of selected antibiotics on biofilm cells
To determine the effects of sub-MICs of antibiotics on S. aureus and P. aeruginosa biofilms, 100 μL of a bacterial biofilm suspension was added to TSB (supplemented with 1% glucose) containing sub-MICs of each antibiotic (for S. aureus: ciprofloxacin 32 μg/ml, cotrimoxazole 32 μg/ml, cefaclor 32 μg/ml, amoxicillin 32 μg/ml; and for P. aeruginosa: ciprofloxacin 8 μg/ml, amikacin 0.003 μg/ml, ceftazidime 32 μg/ml), and the suspension + antibiotic was then incubated at 37°C for 24 hours. After incubation, the antibiotics were removed by washing the tubes three times, and the cells were pelleted for further investigation.
Proteolytic activity assay
Total protease activity of S. aureus and P. aeruginosa in planktonic and biofilm cells was determined by the azocasein assay.Citation20 Briefly, media from each bacterial strain (30 mL) was added to 50 mL azocasein substrate (2% azocasein (Sigma-Aldrich, MI, USA) in 10 mM Tris HCl, 8 mM CaCl2, pH 7.4). The reaction mixture was incubated for 20 hours. Thereafter, 240 mL 10% trichloroacetic acid was added, and the samples were mixed and allowed to stand for 15 minutes to ensure complete precipitation of undigested material. Tubes were centrifuged at 10,600 xg for 10 minutes, and 240 mL of the supernatant was transferred to tubes containing 280 mL 1.0 M NaOH. The absorbance at 440 nm was determined against a blank tube. One unit of enzyme activity corresponds to the absorbance at maximal digestion of 1 mg azocasein/hour.Citation21 The protease activity was expressed as units/106 bacteria/hour.Citation20
LPS extraction and analysis
We followed the LPS extraction kit guidelines (Intron Biotechnology, Kyungki-Do, Republic of Korea) to extract LPSs from P. aeruginosa planktonic and biofilm cells and biofilms induced with sub-MICs of antibiotics. The LPS profile was then determined using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) comprising a 4% stacking gel and a 12% separation gel.Citation22 The LPS gel was then fixed and stained according to the method of Tsai and Frasch.Citation23
Statistical analysis
Analysis was performed using GraphPad Prism software (version 4.0; GraphPad Software, Inc, La Jolla, CA). One-way analyses of variance followed by Dunnett’s post hoc test were used to determine any statistically significant difference. A P-value < 0.05 was considered significant.
Results
The MIC values of selected antibiotics against S. aureus and P. aeruginosa biofilm and planktonic cells were determined ( and ). The MIC values of biofilms were generally higher than their planktonic counterparts.
We determined protease activity of S. aureus and P. aeruginosa in order to evaluate the possible involvement of proteolytic activity in the resistance of the biofilm form of bacteria ( and ). Results demonstrated that control biofilm had significantly higher proteolytic activity than its planktonic counterpart. When biofilms cells were exposed to sub-MICs of selected antibiotics, most showed a slight but not significant increase in their proteolytic activity.
Table 1 Protease activity of Staphylococcus aureus cells
Table 2 Protease activity of Pseudomonas aeruginosa cells
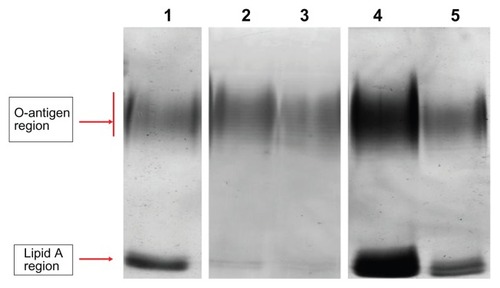
LPSs of the P. aeruginosa cell membrane also have an essential barrier function and directly affect bacterial susceptibility for antibiotics.Citation24 We therefore analyzed the LPS profile by SDS–PAGE and silver stain. LPSs displayed a ladder-like pattern of bands with the slower migrating band of the LPS extract in the O-antigen region and the faster band in the lipid A region (). In comparison to planktonic cells, biofilm-forming cells showed a different LPS profile; the faster migrating band (lipid A) had an increased staining intensity and a slightly decreased number of bands in the O-antigen region. In the presence of (1/8) MIC of ceftazidime, the number of bands in the O-antigen region increased and the faster migrating band (lipid A) decreased to being barely observable when compared with the control biofilm. For (1/4) MIC of ciprofloxacin and (1/8) MIC of amikacin, the number of bands in the O-antigen region decreased slightly and lipid A intensity increased.
Figure 1 Electrophoretic profile of LPS of Pseudomonas aeruginosa.
Notes: Lane 1, LPS extracted from biofilm cells; lane 2, LPS extracted from planktonic cells; lane 3, LPS extracted from biofilm cells treated with (1/8) MIC of ceftazidime; lane 4, LPS extracted from biofilm cells treated with (1/4) MIC of ciprofloxacin; lane 5, LPS extracted from biofilm cells treated with (1/8) MIC of amikacin.
Abbreviations: LPS, lipopolysaccharide; MIC, minimum inhibitory concentration.
Discussion
Biofilm forms of bacteria are responsible for a variety of life-threatening infections. They have the ability to resist attack by host defenses and show resistance to most antibiotics.Citation25,Citation26 A wide range of pathogens, such as P. aeruginosa and S. aureus, are capable of forming biofilms. Both bacterial types are medically significant microbes and can cause implant and prosthetic device infections. Thus, assessment of possible mechanisms for antibiotic resistance in their biofilm form is critical.
Results of this study showed that proteolytic activity increases when bacteria switch from a planktonic to biofilm phenotype. This indicates that biofilms are more virulent and have a greater ability to cause tissue destruction, which correlates with the conclusions of previous studies.Citation27–Citation29 Additionally, the proteolytic potential slightly increased when biofilms were exposed to sublethal concentrations of selected antibiotics. This possibly explains results of clinical studies that show increased severity of disease when subtherapeutic doses or inadequate duration of antibiotics are used.Citation30–Citation33
LPSs are a major constituent of the P. aeruginosa membrane, and changes observed in membrane structure may result in changes to the antibiotic permeability barrier.Citation34,Citation35 For example, the presence of full-length O-antigen renders the LPS smooth, whereas absence or reduction of O-antigen makes the LPS rough. This represents a bacterial shift from an acute to chronic lifestyle, leading to increased persistence of bacteria and a consequent high relapse of disease.Citation36 Results of our study showed decreased O-antigen and increased lipid A in biofilm-forming cells compared to planktonic cells, indicating a phenotypic switch in the LPSs from a smooth form to a rough form.Citation37
Apart from an LPS role in resistance, LPSs are generally considered endotoxins.Citation38 Accordingly, the increased virulence of P. aeruginosa biofilms compared to the planktonic form could be related to an increase in lipid A. In the LPS pattern of P. aeruginosa-treated biofilms, lipid A expression in biofilms exposed to amikacin and ciprofloxacin was up-regulated compared to untreated biofilms. These changes in LPS expression indicate that antibiotic-exposed biofilms had more virulence potential than untreated biofilms. Further studies are required to elucidate the mechanisms by which these antibiotics induce changes in LPSs.
In this study we investigated the effect of certain antibiotics on proteolytic activity of P. aeruginosa and S. aureus and/or membrane LPSs of P. aeruginosa. We chose antibiotics that are most commonly used for the treatment of infections by these two bacterial strains. Future work could cover other important antibiotics and also commonly used antibiotics, such as vancomycin and aztreonam. Studies should also address the possibility of membrane protein involvement in increased virulence of biofilms, especially when challenged with sublethal concentrations of antibiotics.
Collectively, the antibiotic susceptibility results presented in this study showed that biofilms are more tolerant to antimicrobial agents than planktonic forms. Biofilms (control and treated strains) revealed changes in proteolytic activity and LPS patterns that may result in antibiotic resistance. A decrease in O-antigen bands of LPSs could be a mechanism that helps biofilms evade the immune system, while increased lipid A contents may indicate an increase in biofilm endotoxicity. These LPS changes along with increased protease activity indicate that biofilms are more virulent than their planktonic counterparts.
Supplementary tables
Table S1 Minimum inhibitory concentration values of Staphylococcus aureus planktonic and biofilm cells
Table S2 Minimum inhibitory concentration values of Pseudomonas aeruginosa planktonic and biofilm cells
Acknowledgment
This project was supported by a grant (No 37/2010) from the Deanship of Research at the Jordan University of Science and Technology.
Disclosure
The authors report no conflicts of interest in this work.
References
- JohnjulioWFugeLHKadMPostCIntroduction to biofilms in family medicineSouth Med J20121051242922189663
- WolcottRDowdSThe role of biofilms: are we hitting the right target?Plast Reconstr Surg2011127Suppl 128S35S21200270
- BrooksJLJeffersonKKStaphylococcal biofilms: quest for the magic bulletAdv Appl Microbiol201281638722958527
- AparnaMSYadavSBiofilms: microbes and diseaseBraz J Infect Dis200812652653019287843
- LynchASRobertsonGTBacterial and fungal biofilm infectionsAnnu Rev Med20085941542817937586
- WangXWoodTKToxin-antitoxin systems influence biofilm and persister cell formation and the general stress responseAppl Environ Microbiol201177165577558321685157
- WilsonSKCostertonJWBiofilm and penile prosthesis infections in the era of coated implants: a reviewJ Sex Med201291445321951338
- HuangRLiMGregoryRLBacterial interactions in dental biofilmVirulence20112543544421778817
- VlassovaNHanAZenilmanJMJamesGLazarusGSNew horizons for cutaneous microbiology: the role of biofilms in dermatological diseaseBr J Dermatol2011165475175921668434
- DaveyMEO’TooleGAMicrobial biofilms: from ecology to molecular geneticsMicrobiol Mol Biol Rev200064484786711104821
- KydJMMcGrathJKrishnamurthyAMechanisms of bacterial resistance to antibiotics in infections of COPD patientsCurr Drug Targets201112452153021194403
- JensenPOGivskovMBjarnsholtTMoserCThe immune system vs Pseudomonas aeruginosa biofilmsFEMS Immunol Med Microbiol201059329230520579098
- SawhneyRBerryVBacterial biofilm formation, pathogenicity, diagnostics and control: An overviewIndian J Med Sci200963731332119700915
- GaynorMMankinASMacrolide antibiotics: binding site, mechanism of action, resistanceCurr Top Med Chem20033994996112678831
- CantonRMorosiniMIEmergence and spread of antibiotic resistance following exposure to antibioticsFEMS Microbiol Rev201135597799121722146
- ZhangLMahTFInvolvement of a novel efflux system in biofilm-specific resistance to antibioticsJ Bacteriol2008190134447445218469108
- CernohorskaLVotavaMAntibiotic synergy against biofilm-forming Pseudomonas aeruginosaFolia Microbiol (Praha)2008531576018481219
- ChristensenGDSimpsonWAYoungerJJAdherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devicesJ Clin Microbiol198522699610063905855
- Clinical and Laboratory Standards Institute (CLSI)Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standardninth editionVillanova, PA2012
- SchmidtchenAWolffHHanssonCDifferential proteinase expression by Pseudomonas aeruginosa derived from chronic leg ulcersActa Derm Venereol200181640640911859942
- OkamotoTAkaikeTSugaMActivation of human matrix metalloproteinases by various bacterial proteinasesJ Biol Chem19972729605960669038230
- DuanZGYanXJHeXZExtraction and protein component analysis of venom from the dissected venom glands of Latrodectus tredecimguttatusComp Biochem Physiol B Biochem Mol Biol20061453–435035717029995
- TsaiCMFraschCEA sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gelsAnal Biochem198211911151196176137
- HoekstraJLde NeelingAJvan KlingerenVStobberinghEEvan BovenCPResistant strains of Pseudomonas aeruginosa isolated after exposure to several beta-lactam antibioticsEur J Clin Microbiol19876122273106033
- CosPToteKHoremansTMaesLBiofilms: an extra hurdle for effective antimicrobial therapyCurr Pharm Des201016202279229520433417
- KhanWBernierSPKuchmaSLHammondJHHasanFO’TooleGAAminoglycoside resistance of Pseudomonas aeruginosa biofilms modulated by extracellular polysaccharideInt Microbiol201013420721221404215
- HoibyNBjarnsholtTGivskovMMolinSCiofuOAntibiotic resistance of bacterial biofilmsInt J Antimicrob Agents201035432233220149602
- HoibyNCiofuOJohansenHKThe clinical impact of bacterial biofilmsInt J Oral Sci201132556521485309
- SimoesMAntimicrobial strategies effective against infectious bacterial biofilmsCurr Med Chem201118142129214521517762
- FluitACSchmitzFJBacterial resistance in urinary tract infections: how to stem the tideExpert Opin Pharmacother20012581381811336624
- RuppMEHamerKEEffect of subinhibitory concentrations of vancomycin, cefazolin, ofloxacin, L-ofloxacin and D-ofloxacin on adherence to intravascular catheters and biofilm formation by Staphylococcus epidermidisJ Antimicrob Chemother19984121551619533456
- HattJKRatherPNRole of bacterial biofilms in urinary tract infectionsCurr Top Microbiol Immunol200832216319218453276
- FreiEHodgkiss-HarlowKRossiPJEdmistonCEJrBandykDFMicrobial pathogenesis of bacterial biofilms: a causative factor of vascular surgical site infectionVasc Endovascular Surg201145868869621921082
- FernandezLBreidensteinEBSongDHancockRERole of intracellular proteases in the antibiotic resistance, motility, and biofilm formation of Pseudomonas aeruginosaAntimicrob Agents Chemother20125621128113222123702
- BreidensteinEBde la Fuente-NunezCHancockREPseudomonas aeruginosa: all roads lead to resistanceTrends Microbiol201119841942621664819
- AnuntagoolNWuthiekanunVWhiteNJLipopolysaccharide heterogeneity among Burkholderia pseudomallei from different geographic and clinical originsAm J Trop Med Hyg200674334835216525090
- CoulonCVinogradovEFillouxASadovskayaIChemical analysis of cellular and extracellular carbohydrates of a biofilm-forming strain Pseudomonas aeruginosa PA14PLoS One2010512e1422021151973
- SawasdidolnCTaweechaisupapongSSermswanRWTattawasartUTungpradabkulSWongratanacheewinSGrowing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistancePLoS One201052e919620169199