Abstract
Entecavir is a cyclopentyl deoxyguanosine analog that was approved for the treatment of the hepatitis B virus (HBV) in 2005. In Phase III trials, it showed potent HBV suppression with drops of 6- to 7-log copies/mL in HBV DNA at 1 year. In addition, rates of genotypic resistance in nucleos(t)ide-naïve patients are low, reaching only 1.2% after 6 years. Safety and efficacy have been established in compensated cirrhosis and HIV-coinfected patients. Studies in decompensated cirrhosis also show efficacy. Because of potent viral suppression and a large genetic barrier to resistance, entecavir is now a first-line choice in most HBV treatment guidelines and has become an integral part of the HBV treatment armamentarium.
Keywords:
Introduction
During the past decade, four new drugs (adefovir, entecavir, telbivudine, and tenofovir) have been approved for the treatment of chronic hepatitis B virus (HBV), joining lamivudine as options for treatment. These new additions to the HBV arsenal have made decisions about treatment more complex. It has become increasingly important for treating physicians to understand the benefits and limitations of each agent as they decide on an initial or salvage regimen. This review will describe the efficacy of entecavir compared with other available drugs, as well as safety and resistance patterns. These issues are important to consider for drug sequencing in patients with chronic HBV.
Natural history of hepatitis B
Over 350 million people worldwide are chronically infected with HBV.Citation1 The burden of disease is largely geographic, with South-East Asia and Sub-Saharan Africa being endemic.Citation1 In these areas, transmission is primarily vertical or during childhood, where most infections go on to chronicity.Citation2 In nonendemic areas, such as western Europe and the US, transmission is primarily sexual and occurs in early adulthood. When acquired later during life in this situation, 95% of acute HBV cases resolve spontaneously.Citation2 Universal vaccination of infants in both endemic and nonendemic areas will no doubt change this epidemiology over the coming decades.
Once chronicity is established, the patient may undergo a period of immunotolerance, where HBV DNA levels are extremely high yet aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are normal.Citation3 There is little to no inflammation present in the liver. In endemic areas, the immunotolerant period may last years, into the third decade of life. In nonendemic areas, however, the immunotolerant period generally is so short as to not be recognized. Following the immunotolerant period is the time of immune clearance, or active chronic HBV. HBV DNA levels are still high, and ALT and AST levels also become elevated as an indication of active inflammation of the hepatic tissue. Hepatitis B e antigen (HBeAg) is positive. If the patient maintains this state for longer than 6 months (distinguishing acute HBV from chronic HBV), treatment is indicated.
Some patients will pass through the immune clearance phase without treatment and become inactive carriers of HBV, where HBV surface antigen remains positive but HBeAg becomes negative and hepatitis B e antibody (anti-HBe) develops. The HBV DNA levels during this time are low or undetectable, and AST and ALT levels are normal. In many cases, this carrier state persists long term, but, in others, HBV can reactivate as chronic “e-antigen-negative” HBV.Citation3 HBV DNA levels again rise, AST and ALT levels become elevated, and treatment is indicated once again. This is a later stage in the natural history of disease and can progress to cirrhosis more quickly.Citation4 Typically, e-antigen-negative HBV occurs due to a mutation in the precore region that prohibits e-antigen from being produced.Citation5
The endpoints for studies of therapeutics in HBV have been standardized according to a workshop convened by the National Institutes of Health.Citation6 New agents are studied separately in e-antigen-positive patients and e-antigen-negative patients. For both subgroups, endpoints include virologic suppression (drop in HBV DNA), biochemical response (normalization of ALT), and histological response (decrease in Knodell necroinflammatory score by at least two points with no worsening of fibrosis scores). For HBeAg-positive patients, additional endpoints of HBeAg loss and HBeAg seroconversion (development of anti-HBe) are also included. All of these endpoints are surrogate endpoints. More long-term follow-up is needed to determine whether these agents improve mortality or decrease development of end-stage liver disease or hepatocellular carcinoma (HCC).
Mechanism and pharmacokinetics of entecavir
All HBV therapies interrupt HBV replication by acting as nucleos(t)ide analogs. The chemical structure of lamivudine is 2′3′-dideoxy-3′-thiacytidine. Adefovir is a nucleotide analog of adenosine monophosphate. Tenofovir is structurally similar to adefovir. Telbivudine is a l-nucleoside analog of deoxythymidine.
Entecavir is a cyclopentyl guanosine nucleoside analog that has selective activity against the HBV polymerase.Citation7 It is efficiently phosphorylated to its active triphosphate form by host cellular kinases.Citation8 Entecavir blocks HBV replication at three essential steps: priming of the HBV polymerase, elongation of the DNA strand via reverse transcription, and DNA-dependent plus-strand DNA synthesis and polymerization.Citation9 In contrast, lamivudine shows negligible effects on the priming reaction.
In pharmacokinetic studies in healthy subjects, peak plasma concentrations of entecavir were reached in 0.5–1.5 hours, with steady state reached after 6–10 days of once-daily administration.Citation7 The intracellular half-life is approximately 15 hours. It is predominantly excreted by the kidney with urinary recovery of the unchanged drug at steady state ranging from 62% to 73% of the administered dose.Citation7 Because of this, entecavir requires dosage adjustment for patients with creatinine clearance less than 50 mL/min, including those on dialysis.Citation7 No dosage adjustment is required in liver disease. Pharmacokinetic studies have shown that entecavir is not a substrate, inhibitor, or inducer of the cytochrome P450 enzyme system, and therefore the pharmacokinetics of entecavir are unlikely to be affected by coadminstration of agents that are metabolized by, inhibit, or induce these enzymes.Citation7
Clinical studies of efficacy
Phase I dose-ranging studies of entecavir demonstrated rapid and potent dose-related virologic suppression of HBV DNA.Citation10,Citation11 Because of shared resistance mutations between entecavir and lamivudine, clinical trials evaluated its use in both nucleos(t)ide-naïve patients and lamivudine-refractory patients. Efficacy was reduced in lamivudine-refractory patients harboring mutations in the tyrosine–methionine–aspartate–aspartate (YMDD) motif of the HBV polymerase; thus, a higher dosage of 1 mg daily was selected for development in these patients, compared with 0.5 mg/day for nucleos(t)ide-naïve patients.
Two multinational Phase III trials were conducted among nucleos(t)ide-naïve patients in HBeAg-positive patientsCitation12 and HBeAg-negative patients,Citation13 both compared with lamivudine 100 mg daily. A Phase III trial was also carried out in China in a mixed population of both HBeAg-positive and HBeAg-negative patients.Citation14 A smaller Phase III study compared entecavir with adefovir in patients with baseline HBV DNA levels >8 log copies/mL.Citation15 Among HBeAg-positive patients, results at 48 weeks were similar across all studies with 68%–89% of patients achieving ALT normalization, and 58%–74% having an undetectable HBV DNA by sensitive polymerase chain reaction (PCR) assay (). The mean drop in HBV DNA over 48 weeks was 6–7 log copies/mL. On all of these parameters, entecavir outperformed the comparator drug. Rates of HBeAg loss were 18%–22% and HBeAg seroconversion 15%–21%, which were no different from rates seen in the lamivudine arms of the trial. Only one study reported on histologic response (its primary endpoint), with 72% of entecavir-treated patients responding favorably.Citation12
Table 1 Summary of clinical trials of entecavir
Results at 48 weeks were similar among nucleoside-naïve HBeAg-negative patients, though the number of patients treated in the Chinese study was small (n = 33).Citation13,Citation14 High rates of ALT normalization (78%–94%), undetectable HBV DNA by PCR assay (90%–94%), and histologic response (70%) were seen. The mean drop in HBV DNA was approximately 5 log copies/mL.
Phase II and III studies in the more difficult-to-treat lamivudine-refractory population were completed in multinationalCitation12,Citation18 and Asian cohorts.Citation19,Citation20 Week 48 HBV DNA suppression was not as robust as seen in naïve patients, with mean decreases of about 5 log copies/mL. ALT normalization was seen in 61%–85% of patients, and HBV DNA undetectability by PCR assay in just 19%–33% of subjects at the end of 1 year. Among HBeAg-positive patients, HBeAg loss was seen in 10%–11%, and HBeAg seroconversion in 4%–8%. Histologic responses were seen in 55%–60% of subjects.Citation18,Citation20
The design of the entecavir trials makes determinations of efficacy beyond 48 weeks confusing. In most trials, patients who were classified as “responders” (in HBeAg-positive patients: HBV DNA < 0.7 mEq/mL [∼700,000 copies/mL], loss of HBeAg; in HBeAg-negative patients: HBV DNA < 0.7 mEq/mL) were removed from the study drug at 48 weeks and monitored off therapy for sustained response without the now standard period of consolidation therapy following HBeAg seroconversion.Citation23 Nonresponders (HBV DNA > 0.7 mEq/mL at week 48) were also discontinued from therapy. Therefore, those continuing on entecavir for 2 years and beyond represent a biased cohort of patients from which conclusions regarding entecavir efficacy must be made with caution. However, published data suggest that entecavir maintains virologic suppression and ALT normalization through up to 5 years of treatment, with additional cumulative HBeAg seroconversion and a small chance of HBV surface antigen (HBsAg) loss.Citation16,Citation17,Citation21,Citation22,Citation24
Compared with other available nucleos(t)ide analogs, entecavir achieves more potent HBV DNA suppression than all agents except perhaps tenofovir, which is equivalent. HBeAg clearance and HBeAg seroconversion are comparable with lamivudine. As with most oral agents, surface antigen loss is rare. Comparative efficacies of available agents at 1 year are shown in .
Table 2 Comparative efficacies at 1 year of available nucleos(t)ide analogs for the treatment of hepatitis B
Entecavir resistance
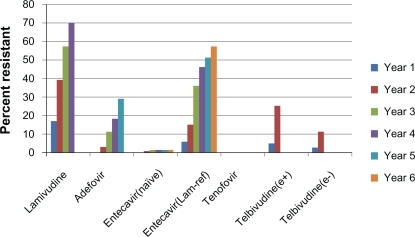
One disadvantage of nucleos(t)ide analogs is development of antiviral resistance with prolonged use. Resistance is accompanied by virologic and biochemical breakthrough, and, in rare cases, hepatic decompensation and death.Citation34,Citation35 Rates of lamivudine resistance exceed 70% by year 4 of treatment,Citation36 limiting its usefulness for first-line therapy. Resistance rates for adefovir and telbivudine are lower, yet still clinically significant (). Tenofovir resistance has not yet been well defined but appears to be rare, as no signature mutation has been defined and genotypic resistance through 4 years of therapy has not been reported.Citation41
Figure 1 Resistance rates of available nucleos(t)ide analogs in hepatitis B patients. Note that no tenofovir resistance has been seen through 4 years of follow-up.Citation31,Citation36–Citation42
Abbreviations: e+, hepatitis B e-antigen positive; e−, hepatitis B e-antigen negative; lam-ref, lamivudine refractory.
Resistance to HBV antivirals is conferred by mutations in the HBV polymerase, which render the agent ineffective. For lamivudine, the signature mutation is a change in the reverse transcriptase at position 204 from methionine to valine or isoleucine (rtM204V/I).Citation43 The rtM204I mutation also confers resistance to telbivudine.Citation42 Lamivudine-resistant patients may develop a compensatory mutation at position 180 from leucine to methionine (rtL180M), which restores viral fitness. Adefovir does not show cross-resistance with lamivudine or telbivudine, with its signature resistance mutations a change from asparagine to threonine at position 236 (rtN236T)Citation44 or alanine to valine or threonine at position 181 (rtA181V/T).Citation45
Entecavir resistance is unique because it requires up to three mutations for full resistance to develop.Citation46 The rtM204V/I mutation, lamivudine’s signature mutation, is necessary but not sufficient for entecavir resistance, causing an 8- to 10-fold decrease in susceptibility to entecavir compared with wild-type HBV. Additional mutations at positions rtI169, rtT184, rtS202, and rtM250 confer additional decreases in entecavir susceptibility.Citation46,Citation47 These mutations alone have only a minimal impact on entecavir efficacy, but in the presence of an rtM204 mutation, the addition of one of these mutations leads to a 10- to 250-fold decrease in entecavir susceptibility and two mutations to a 500- to 1000-fold decrease.Citation46,Citation47
The large genetic barrier to resistance has predictably led to low observed rates of clinical genotypic entecavir resistance in trials of nucleos(t)ide-naïve HBV patients. In the registration trials of entecavir, rates of genotypic resistance after 1 year were 0% in both HBeAg-positive and HBeAg-negative patients.Citation12,Citation13 A long-term resistance cohort from these trials has demonstrated that, after 4 years, cumulative resistance in naïve patients was 0.8% and, after 6 years, 1.2%.Citation37 Cumulative genotypic resistance rates among lamivudine-refractory patients are much higher: 6%, 15%, 36%, 47%, 51%, and 57% at years 1–6, respectively.Citation37 Although entecavir has been studied in lamivudine-refractory patients, its use as a salvage drug in this setting is generally not recommended when other options are available, because of these higher resistance rates. This demonstrates the superiority of entecavir when used as a first-line agent rather than following lamivudine failure, and highlights the importance of considering antiviral sequencing when selecting initial HBV therapy.
Special populations
Advanced fibrosis, cirrhosis, and transplanted patients
Approximately 15% of the subjects enrolled in the registration trials of entecavir had advanced fibrosis, defined as an Ishak score of 4–6. All patients had compensated liver disease. Results in this subgroup of patients were published separately.Citation48 For all endpoints examined in the parent studies, subjects with advanced fibrosis achieved comparable efficacy with the overall study population, including HBeAg-positive, HBeAg-negative, and lamivudine-refractory populations. Up to 59% of patients experienced an improvement in Ishak fibrosis score, which was better than that seen in the study population at large. Patients with advanced fibrosis or cirrhosis did not experience any more adverse events than the overall study population. No patient discontinued entecavir due to adverse events. There was a single ALT flare in the entecavir group. All deaths in the entecavir arm during the trial occurred in the subgroup with advanced fibrosis or cirrhosis, but no death was deemed related to study drug. One patient died from a gastrointestinal bleed secondary to a splenic lymphoma, one from hepatocellular carcinoma, and one from multiorgan failure from diabetes mellitus and cirrhosis.Citation48
Experience with entecavir in decompensated cirrhosis has shown improvements in Child–Turcotte–Pugh (CTP) scores (from 8.1 to 6.6) and Model for End-stage Liver Disease (MELD) scores (from 11.1 to 8.8) after 1 year of therapy.Citation49 In this single-center study, all patients were nucleos(t)ide naïve, as well as naïve to interferon. Compared with patients with compensated liver disease, those with decompensated cirrhosis had comparable rates of undetectable HBV DNA at month 12 (89.1% versus 78.5%, P = 0.104), HBeAg sero-conversion (22.2% versus 24.4%, P = 0.812), HBeAg loss (48.1% versus 41.1%, P = 0.512), and ALT normalization (76.4% versus 75.0%, P = 0.535).Citation49 There were no virologic breakthroughs on therapy. The cumulative incidence of mortality was 17% and the cumulative incidence of HCC or liver transplant was 6.9% among the decompensated cirrhotics, but no control group was reported.
Entecavir has also been compared with adefovir in a Phase IIIb study in subjects with evidence of hepatic decompensation.Citation50 At baseline, the mean HBV DNA in the entecavir group was 7.53 log copies/mL, mean MELD 17.1, and mean CTP score 8.81. Thirty-six percent of subjects were lamivudine resistant, and 54% were HBeAg positive. In an interim analysis of the first 100 patients to complete 96 weeks of treatment, entecavir had a statistically significant higher rate of HBV DNA < 300 copies/mL at week 48 compared with adefovir (57% versus 20%, respectively, P < 0.0001). Entecavir also had higher rates of ALT normalization (63% versus 46%, P = 0.0425) at week 48. Rates of HBeAg loss (11% versus 18%), HBeAg seroconversion (6% versus 10%), and HBsAg loss (5% versus 0%) were not statistically different. As shown in the prior study, MELD scores improved by −2.6 in the entecavir group. Thirty-five percent of entecavir-treated patients had a reduction in their CTP score by two or more points at week 48. Serious adverse events occurred in over 65% of both treatment groups, but the details of these events were not reported. Adverse events led to drug discontinuation in 7% of entecavir patients and 6% of adefovir patients. Further follow-up of these patients is needed to determine the effects of antivirals on patients’ mortality and development of HCC. Although no major events were reported in these trials of entecavir in decompensated patients, caution is advised with its use in these fragile patients, as later case reports demonstrated fatal lactic acidosis.
There is little published information on entecavir in the post-liver transplant population. A single-center retrospective case-control study of 30 patients treated with entecavir compared with lamivudine showed that entecavir achieved undetectable HBsAg levels earlier than lamivudine (median 3 days versus 5 days).Citation51 There were fewer HBV recurrences in the entecavir group (0% versus 11.1% in the lamivudine group), but cumulative survival rates after 30 months were similar in both groups (actuarial survival 81%). Larger, prospective trials are needed in this population, but the limited data available are favorable. Given the long-term therapy necessary post-transplant, the low resistance rates associated with entecavir make it an attractive option for post-transplantation prophylaxis.
HIV
Entecavir has been studied in HIV-positive patients failing lamivudine-containing antiretroviral regimens.Citation52 Ninety-five percent of these patients had lamivudine resistance at baseline, and the 1 mg dose of entecavir was used. At 48 weeks, HBV DNA decreased by 4.2 log copies/mL, and 8% of subjects had an undetectable HBV DNA. ALT normalization occurred in 37%, and only 2% achieved HBeAg loss/HBeAg seroconversion.
During clinical development, entecavir was not shown to have any activity against HIV. It therefore became the treatment of choice for HIV patients requiring treatment of HBV who did not meet criteria for antiretroviral therapy for HIV. Following its approval, reports of entecavir’s effects on HIV replication and resistance were reported.Citation53,Citation54 In these reports, entecavir decreased HIV viral loads by about 1 log copies/mL. More sensitive in vitro assays using primary CD4+ T cells rather than transformed cell lines confirmed entecavir inhibition of HIV in the low nanomolar range.Citation53 In addition to effects on HIV viral load, entecavir also selected for the methionine to valine mutation at position 184 (M184V) of the HIV reverse transcriptase, which is the signature mutation for HIV resistance to lamivudine. This occurred in three patients in the absence of any exposure to antiretrovirals.Citation54 Because of these reports, entecavir is not recommended as monotherapy in HIV/HBV-coinfected patients who are not receiving a fully active antiretroviral regimen. Most recent HIV treatment guidelines from the United States Department of Health and Human ServicesCitation55 and the International AIDS Society-USACitation55,Citation56 list active chronic HBV as an indication for antiretroviral therapy in HIV patients, regardless of CD4 count or HIV viral load. This recommendation stems in part from these findings related to entecavir activity.
Adverse effects
During the Phase III trials of entecavir, there were no adverse effects of the drug that occurred more often than with the comparator drug. The most common adverse events were elevations in ALT, which were associated in all cases with reductions in HBV DNA.Citation12,Citation13 In most cases, these were self-limiting with continued treatment with entecavir. Among nucleoside-naïve HBeAg-positive patients, ALT flares over twice baseline and > 10 times upper limit of normal (ULN) occurred in 3% of entecavir-treated patients, and ALT flares over twice baseline and more than five times ULN occurred in 10% of subjects.Citation12 These numbers in naïve HBeAg- negative subjects were < 1% and 2%, respectively.Citation13 Discontinuation due to adverse events was 2% or less in both studies at 1 year. There were no unusual adverse effects during the Phase II and III trials that were concerning.
Experience with nucleoside analogs in the treatment of HIV demonstrated an association with certain drugs and lactic acidosis variably accompanied by hepatic steatosis, pancreatitis, peripheral neuropathy, and myopathy. The dideoxy-nucleotide analogs were particularly implicated (didanosine [ddI], zalcitabine [ddC]). Development of an investigational drug for HBV, fialuridine (FIAU), was halted after seven patients in a Phase II trial developed severe lactic acidosis after a minimum of 9 weeks of therapy.Citation57 Five patients in the study died, and two survived only after emergent liver transplant. All seven had pancreatitis with severe lactic acidosis and hepatic failure. Histologic examination showed marked macro- and microvesicular steatosis and cholestasis. Mitochondrial anatomy was distinctly abnormal on electron microscopy. In part because of these clinical reports, all nucleos(t)ide analogs, including entecavir, carry a black-box warning against the occurrence of lactic acidosis and severe hepatomegaly with steatosis.
Further study into the mechanisms for both HIV antiret-roviral and FIAU toxicity implicate damage to mitochondria as the reason for metabolic perturbation.Citation58–Citation60 In addition to inhibition of the viral polymerase, nucleoside analogs also inhibit the polymerase function of the human DNA polymerase-γ, which is responsible for replication of mitochondrial DNA.Citation60 Depletion of mitochondrial DNA leads to mitochondrial dysfunction, as synthesis of proteins essential for oxidative phosphorylation is inhibited. Mutations in mitochondrial DNA and oxidative stress within the mitochondria may also contribute to this dysfunction. Electron leakage from the electron transport chain occurs with disruption of oxidative phosphorylation, increasing production of reactive oxygen species.Citation60
In vitro studies of entecavir did not show any evidence for mitochondrial toxicity.Citation61 Incubation of hepatoma HepG2 cells with entecavir at 10 times maximum concentration produced no nonspecific cell cytotoxicity. Entecavir also did not affect extracellular lactate levels at the highest concentrations and durations tested. In assays of mitochondrial DNA, levels were not decreased on exposure to entecavir when compared with controls. Even at 100 times achievable concentrations in vivo, there were no changes in mitochondrial protein levels. Assays to assess DNA polymerase-γ showed that entecavir did not utilize or inhibit function.
Despite these in vitro studies, postmarketing case reports of severe lactic acidosis during treatment with entecavir were published.Citation62,Citation63 In the first report, entecavir was used in combination with adefovir to treat a newly recognized HBV patient who developed a severe flare of hepatitis following chemotherapy for chronic lymphocytic leukemia.Citation63 At the time of treatment initiation, his MELD score was approximately 24. His ALT was 3521 U/L, AST 2853 U/L, and bilirubin 20.9 mg/dL. After 10 days of combination therapy, he presented with dyspnea, hypoxia, and profound metabolic lactic acidosis with a lactate level of 9.5 mEq/L, pH 6.95, and carbon dioxide level 8 mmol/L. Entecavir and adefovir were discontinued. Despite aggressive measures, the patient expired within 24 hours of admission.
A second report describes a case series of 16 patients with advanced liver disease/decompensated cirrhosis treated with entecavir, of whom five developed lactic acidosis.Citation62 In three cases, the syndrome was severe, and one of these cases died. Of the severe cases, lactic acidosis developed at 5 days, 8 days, and 8 months after initiation of entecavir. One of these cases was a post-liver transplant patient with recurrent HBV. Two additional patients had less severe lactic acidosis, which remained compensated. They developed symptoms at 4 and 31 days after starting entecavir therapy. In this series, all of the patients who developed lactic acidosis had an MELD score greater than 22 at the time of entecavir initiation. None of the patients whose MELD was lower than 18 developed lactic acidosis.
Neither of these reports provided histologic or microscopic evidence of mitochondrial damage. Only one patient in the second series was reported to have hepatic steatosis. Whether these reports of lactic acidosis represent manifestations of mitochondrial toxicity cannot be determined, although phenotypically they are suspicious. Of note, toxicity occurred much earlier in treatment than is usually seen with mitochondrial toxicity with other nucleoside analogs, suggesting a possible alternative mechanism for the lactic acidosis seen in these cases. These reports are heterogeneous, and a clear association with entecavir has not been well established. Entecavir is generally considered safe in decompensated cirrhosis, and both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) guidelines recommend it in this setting.
Use of entecavir in clinical practice
From the data discussed above, entecavir has been proven to potently suppress HBV replication with low rates of genotypic resistance even with long-term therapy in the nucleos(t)ide-naïve patient. In lamivudine-refractory patients, its success rate is compromised, illustrating the importance of drug sequencing when considering initial HBV therapy. Several professional societies around the world have made recommendations regarding initial therapy, taking these factors into consideration. The AASLD recommends entecavir or tenofovir as first-line oral agents for nucleos(t)ide-naïve patients, as both combine potency and low resistance rates.Citation23 Recommendations from the EASL are concordant.Citation64 The Asian Pacific Association for the Study of the Liver considers all agents to be first line, recognizing that the higher cost of entecavir and tenofovir may be prohibitive in some cases.Citation65 Therefore, lamivudine remains a first-line option in this setting. Finally, the US Treatment AlgorithmCitation66 also recommends entecavir or tenofovir as first-line agents but describes a “road-map” approach, whereby therapy is modified at week 24 or 48 as determined by on-therapy virologic response. Patients who fail entecavir should be switched to tenofovir or tenofovir–emtricitabine according to this algorithm.
For patients who already express lamivudine resistance, entecavir is not the optimal salvage drug, as entecavir resistance develops more quickly. Telbivudine is also cross-resistant, and patients failing this drug are also not good entecavir candidates. For patients failing lamivudine or telbivudine, tenofovir is a better alternative. For failures of adefovir or tenofovir, entecavir is predicted to maintain full activity and is a reasonable salvage choice.
Summary
The approval of entecavir in 2005 began a new era in the treatment of HBV where long-term virologic suppression was both realistic and safe. The low risk of entecavir resistance in naïve patients, in part due to its rapid and potent suppression of HBV DNA, makes it an optimal choice for the initial therapy of both HBeAg-positive and HBeAg-negative patients. It requires three mutations to develop full resistance, the first of which is selected by lamivudine; hence, its activity is reduced in patients with genotypic lamivudine resistance. This is partly overcome by the use of a larger dose in lamivudine-refractory patients. There are few adverse effects associated with entecavir, although fatal cases of lactic acidosis have been reported with its use in decompensated cirrhotics, particularly when the MELD score exceeds 22. It should be used with caution in this population. Its efficacy in HIV/HBV coinfected patients has been established, although it should not be used without a fully suppressive antiretroviral regimen, as anti-HIV activity has been described in vitro and in vivo. Its impact on end-stage liver disease and mortality has yet to be delineated, as it is yet too early to see the benefits of long-term viral suppression.
Disclosure
The author reports no conflicts of interest in this work.
References
- LavanchyDHepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measuresJ Viral Hepat20041129710714996343
- HyamsKCRisks of chronicity following acute hepatitis B virus infection: a reviewClin Infect Dis199520499210007795104
- YimHJLokASNatural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005Hepatology2006432 Suppl 1S173S18116447285
- HadziyannisSJPapatheodoridisGVHepatitis B e antigen-negative chronic hepatitis B: natural history and treatmentSemin Liver Dis200626213014116673291
- CarmanWFJacynaMRHadziyannisSMutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infectionLancet1989286635885912570285
- HoofnagleJHDooELiangTJManagement of hepatitis B: summary of a clinical research workshopHepatology20074541056107517393513
- Baraclude Package InsertWallingford, CTBristol-Myers Squibb http://packageinserts.bms.com/pi/pi_baraclude.pdf. Accessed December 4, 2010.
- YamanakaGWilsonTInnaimoSMetabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virusAntimicrob Agents Chemother19994311901939869593
- SeiferMHamatakeRKColonnoRJStandringDNIn vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavirAntimicrob Agents Chemother19984212320032089835515
- ChangTTGishRGHadziyannisSJA dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patientsGastroenterology200512941198120916230074
- De ManRAWoltersLMNevensFSafety and efficacy of oral entecavir given for 28 days in patients with chronic hepatitis B virus infectionHepatology200134357858211526545
- ChangTTGishRGde ManRA comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis BN Engl J Med2006354101001101016525137
- LaiCLShouvalDLokASEntecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis BN Engl J Med2006354101011102016525138
- YaoGChenCLuWEfficacy and safety of entecavir compared to lamivudine in nucleoside-naive patients with chronic hepatitis B: a randomized double-blind trial in ChinaHepatol Int20071336537219669331
- LeungNPengCYHannHWEarly hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: a randomized international study of entecavir versus adefovirHepatology2009491727919065670
- GishRGLokASChangTTEntecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis BGastroenterology200713351437144417983800
- ShouvalDLaiCLChangTTRelapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapyJ Hepatol200950228929519070393
- ShermanMYurdaydinCSollanoJEntecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis BGastroenterology200613072039204916762627
- YaoGZhouXXuDEntecavir for the treatment of lamivudine-refractory chronic hepatitis B patients in ChinaHepatol Int20071337338119669332
- SuzukiFToyodaJKatanoYEfficacy and safety of entecavir in lamivudine-refractory patients with chronic hepatitis B: randomized controlled trial in Japanese patientsJ Gastroenterol Hepatol20082391320132618554238
- ShermanMYurdaydinCSimsekHEntecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeksHepatology20084819910818537189
- YaoGBXuDZRenHThree years of continuous entecavir treatment in Chinese patients who had previously failed lamivudine: results from studies ETV-056 and ETV-050 (Abstract)Hepatology200848Suppl 2S267
- LokASMcMahonBJChronic hepatitis B: update 2009Hepatology200950366166219714720
- ChangTTLaiCLKew YoonSEntecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis BHepatology201051242243020049753
- LaiCLChienRNLeungNWA one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study GroupN Engl J Med1998339261689654535
- DienstagJLPerrilloRPSchiffERA preliminary trial of lamivudine for chronic hepatitis B infectionN Engl J Med199533325165716617477217
- MarcellinPChangTTLimSGAdefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis BN Engl J Med2003348980881612606735
- ZengMMaoYYaoGA double-blind randomized trial of adefovir dipivoxil in Chinese subjects with HBeAg-positive chronic hepatitis BHepatology200644110811616799983
- ChanHLHeathcoteEJMarcellinPTreatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trialAnn Intern Med20071471174575417909201
- HouJYinYKXuDTelbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trialHepatology200847244745418080339
- LaiCLGaneELiawYFTelbivudine versus lamivudine in patients with chronic hepatitis BN Engl J Med2007357252576258818094378
- MarcellinPHeathcoteEJButiMTenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis BN Engl J Med2008359232442245519052126
- HadziyannisSJTassopoulosNCHeathcoteEJAdefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis BN Engl J Med2003348980080712606734
- LiawYFChienRNYehCTAcute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapyHepatology199930256757210421670
- FungSKAndreonePHanSHAdefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensationJ Hepatol200543693794316168522
- LeungNWLaiCLChangTTExtended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapyHepatology20013361527153211391543
- TenneyDPokornowskiKRoseREEntecavir maintains a high genetic barrier to HBV resistance through 6 years in naive patients (Abstract)Hepatology200950Suppl 2S10
- TenneyDJRoseREBaldickCJLong-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapyHepatology20094951503151419280622
- HadziyannisSJTassopoulosNCHeathcoteEJLong-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis BN Engl J Med2005352262673268115987916
- HadziyannisSJTassopoulosNCHeathcoteEJLong-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 yearsGastroenterology200613161743175117087951
- Snow-LampartAKitrinosKChappellBJNo resistance to tenofovir disoproxil fumarate (TDF) detected following 192 weeks of treatment in subjects mono-infected with chronic hepatitis B virus (Abstract)Hepatology201052Suppl 1977A
- LiawYFGaneELeungN2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis BGastroenterology2009136248649519027013
- TipplesGAMaMMFischerKPMutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivoHepatology19962437147178781348
- AngusPVaughanRXiongSResistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymeraseGastroenterology2003125229229712891527
- Borroto-EsodaKMillerMDArterburnSPooled analysis of amino acid changes in the HBV polymerase in patients from four major adefovir dipivoxil clinical trialsJ Hepatol200747449249817692425
- TenneyDJLevineSMRoseREClinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to LamivudineAntimicrob Agents Chemother20044893498350715328117
- BaldickCJEggersBJFangJHepatitis B virus quasispecies susceptibility to entecavir confirms the relationship between genotypic resistance and patient virologic responseJ Hepatol200848689590218362040
- SchiffESimsekHLeeWMEfficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosisAm J Gastroenterol2008103112776278318721244
- ShimJHLeeHCKimKMEfficacy of entecavir in treatment-naive patients with hepatitis B virus-related decompensated cirrhosisJ Hepatol201052217618220006394
- LiawYFRaptopoulou-GigiMChienquerHEfficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with evidence of hepatic decompensation (Abstract)Hepatology200950Suppl 4505A
- XiZFXiaQZhangJJThe role of entecavir in preventing hepatitis B recurrence after liver transplantationJ Dig Dis200910432132719906113
- PessoaMGGazzardBHuangAKEfficacy and safety of entecavir for chronic HBV in HIV/HBV coinfected patients receiving lamivudine as part of antiretroviral therapyAIDS200822141779178718753861
- McMahonMAJilekBLBrennanTPThe HBV drug entecavir: effects on HIV-1 replication and resistanceN Engl J Med2007356252614262117582071
- SasadeuszJAudsleyJMijchAThe anti-HIV activity of entecavir: a multicentre evaluation of lamivudine-experienced and lamivudine-naive patientsAIDS200822894795518453854
- Department of Health and Human ServicesGuidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescentsOffice of AIDS Research Advisory Council (OARAC)1212009 http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. Accessed December 12, 2010.
- HammerSMEronJJJrReissPAntiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panelJAMA2008300555557018677028
- McKenzieRFriedMWSallieRHepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis BN Engl J Med199533317109911057565947
- BrinkmanKter HofstedeHJBurgerDMAdverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathwayAIDS19981214173517449792373
- ColacinoJMMechanisms for the anti-hepatitis B virus activity and mitochondrial toxicity of fialuridine (FIAU)Antiviral Res1996292–31251398739593
- LewisWDayBJCopelandWCMitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspectiveNat Rev Drug Discov200321081282214526384
- MazzuccoCEHamatakeRKColonnoRJTenneyDJEntecavir for treatment of hepatitis B virus displays no in vitro mitochondrial toxicity or DNA polymerase gamma inhibitionAntimicrob Agents Chemother200852259860518056280
- LangeCMBojungaJHofmannWPSevere lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver functionHepatology20095062001200619937695
- CohenSMLevyRMJovanovichJFAhnJFatal lactic acidosis associated with the use of combination oral medications to treat reactivation of hepatitis BJ Clin Gastroenterol200943101008101019461528
- European Association for the Study of the LiverEASL clinical practice guidelines: management of chronic hepatitis BJ Hepatol200950222724219054588
- LiawYFLeungNKaoJHAsian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 updateHepatol Int2008226328319669255
- KeeffeEBDieterichDTHanSHA treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 updateClin Gastroenterol Hepatol20086121315134118845489