Abstract
Purpose
The E. coli ST167 clone is the globally dominant ST among extraintestinal pathogenic E. coli (ExPEC) and is frequently associated with carbapenem resistance. This study reports genomic characterization of a pandrug-resistant E. coli ST167 isolate (ECO3183) and the possibility of the type strains’ transmission.
Materials and Methods
Antibiotic susceptibility testing was performed using disk diffusion and the VITEK 2 automated system. The E. coli ECO3183 genome was sequenced. We used the genome to analyze the phylogenetic relationship, phylogenetic group, sequence type (ST), acquired antibiotic resistance genes (ARGs), IS elements, genomics islands, the replicon type and transferability of the plasmids. The conjugative transfer of plasmids was assessed using filter mating experiments.
Results
ECO3183 contained a 4.87-Mb chromosome and two plasmids [pECO3183-1 (167.63 Kb) and pECO3183-2 (46.16 Kb)]. It belonged to phylogenetic group A, clonal complex 10 (CC10), and ST167. ECO3183 is a pandrug-resistant strain nonsusceptible to 24 tested antimicrobials representing 8 different antimicrobial classes. Among 55 E. coli isolates phylogenetically related to ECO3183, 47% (26/55) were from humans, while 35% (19/55) were from animals. Further analysis revealed that among 1140 ST167 isolates (in the EnteroBase database), 4% (47/1140) originated from environments, 17% (192/1140) were isolated from humans, and 78% (890/1140) were obtained from animals. The pECO3183-1 contained two identical repeats of a 9633 bp region (IS6100-sul1-ΔaadA16-dfrA27-arr-3-aac(6′)-Ib-cr-IS26) and a 17.88-kb resistance island (sul2-aph(3″)-Ib-aph(6)-Id-IS26-Δaph(3′)-Ia-IS26-tet(A)-ΔfloR-ΔISVsa3-IS26-Δaac(3)-IId-IS26-mph(A)), and these three regions contained most of ECO3183 carrying ARGs. It was identified as a conjugative plasmid, which confers MDR resistance and has the potential to spread.
Conclusion
ECO3183 exhibited pandrug-resistance phenotype that was mediated by pECO3183-1 carrying MDR ARGs and pECO3183-2 carrying blaNDM-5. Source analysis of strains indicated that ST167 E. coli might be transmitted between species from animals to humans, which needs continued monitoring.
Introduction
Infections caused by Enterobacterales, including Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis, are associated with a variety of clinical illnesses, such as urinary tract infections, septicemia, pneumonia, intra-abdominal infections, and meningitis.Citation1 Multidrug-resistant (MDR) and carbapenem-resistant Enterobacterales are not only resistant to a majority of commonly used antibiotics, but they are also known to rapidly spread from person to person, making them harder to control.Citation1 The emergence of multidrug-resistant (MDR) and carbapenem-resistant Enterobacterales poses a serious challenge for clinical treatment.
E. coli, a predominant member of Enterobacterales, is frequently encountered and isolated in clinical laboratories. Based on the data obtained from the China Antimicrobial Resistance Surveillance System (http://www.carss.cn/), E. coli exhibited an average isolation rate of 20.6% in recent five years (2017–2021), with 1.6% of the strains being carbapenem-resistant. In recent years, the E. coli sequence type (ST) 167 clone, as the globally dominant ST among extraintestinal pathogenic E. coli (ExPEC), was frequently reported for its association with carbapenem resistance.Citation2 It has been documented that carbapenem-resistant E. coli ST167 strains harboring blaNDM-5 are capable of infecting both humans and animals.Citation3 However, current knowledge regarding the transmission risk of E. coli ST167 strains between animals and humans remains limited. A total of four ST167 isolates exhibited MDR phenotypes among 411 E. coli isolates in our previous study.Citation4 In this study, we sequenced the entire genome of a pandrug-resistant E. coli ST167 isolate (ECO3183), analyzed antibiotic resistance genes (ARGs) conferring antibiotic resistance, and explored its potential dissemination risk.
Materials and Methods
Bacterial Isolate
Similar to our previous study,Citation4 the E. coli strain ECO3183 was isolated from a urine sample of a urinary tract-infected patient in the Second Hospital of Tianjin Medical University (Tianjin, China). The strain was identified using the VITEK MS system (bioMérieux, Marcy l’Etoile, France).
Antimicrobial Susceptibility Testing (AST)
We used a Gram-negative antimicrobial susceptibility testing card (AST-GN13 and AST-GN334) on the VITEK 2 system to perform the susceptibility tests. The Kirby-Bauer disc diffusion assay (K-B) was additionally employed to detect kanamycin, chloramphenicol, polymyxin B, and tetracycline. E. coli ATCC 25922 was used as a control strain. Interpretation of the results was performed using the guidelines from the Clinical & Laboratory Standards Institute (CLSI, 2022)Citation5 or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints (2022, https://www.eucast.org/).
Whole Genome Sequencing and Analysis
The whole-genome sequence of ECO3183 was sequenced using Pacific Biosciences (PacBio) Sequel sequencing platform and high-throughput Illumina sequencing platform at the Beijing Novogene Bioinformatics Technology Co., Ltd. Sequence reads were generated from 10 kb SMRT Bell library and 350 bp library. The SMRT Link v.5.0.1 program (Pacific Biosciences of California, Inc., Menlo Park, CA, USA) was used for preliminary genome assembly, Arrow in the SMRT analysis software suite (https://www.pacb.com/support/software-downloads/) was used to align the initial assembly results, and complete circular contigs without any gaps were generated. The assembled genome was submitted to ClermonTypingCitation6 (http://clermontyping.iame-research.center/), the Escherichia typing database (https://pubmlst.org/bigsdb?db=pubmlst_escherichia_seqdef), ResFinder 4.1Citation7–9 (https://cge.food.dtu.dk/services/ResFinder/), ISfinderCitation10 (https://isfinder.biotoul.fr/) to identify phylogenetic group, sequence type (ST), acquired antibiotic resistance genes (ARGs), and IS elements, respectively. Core genome multilocus sequence typing (cgMLST) approaches with a 100 threshold for phylogenetic analysis were used to investigate the phylogenetic relationship between ECO3183 and other E. coli isolates currently available on the BacWGSTdb serverCitation11 (http://bacdb.cn/BacWGSTdb/). The replicon type and transferability of the plasmids were predicted using PlasmidFinder 2.1Citation9,Citation12 (https://cge.food.dtu.dk/services/PlasmidFinder/) and OriTfinderCitation13 (https://tool-mml.sjtu.edu.cn/oriTfinder/oriTfinder.html), respectively. Genomics islands were predicted by the IslandPath-DIOMB method implemented in IslandViewer 4Citation14 (https://www.pathogenomics.sfu.ca/islandviewer/).
Conjugation
ECO3183 was subjected to conjugation via filter mating with the sodium azide-resistant E. coli J53 as the recipient, following the procedure described previously.Citation15 Transconjugants were selected on LB agar supplemented with 100 mg/L sodium azide and 50 mg/L tetracycline. Plasmid DNA was purified from the E. coli J53 transconjugant using the EZNA® BAC/PAC DNA kit (Omega Bio-Tek Inc., Norcross, GA, USA). PCR was performed to detect the presence of pECO3183-1 among these transconjugants using two sets of primers specific to pECO3183-1 (Table S1). For all reactions, the reaction mixture (25 μL) contained 1 μL of plasmid DNA, 1 μL each of primer, 9.5 μL of ddH2O and 12.5 μL of 2 × Taq PCR Master Mix (Sangon Biotech Co., Ltd., Shanghai, China) under the following conditions: initial denaturation at 94°C for 4 min, 35 cycles of incubation at 94°C for 30s, 51°C for 30s and 72°C for 18s, and a final extension at 72°C for 5 min. The following primer pairs were used to generate PCR products of different length: aph(6)-Id_F/aph(6)-Id_R (238 bp) and aph(3″)-Ib_F/aph(3″)-Ib_R (207 bp).
Results and Discussion
E. coli strain ECO3183 is a pandrug-resistant strain nonsusceptible to 24 tested antimicrobials. The 24 antimicrobials tested represent a total of 8 different antimicrobial classes, including gentamicin, kanamycin, tobramycin, amoxicillin/clavulanate, ampicillin, ampicillin/sulbactam, ceftriaxone, cefazolin, cefepime, cefoperazone/sulbactam, cefotetan, cefoxitin, ceftazidime, cefuroxime, ertapenem, imipenem, piperacillin/tazobactam, chloramphenicol, ciprofloxacin, levofloxacin, polymyxin B, nitrofurantoin, trimethoprim/sulfamethoxazole, and tetracycline. The minimum inhibitory concentration (MIC) of the antibiotics tested and associated ARGs are presented in . The phylogenetic group was determined by querying ClermonTyping with the genome sequence, while the clonal complex and ST were concurrently determined by querying the Escherichia typing database with the same genome sequence. ECO3183 belonged to phylogenetic group A, clonal complex 10 (CC10) and ST167, which has been reported to be the 14th of top 20 ExPEC STs.Citation16 ECO3183 contained a 5.08-Mb genome, including a 4.87-Mb chromosome and two plasmids (pECO3183-1 and pECO3183-2). We analyzed the phylogenetic relationship between ECO3183 and other E. coli isolates currently deposited in the BacWGSTdb server (24 April 2023; ). A total of 55 phylogenetically related strains (with 24–100 different cgMLST alleles) were identified in the database (Table S2), all belonging to ST167, with 69% (38/55) from China, 47% (26/55) from humans, and 35% (19/55) from animals. This data indicates the potential transmission of these 55 strains between animals and humans. More specifically, the closest relatives of ECO3183 (only 24 different cgMLST alleles) were another three ST167 E. coli strains carrying blaNDM-9 (490, 494, 707; Accession No. SAMN05928947, SAMN05928958, SAMN05928933), which were isolated from flies in a chicken farm in Shandong, China.Citation17 The published study linked flies to the widespread dissemination of blaNDM.Citation17 Similarly, a recent study also reported the clustering of six blaNDM-5-positive ST167 E. coli isolates (ECO167624, LR880734.1, 562.30390, 562.50775, 562.50948, and 562.50949) from both human and animal sources within a clade on the phylogenetic tree.Citation18 The upper and lower right corners of the phylogenetic tree () showed that animal-origin ST167 E. coli isolates (upper cluster: 490, 494, 707, 598, 601; lower cluster: ECOL_18, LJ037ch) cluster closely with human-origin isolates (upper cluster: E4903, ECO3183, ECM_49; lower cluster: L665, L610, L612, FDAARGOS_434), suggesting a potential transmission between animals and humans. To further explore the possibility of the type strains’ transmission between animals and humans within a larger cohort of ST167 isolates, we conducted an analysis of the isolation sources for all 1480 ST167 E. coli isolates found in the EnteroBase database (https://enterobase.warwick.ac.uk/; 7 May 2023; Table S3). Out of the total of 1480 isolates, 1140 strains had well-documented isolation sources. Among these 1140 ST167 isolates, 4% (47/1140) were from environments, 17% (192/1140) from humans, and 78% (890/1140) from animals. The data also suggested that ST167 E. coli strains might be transmitted between species from animals to humans.
Figure 1 Phylogenetic tree of ECO3183 and other E. coli isolates retrieved from the BacWGSTdb server (http://bacdb.cn/BacWGSTdb/). The line length connecting each circle depicts clonal relationships between different isolates. The fill and stroke color of the circle denotes a specific country and host, respectively. The numbers in square brackets are the number of corresponding isolates.
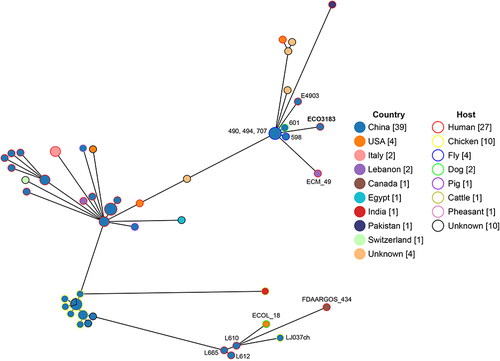
Table 1 Antimicrobial Susceptibility Patterns and Resistance Genes of ECO3183
Two plasmids pECO3183-1 (167,630 bp) and pECO3183-2 (46,155 bp) belonged to different incompatibility groups IncC and IncX3, respectively. The pECO3183-1 was most similar to another four multidrug resistance plasmids (≥ 99.9% identity and ≥ 84% coverage) available on the NCBI nucleotide collection (nr/nt) database (24 April 2023) (). Two plasmids pECY53 (GenBank accession no. KT997783.1) and pUnnamed3 (CP083494.1) from E. coli clinical isolates contained the same ARGs (aph(6)-Id, aph(3″)-Ib, aac(3)-IId, aph(3′)-Ia, floR, sul2, and tet(A)), while the other two plasmids pJNE2-NDM (CP096170.1) and pJNE10-2-NDM (CP096164.1) from environmental isolates of S. putrefaciens also contained the same ARGs (aph(6)-Id, aph(3″)-Ib, aac(3)-IId, aph(3′)-Ia,blaNDM-1, mph(A), sul1, sul2, arr-3, aadA5, and dfrA17).Citation22 Interestingly, the plasmid pECO3183-1 contained two identical repeats of a 9633 bp region (IS6100-sul1-ΔaadA16-dfrA27-arr-3-aac(6′)-Ib-cr-IS26) and a 17.88-kb resistance island (sul2-aph(3″)-Ib-aph(6)-Id-IS26-Δaph(3′)-Ia-IS26-tet(A)-ΔfloR-ΔISVsa3-IS26-Δaac(3)-IId-IS26-mph(A)), and the three regions contributed most of the ARGs (). IS26, IS6100, and ΔISVsa3 were interspersed between these ARGs, highlighting their possible role in the dissemination of these MDR ARGs. The plasmid pECO3183-1 was defined as a putative conjugative plasmid because it carried the oriTfinder-predicted oriT region and genes encoding relaxase, T4CP, and T4SS. Filter mating experiments further showed that E. coli J53 transconjugant exhibited a multidrug resistance profile (Table S4). PCR amplification of fragments from the aph(3″)-Ib and aph(6)-Id genes of pECO3183-1 confirmed that the antibiotic resistance dissemination transfer was mediated by pECO3183-1 (Figure S1). The literature also suggests that the large and broad host range of IncC plasmids is a significant contributor to the spread of ARGs.Citation23 In addition, the plasmid pECO3183-2 sequence was BLASTed against the NCBI nucleotide collection (nr/nt) database using blastn via the NCBI BLAST web interface (https://blast.ncbi.nlm.nih.gov/Blast.cgi) with default parameter values (12 July 2023). It displayed a high similarity (≥ 99% identity and coverage) to 200 plasmids (containing pECO3183-2). Out of these 200 plasmids, 122 are labeled with the abbreviation “NDM” in their names, indicating their possession of the blaNDM gene (data not shown). Due to pECO3183-2 belonging to the IncX3 incompatibility group, most of the 122 highly homologous plasmids to pECO3183-2 also belong to IncX3. Consistent with our analysis findings, multiple reports in the literature have documented the dissemination of blaNDM via the IncX3 plasmids.Citation24–26
Figure 2 (A) A 17.88-kb resistance island carrying 8 ARGs and 5 IS elements was predicted using the IslandPath-DIOMB method. (B) BRIG analysis of the conjugative multidrug resistance plasmid pECO3183-1. Comparative analysis of pECO3183-1 with four closely related plasmids was performed using the BLAST Ring Image Generator (BRIG).Citation27 The concentric rings display similarity between the pECO3183-1 sequence in the inner ring and the other sequences in the outer rings. The various color levels indicate a BLAST result with a matched degree of shared regions, as shown to the right of the ring. The predicted ARGs are shown in red, and the IS elements are shown in blue.
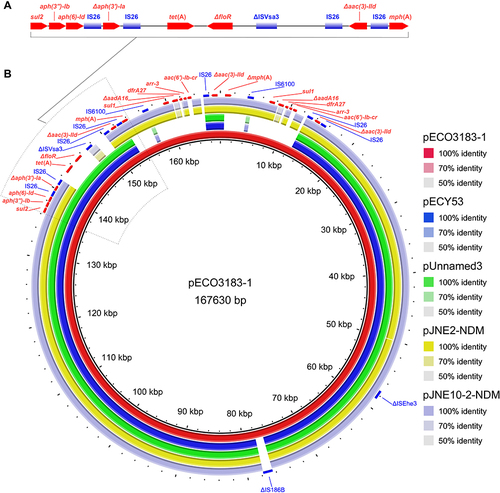
In conclusion, E. coli strain ECO3183 is a pandrug-resistant strain nonsusceptible to 24 tested antimicrobials representing 8 different antimicrobial classes. Its pandrug-resistance phenotype was mediated by pECO3183-1 carrying MDR ARGs and pECO3183-2 carrying blaNDM-5 (). The plasmid pECO3183-1 contained two identical repeats of a 9633 bp region and a 17.88-kb resistance island, and the three regions contributed most of the ARGs. Source analysis of strains indicated that ST167 E. coli might be transmitted between species from animals to humans, which needs continued monitoring.
Ethics Statement
The ECO3183 strain was generated as part of routine clinical laboratory procedures. This study complied with the Declaration of Helsinki and the Ethics Committee of the Second Hospital of Tianjin Medical University exempted this study from review because it focused only on bacteria.
Nucleotide Sequence Accession Numbers
The ECO3183 chromosome, pECO3183-1, and pECO3183-2 plasmid sequences were deposited under GenBank accession numbers CP104721, CP104722, and CP104723, respectively.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Tilahun M, Kassa Y, Gedefie A, Ashagire M. Emerging carbapenem-resistant Enterobacteriaceae infection, its epidemiology and novel treatment options: a review. Infect Drug Resist. 2021;14:4363–4374. doi:10.2147/IDR.S337611
- Garcia-Fernandez A, Villa L, Bibbolino G, et al. Novel insights and features of the NDM-5-producing Escherichia coli sequence type 167 high-risk clone. mSphere. 2020;5(2):e00269–00220. doi:10.1128/mSphere.00269-20
- Grönthal T, Österblad M, Eklund M, et al. Sharing more than friendship – transmission of NDM-5 ST167 and CTX-M-9 ST69 Escherichia coli between dogs and humans in a family, Finland, 2015. Euro Surveill. 2018;23(27):1700497. doi:10.2807/1560-7917.ES.2018.23.27.1700497
- Duan Y, Gao H, Zheng L, et al. Antibiotic resistance and virulence of extraintestinal pathogenic Escherichia coli (ExPEC) vary according to molecular types. Front Microbiol. 2020;11:598305. doi:10.3389/fmicb.2020.598305
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. In: CLSI Supplement M100. 32nd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
- Beghain J, Bridier-Nahmias A, Le Nagard H, Denamur E, Clermont O. ClermonTyping: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb Genom. 2018;4(7):e000192. doi:10.1099/mgen.0.000192
- Bortolaia V, Kaas RS, Ruppe E, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75(12):3491–3500. doi:10.1093/jac/dkaa345
- Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother. 2017;72(10):2764–2768. doi:10.1093/jac/dkx217
- Camacho C, Coulouris G, Avagyan V, Ma N, Madden TL. BLAST+: architecture and applications. BMC Bioinform. 2009;10(1):421. doi:10.1186/1471-2105-10-421
- Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34(suppl_1):D32–D36. doi:10.1093/nar/gkj014
- Feng Y, Zou S, Chen H, Yu Y, Ruan Z. BacWGSTdb 2.0: a one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021;49(D1):D644–D650. doi:10.1093/nar/gkaa821
- Carattoli A, Zankari E, García-Fernández A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–3903. doi:10.1128/AAC.02412-14
- Li X, Xie Y, Liu M, et al. oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 2018;46(W1):W229–W234. doi:10.1093/nar/gky352
- Bertelli C, Laird MR, Williams KP, et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45(W1):W30–W35. doi:10.1093/nar/gkx343
- Khajanchi BK, Kaldhone PR, Foley SL. Protocols of conjugative plasmid transfer in Salmonella: plate, broth, and filter mating approaches. In: Ricke S, Park S, Davis M, editors. Microbial Transposon Mutagenesis. Methods in Molecular Biology. Vol. 2016. New York: Humana; 2019:129–139. doi:10.1007/978-1-4939-9570-7_12
- Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout JDD. Global extraintestinal pathogenic Escherichia coli (ExPEC) lineages. Clin Microbiol Rev. 2019;32(3):e00135–18. doi:10.1128/CMR.00135-18
- Wang Y, Zhang R, Li J, et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol. 2017;2(4):16260. doi:10.1038/nmicrobiol.2016.260
- Biffignandi GB, Piazza A, Marchesini F, et al. Genomic characterization of an O101:H9-ST167 NDM-5-producing Escherichia coli strain from a kitten in Italy. Microbiol Spectr. 2022;10(3):e00832–e00822. doi:10.1128/spectrum.00832-22
- Jones RN, Guzman-Blanco M, Gales AC, et al. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011). Braz J Infect Dis. 2013;17:672–681. doi:10.1016/j.bjid.2013.07.002
- Yang Q, Zhang H, Cheng J, et al. In vitro activity of flomoxef and comparators against Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis producing extended-spectrum β-lactamases in China. Int J Antimicrob Agents. 2015;45:485–490. doi:10.1016/j.ijantimicag.2014.11.012
- Robicsek A, Strahilevitz J, Jacoby GA, et al. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med. 2006;12:83–88. doi:10.1038/nm1347
- Li R, Zhang L, Lu X, et al. Occurrence and characterization of NDM-1-producing Shewanella spp. and Acinetobacter portensis co-harboring tet(X3) in a Chinese dairy farm. Antibiotics. 2022;11(10):1422. doi:10.3390/antibiotics11101422
- Ambrose SJ, Harmer CJ, Hall RM. Evolution and typing of IncC plasmids contributing to antibiotic resistance in gram-negative bacteria. Plasmid. 2018;99:40–55. doi:10.1016/j.plasmid.2018.08.001
- Zhao Q, Berglund B, Zou H, et al. Dissemination of blaNDM-5 via IncX3 plasmids in carbapenem-resistant Enterobacteriaceae among humans and in the environment in an intensive vegetable cultivation area in eastern China. Environ Pollut. 2021;273:116370. doi:10.1016/j.envpol.2020.116370
- Yuan Y, Li Y, Wang G, et al. blaNDM-5 carried by a hypervirulent Klebsiella pneumoniae with sequence type 29. Antimicrob Resist Infect Control. 2019;8(1):140. doi:10.1186/s13756-019-0596-1
- Krishnaraju M, Kamatchi C, Jha AK, et al. Complete sequencing of an IncX3 plasmid carrying blaNDM-5 allele reveals an early stage in the dissemination of the blaNDM gene. Indian J Med Microbiol. 2015;33(1):30–38. doi:10.4103/0255-0857.148373
- Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genom. 2011;12(1):402. doi:10.1186/1471-2164-12-402
