Abstract
Background
Qiguiyin decoction (QGYD) is a traditional Chinese medicine (TCM) and its combined application with levofloxacin (LVFX) has been confirmed effective in the clinical treatment of multidrug-resistant Pseudomonas aeruginosa (MDR PA) infection. This study investigated the therapeutic effect and possible mechanism of QGYD in sensitizing LVFX against MDR PA infection.
Materials and Methods
Pulmonary infections were induced in rats by MDR PA. The changes in pharmacokinetics-pharmacodynamics (PK-PD) parameters of LVFX after combined with QGYD were investigated in MDR PA-induced rats. Subsequently, the correlation between PK and PD was analyzed and PK-PD models were established to elucidate the relationship between QGYD-induced alterations in LVFX metabolism and its sensitization to LVFX. Antibody chip technology was used to detect the levels of inflammatory factors, suggesting the relationship between the beneficial effect of immune regulation and the sensitization of QGYD.
Results
QGYD significantly enhanced the therapeutic efficacy of LVFX against MDR PA infection. The combination of QGYD changed the PK parameters of LVFX such as Tmax, t1/2, MRT, Vd/F, CL/F and PD parameters such as MIC, AUC0-24h/MIC. Predicted results from PK-PD models demonstrated that the antibacterial effect of LVFX was significantly enhanced with the combination of QGYD, consistent with experimental findings. Antibody chip results revealed that the combination of QGYD made IL-1 β, IL-6, TNF- α, IL-10, and MCP-1 levels more akin to those of the blank group.
Conclusion
These findings indicated that QGYD could change the PK-PD behaviors of LVFX and help the body restore immune balance faster. This implied that a potential drug interaction might occur between QGYD and LVFX, leading to improved clinical efficacy when combined.
Introduction
The overuse of levofloxacin (LVFX) has resulted in a shift in the susceptibility of Pseudomonas aeruginosa (PA) to LVFX, contributing to the rise of multidrug-resistant (MDR) PA strains, posing a significant threat to patient health.Citation1–4 GuoCitation5 analyzed drug sensitivity data from pulmonary tuberculosis patients with PA infection between 2016 to 2020, revealing a notable increase in PA resistance to LVFX in 2020. This underscores the challenge of achieving optimal efficacy with LVFX monotherapy, prompting the adoption of combination therapy strategies for the treatment of MDR PA infections.Citation6
Traditional Chinese medicine (TCM) has played an important role in preventing and treating infected diseases since ancient times.Citation7 In the clinical treatment of MDR PA infection, numerous Chinese medicinal treatments, along with their concurrent use with antibacterial drugs, have demonstrated efficacy.Citation8,Citation9 Qiguiyin decoction (QGYD) has a good anti-infection effect combined with antibacterial drugs in clinical practice,Citation10 which consists of five Chinese medicines including Astragalus membranaceus (Fisch.) Bge, Lonicera japonica Thunb., Angelica sinensis (Oliv.) Diels, Artemisia annua L., and Polygonum cuspidatum Sieb. et Zucc. Our previous studies have shown that QGYD could delay or reverse PA resistance to antibacterial drugs, regulate inflammation and immune dysregulation resulting from PA infection, and enhance therapeutic outcomes when combined with LVFX.Citation11,Citation12 Nonetheless, the impact of QGYD on the pharmacokinetic-pharmacodynamic (PK-PD) profile of LVFX in PA-infected rats, as well as the mechanism underlying QGYD’s sensitization of LVFX, remains obscure.
Following PA infection, the body often experiences a disruption in inflammatory factors. MDR PA infections are more prone to inducing or exacerbating inflammatory immune imbalances compared to typical bacterial infections.Citation13 Some studies have shown that TCM can improve the body’s immunity.Citation14 Consequently, we hypothesize that the co-administration of QGYD influences the PK-PD parameters of LVFX and immune alterations in vivo, thereby facilitating the sensitization of LVFX against MDR PA.
We undertook three main tasks in this study. Firstly, we investigated the PK parameters of LVFX administered alone or in conjunction with QGYD in pneumonia rat models induced by MDR PA, employing ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQ/MS). Subsequently, the bacteriostatic activity of drug-containing serum in pneumonia rats was examined in vitro. Furthermore, PK/PD models were established to assess the efficacy of LVFX as monotherapy or in combination with QGYD against MDR PA, offering insights into the integration of Chinese and Western medicine in clinical practice. Additionally, we employed antibody chips to analyze alterations in serum inflammatory factors in pneumonia rats following treatment with LVFX alone or in combination with QGYD, aiming to elucidate the mechanism by which QGYD sensitizes LVFX from an immunological perspective and present a novel clinical option for enhancing treatment against MDR PA resistance.
Materials and Methods
Materials
Drugs and Chemicals
Levofloxacin (Batch No: Y02N7C23971, purity > 99%), and ciprofloxacin hydrochloride (Batch No: SM0325YG13, purity > 98%) were purchased from Shanghai Yuan-ye Biological Technology Co., Ltd. (Shanghai, China). Levofloxacin tablets (Batch No: BY008A1) were purchased from Daiichi Sankyo Pharmaceutical Co., Ltd. (Beijing, China). QGYD is composed of five traditional Chinese medicines including Astragalus membranaceus (Fisch.) Bunge. (Huangqi), Angelica sinensis (Oliv.) Diels. (Danggui), Lonicera japonica Thunb. (Jinyinhua), Polygonum cuspidatum Sieb. et Zucc. (Huzhang), and Artemisia annua L. (Qinghao), which were purchased from Beijing Sanhe Pharmaceutical Co., Ltd. (Beijing, China) and authenticated by Professor Yaojun Yang (School of Chinese Materia Medica, Beijing University of Chinese Medicine). LC-MS grade methanol, acetonitrile, and formic acid were purchased from Thermo Fisher Scientific (New Jersey, USA). Ultra-pure water was purified by a Milli-Q water system (MA, USA). Cytokine Antibody Chip Kit was from RayBiotech, Inc. (Atlanta, USA).
Animals and Bacterial Suspension
Forty healthy male Wistar rats, (4–5 months old, SPF grade), weighing around 220–260 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), license number: SCXK (jing) 2021–0011. All rats were adaptively fed with a standard diet and water for 3 days before the experiments. The environmental conditions were maintained at a temperature of 23 ± 2 °C, with a relative humidity of 60 ± 5%, and a 12-hour light-dark cycle. This study adhered to the Guide for the Care and Use of Laboratory Animals (National Institute) and received approval from the Animal Experimental Medical Ethics Review Committee of Beijing University of Chinese Medicine (No: BUCM-4-2022030401-1033).
The MDR PA strain (No.1912109) was sourced from the Department of Clinical Laboratory of Dongzhimen Hospital, affiliated with Beijing University of Chinese Medicine. The strain was cultured on Mueller-Hinton Agar (MHA) medium at 37°C for 16–20 hours. Subsequently, the MDR PA suspension was adjusted to a concentration of 6×108 CFU/mL using a turbidimetric method with sterile saline, with an absorbance value at 625 nm (OD625) ranging at 0.3–0.4.
Preparation of QGYD Extract
The extraction method for QGYD had been previously established and its stability had been confirmed through fingerprint analysis. Thus the extraction method was directly applied in this experiment. QGYD is comprised of five traditional Chinese medicines, including Huangqi, Danggui, Jinyinhua, Huzhang, and Qinghao, with the respective compounds mixed in proportions of 12:2:3:2:3. The preparation process involved extracting QGYD three times using deionized water at ratios of 12, 10, and 10 folds (v/w), each extraction lasting 1 hour. The combined solutions were then concentrated to a concentration of 1.0 g/mL, followed by the addition of ethanol to achieve a concentration of 60%. The mixture was left to stand for 24 hours at 4 °C. The supernatant was collected after suction filtration, dried under vacuum at 60 °C, resulting in the QGYD extract (yield: 26.13%).
Animal Experimental Design
Forty rats were randomly assigned to four groups: control group, model group, LVFX group, and QGYD-LVFX group. The rat infection model was established following a previously report.Citation12 The MDR PA-induced pneumonia rat model was established by endotracheal intubation. Each rat received 100 μL of MDR PA suspension at a concentration of 6×108 CFU/mL, while the control group received an equal volume of sterile saline. Following modeling, the control group was housed separately from the MDR PA-infected groups. The rat dosage was scaled to be equivalent to 6.25 times that for a 60 kg adult. An hour after infection, rats in the LVFX group received oral administration of LVFX (0.078 g/kg/d), while rats in the QGYD-LVFX group received co-administration of QGYD (11.5 g/kg/d) and LVFX (0.078 g/kg/d). The control and model groups received equivalent amounts of distilled water.
The general condition of the rats was monitored daily after modeling. The rats fasted for 12 hours before the final administration but drank water freely. After five days of continuous gavage, blood samples were collected from the orbital venous plexus at 0 h, 0.25 h, 0.5 h, 1 h, 1.5 h, 2h, 4 h, 8 h, 12 h, and 24 h after the last administration. The collected blood samples were placed in EP tubes containing a coagulant and centrifuged at 3500 rpm at 4 °C for 15 min following 1 h at room temperature. The supernatant was collected and stored at −80 °C for later use.
Determination of Levofloxacin Concentrations in Rat Serum Samples
A validated ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was employed to quantify LVFX concentrations from serum samples. Frozen plasma samples were thawed at 4 °C before use. The 5 μL of internal standard (IS) solution (20 μg/mL CPFX) was added to a 20 µL plasma sample, followed by the addition of 55 μL methanol to precipitate proteins. The mixture was vortexed for 5 min and then centrifuged at 14,000 rpm at 4 °C for 15 min. The supernatant was collected and dried under a nitrogen stream. Subsequently, the residues were redissolved in 1 mL of initial proportion of the mobile phase, vortexed for 30s, and then, centrifuged at 14,000 rpm for 15 min at 4 °C. The final supernatant was injected into UPLC-TQ/MS for analysis.
The chromatographic separation was achieved on ultra-high performance liquid chromatography (UPLC) with an ACQUITY UPLC BEH C18 column (2.1×100 mm, 1.7 µm; Waters Corp., USA) at a column temperature of 40 °C. The temperature of the sample chamber was 10 °C. The mobile phase was composed of 0.1% formic acid aqueous solution (A) and acetonitrile (B). The gradient was set as follows: 0–0.1 min, 90–85% A; 0.1–3.0 min, 85–20% A; 3.0–3.5 min, 20–90% A. The flow rate was 0.30 mL/min, and the injection volume was 1 μL.
For mass spectrum acquisition, a triple quadrupole tandem mass spectrometry (TQ/MS) (Thermo Fisher, USA) was used in the positive ion (ESI+) mode by multiple reaction monitoring (MRM) of the transition of m/z 362.2395-m/z 261.1767 for LVFX and m/z 332.1651-m/z 245.1919 for CPFX (internal standard). The optimal MS parameters were as follows: the capillary voltage was set at 2.5 kV; desolvation gas flow rate was set at 650 L/h; source and desolvation temperatures maintained at 150 °C and 350 °C, respectively; the optimized cone voltage and collision energy were set at 44 V and 22 eV for LVFX, and 42 V and 20 eV for CPFX, respectively. Data acquisition and processing were performed using MassLynx 4.2 software (Waters Corp., USA).
The lower limit of quantification for levofloxacin was 2 ng/mL, and the standard curve exhibited linearity within the range of 2–400 ng/mL, with a coefficient of determination (R2) of 0.9997.
Pharmacokinetic Analysis
PK parameters were determined by DAS 2.0 software. Based on the actual serum concentration-time data of rats in each group, PK parameters were calculated using the non-compartmental model, such as time to peak time (Tmax), peak concentration (Cmax), area under the concentration-time curve (AUC), mean residence time (MRT), half life (t1/2), volume of distribution adjusted for bioavailability (Vd/F) and clearance adjusted for bioavailability (CL/F). The akaike information criterion (AIC) and R2 were used to determine the optimal atrioventricular model.
Susceptibility Testing
Determination of Minimum Inhibitory Concentration of Levofloxacin and Qiguiyin Decoction
The minimum inhibitory concentration (MIC) of LVEX and QGYD against MDR PA (No.1912109) were determined using the broth microdilution method according to CLSI guidelines. Twofold serial dilution of LVFX (1024 ug/mL) or QGYD (2 g/mL) in Mueller-Hinton Broth (MHB) was prepared in 24 -well microtitre plate and the volume in each well was 1 mL. Besides, the positive and negative control wells were set. 10 μL of the prepared MDR PA bacterial solution was added to each well (except the negative control well) with a concentration of 1.5×108 CFU/mL, and the 24-well plate was placed in the incubator at 37 °C for 24 h. Record the value of MIC.
Determination of Fractional Inhibitory Concentration Index of Levofloxacin and Qiguiyin Decoction Using Broth Microdilution Method
According to the checkerboard method design, the combined bacteriostatic effect of LVFX and QGYD with different concentrations (1/16MIC-2MIC) on the MDR PA strain was determined by the broth microdilution method. 50 uL of twofold serial dilution of LVFX (64 ug/mL) and QGYD (2 g/mL) in MHB was prepared in a 96-well microtitre plate. There are also positive and negative control wells. 100 μL of this suspension with a concentration of 3×106 CFU/mL was inoculated into the broths except for the negative control wells. The final volume in each well was 200 uL After being incubated at 37 °C for 24 h in the incubator, the fractional inhibitory concentration index (FICI) was calculated using the following formula:
Interpretation criteria: FICI ≤ 0.5 denotes “synergistic”; 0.5 < FICI ≤ 1 denotes “additivity”; 1 < FICI≤ 4 denotes “no interaction”; FICI > 4 denotes “antagonism”.
Determination of Antibacterial Activity in the Serum of Rats Administered with Levofloxacin Alone and in Combination with Qiguiyin Decoction
The antibacterial activity in rat serum of all groups was determined by adding 50 uL of serum at various time points to the 96-well plate containing 130 uL of MHB. Then 20 μL of bacterial solution (1.5 × 107 CFU/mL) was added. In addition, positive and negative control holes containing blank serum were set for parallel control. After being incubated in a 37 °C constant temperature incubator for 18–24 hours, the result was detected at 600 nm by the microplate reader, and the antibacterial rate of drug-containing serum at different time points was calculated according to the OD value.
Fitting and Analyzing of PK-PD Model
The dynamic studies between the semi-body parameter AUC24h/MIC and the bacteriostatic effect were modeled and analyzed using Emax Model in Phoenix WinNonlin software. The standard error (Stderr) and coefficient of variation (CV%) were used to evaluate the accuracy of fit of the model and the AIC value was used to evaluate the model’s goodness of fit. The optimal model was comprehensively compared and determined.
Determination of Serum Inflammatory Cytokines
The QAM-INF-1 Kit was used to simultaneously detect 5 common inflammatory cytokines including IL-1β, IL-6, IL-10, MCP-1, and TNF-α in rat serum in each group. The operation steps are as follows: 100 μL of sample diluent was added and incubated in a shaking table at room temperature for 1 h to close the chip and then discarded. And 100 μL of sample solution or standard solution was added and incubated in a shaking table at 4 °C. The chips were washed by washing solutions I and II after being diluted with deionized water. After adding 80 mL test antibodies, it was incubated in a 37 °C shaker for 2 hours. After being washed as above, 80 uL of Cy3-Streptavidin was added and then incubated in a 37 °C shaker for 1 hour. After being washed and dried at room temperature, the chips were detected at 532 nm with Innoscan 300 scanner. QAM-INF-1 software was used to analyze the changes of the above inflammatory cytokines and search for differential inflammatory factors in different groups of rats.
Statistical Analysis
The data were expressed as mean ± standard deviation (mean ± SD) in figures and tables. The differences of PK parameters and levels of inflammatory factors were analyzed by one-way ANOVA with SPSS 21.0 software. P < 0.05 was considered to be statistically significant. GraphPad Prism 8.0 software was used for making charts.
Results
Susceptibility Testing
The MICs of LVEX and QGYD against MDR PA were determined using the broth microdilution method. The results showed that the combination of QGYD reduced the MIC of LVFX from 8 µg/mL to 4 µg/mL, and the MIC of QGYD decreased from 250 mg/mL to 125 mg/mL compared to QGYD alone. Compared to the single treatment group, the MIC of LVFX and OGYD decreased in the combined treatment group, indicating an increased bacteriostasis rate following the combination of these two drugs. Furthermore, the FICI of QGYD and LVFX in combination against MDR PA-induced pneumonia rats was calculated as 1, indicating an additive effect against the MDR PA strain.
Effects of Qiguiyin Decoction on Pharmacokinetics of Levofloxacin in Serum of Pneumonia Rats
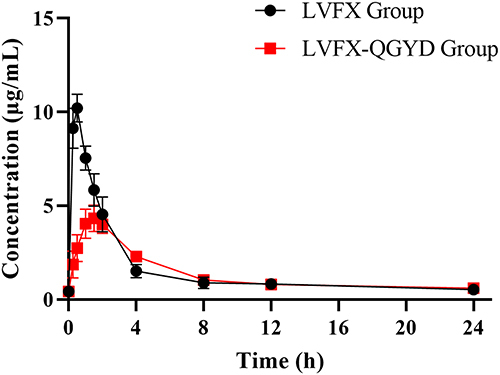
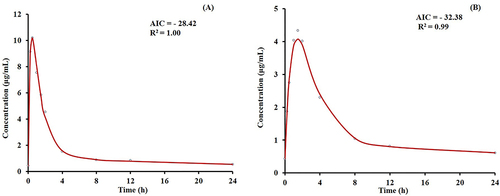
The PK parameters of LVFX group and LVFX-QGYD group in the MDR PA-infected rat are shown in . The PK of LVFX in pneumonia rats followed a primary absorption rate and a two-compartment model. The model-fitting results are shown in . The results basically corresponded with those reported in the previous literature,Citation15,Citation16 indicating the determination of LVFX concentration in rat serum was accurate and reliable.
Table 1 The PK Parameters of LVFX Group and LVFX-QGYD Group in the MDR PA-Infected Rats. (n=6, Mean±SD)
Figure 1 The model-fitting results of LVFX group (A) and LVFX-QGYD group (B). (AIC, akaike information criterion; R², determination coefficient).
Plasma concentration-time profiles of the LVFX group and LVFX-QGYD group in MDR PA-infected rats are shown in . The results showed that the combination of QGYD resulted in prolonged MRT and Tmax of LVFX in pneumonia rats. Moreover, the combination of QGYD reduced Vd/F and t1/2, and increased CL/F in pneumonia rats. These results suggested that the combination of QGYD might affect the absorption, distribution, metabolism, and excretion process of LVFX in vivo by changing its PK parameters.
Effects of Qiguiyin Decoction on Pharmacodynamics of Levofloxacin in Serum of Pneumonia Rats
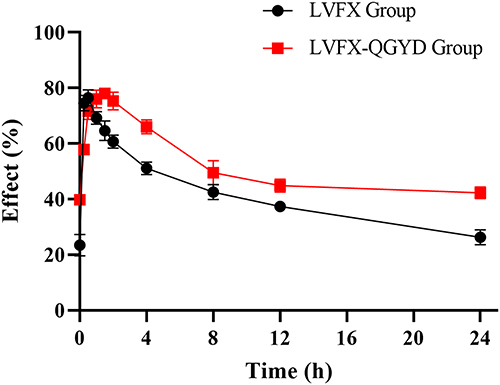
The average bacteriostatic effect-time curves of the LVFX group and LVFX-QGYD group are shown in , revealing a higher initial bacteriostatic rate and longer Tmax following the combination of QGYD. Furthermore, the serum bacteriostatic rate of the combination group remained consistently higher than that of LVFX alone 1 h after administration, indicating a significant enhancement in the inhibition of LVFX on MDR PA-induced rats by the combination with QGYD.
Establishment of PK/PD Modelings
The semi-body parameter AUC24h/MIC was selected as PK/PD parameter to describe the antibacterial effect of LVFX alone or in combination with QGYD on MDR PA-induced rats. Compared with the LVFX single group, the combination of QGYD significantly increased the AUC0-24h/MIC of LVFX from 4.56±0.63 to 7.99±0.16. The PK-PD parameters of LVFX alone and in combination with QGYD are presented in and . It was concluded that the PK/PD model of LVFX singly was E=(81.25×C0.93)/(0.750.93+C0.93), while the PK/PD model of LVFX-QGYD was E= 38.30+(46.80×C2.06)/(2.942.06+C2.06). Therefore, the antibacterial effect of LVFX singly was (68.34±1.36) %, and LVFX-QGYD was (79.81±0.19) % (P < 0.01).
Table 2 The PK-PD Parameters of LVFX Group
Table 3 The PK-PD Parameters of LVFX-QGYD Group
Effects of Qiguiyin Decoction Combined with Levofloxacin on Inflammatory Cytokines in Serum of Pneumonia Rats
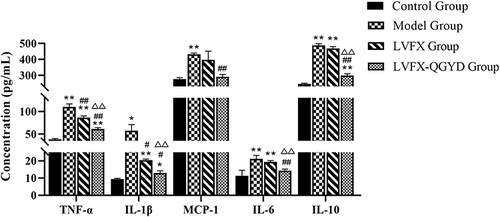
It is well established that inflammatory cytokines play a pivotal role in promoting the occurrence and development of inflammatory immunity in the body. Dynamic changes in inflammatory factors during the modeling and administration process were analyzed using antibody chip. As shown in , our study revealed that the levels of 5 inflammatory factors in the model group were elevated compared to those in the control group. These findings suggested that MDR PA infection could significantly enhance the expressions of IL-1β, IL-6, TNF-α, IL-10, and MCP-1, leading to a disruption in the body’s immune homeostasis. Following intervention with LVFX, a significant decrease in the expressions of IL-1β and TNF-α was observed, and the expressions of IL-6, IL-10, and MCP-1 showed a slight reduction compared to the model group. Notably, the combined treatment of LVFX with QGYD resulted in expressions of IL-1β, IL-6, TNF-α, IL-10, and MCP-1 that were closer to those in the control group than LVFX administered alone. Consequently, QGYD-LVFX treatment demonstrated a significant regulatory effect on the expressions of inflammatory factors in MDR PA-induced rats, facilitating the restoration of immune balance.
Discussion
Traditionally, PK-PD studies are conducted using healthy laboratory animals.Citation17 However, disparities exist in the PK and PD parameters of antibacterial drugs between normal and infected animal models.Citation18 The PK-PD parameters of LVFX in combination with QGYD in infected models hold greater significance and clinical relevance compared to those in normal models. Therefore, we established a pneumonia model induced by MDR PA in rats and further investigated the PK-PD of LVFX alone or in combination with QGYD, providing a robust foundation for the combined use of Chinese and Western medicines in the treatment of MDR PA infections.
PK experiments revealed that the combination of QGYD slowed down the release of LVFX in rats. And the distribution of LVFX in various tissues was reduced, its access into the vascular ventricle was increased, and the clearance rate was enhanced. Moreover, the AUC0-24h/MIC ratio of LVFX significantly increased after combining with QGYD, suggesting a potential enhancement in the pharmacological activity of LVFX. Model-predicted results revealed that the serum bacteriostatic effect was significantly augmented in the combined group compared to the LVFX group, indicating the combination of QGYD could enhance the efficacy of LVFX against MDR PA infection in rats These findings were also consistent with previous researches.Citation10,Citation19
Furthermore, we used the antibody chip to investigate the mechanism of QGYD restoring LVFX sensitivity to MDR PA. Pneumonia infection caused by PA involves multiple inflammatory pathways. The results demonstrated a significant increase in the serum inflammatory cytokine IL-1β, IL-6, TNF-α, IL-10, and MCP-1 levels in the model group, consistent with previous studies.Citation12,Citation20 Compared to LVFX alone, the QGYD-LVFX combination treatment normalized some pro-inflammatory factors and anti-inflammatory factors towards levels observed in the control group, suggesting that QGYD-LVFX therapy could inhibit the further expansion of inflammatory response and help the body recover to normal immune balance more quickly. TCM has also been proven to promote inflammation recovery.Citation21 Consequently, the combination of QGYD and LVFX not only improved antibacterial efficacy by influencing pharmacokinetics but also enhances overall immunity.
Therefore, our study demonstrated that the combination of QGYD significantly enhanced the antibacterial effect of LVFX in pneumonia rats, potentially through herb-drug interactions that improved anti-infective efficacy.
Conclusion
In conclusion, the combination of QGYD changed the PK/PD parameters of LVFX in pneumonia rats, enhancing its activity against MDR PA. PK/PD model fitting further confirmed the superior antibacterial effects of the LVFX-QGYD combination over LVFX alone, providing additional evidence for the efficacy of QGYD in combination with LVFX against MDR PA. From the perspective of immunity, compared to LVFX alone, the combination of QGYD could significantly regulate the expressions of IL-1β, TNF-α, IL-6, and IL-10 caused by MDR PA infection. This modulation promoted the restoration of immune balance in pneumonia rats and enhanced the sensitizing effect of LVFX.
Abbreviations
AIC, Akaike information criterion; AUC, area under the concentration-time curve; Cmax, peak concentration; CL/F, clearance adjusted for bioavailability; CV%, coefficient of variation; FICI, fractional inhibitory concentration index; IS, internal standard; LVFX, levofloxacin; MDR PA, multidrug-resistant Pseudomonas aeruginosa; MHB, Mueller-Hinton Broth; MIC, minimum inhibitory concentration; MRT, mean residence time; PK-PD, pharmacokinetics-pharmacodynamics; QGYD, Qiguiyin decoction; Stderr, standard error; SPF, specific pathogen free; t1/2, half life; Tmax, peak time; TCM, traditional Chinese medicine; UPLC-MS/MS, ultra-performance liquid chromatography-tandem mass spectrometry; UPLC-TQ/MS, ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry; Vd/F, volume of distribution adjusted for bioavailability.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors acknowledge the funding of the National Natural Science Foundation of China (No. 81873127) and the support from the High-Level Traditional Chinese Medicine Key Discipline Construction Project of Chinese Medicine Analysis (No. 90010951310016).
References
- Zhao L, Wang S, Li X, He X, Jian L. Development of in vitro resistance to fluoroquinolones in Pseudomonas aeruginosa. Antimicrob Resist Infect Control. 2020;9(1):124. doi:10.1186/s13756-020-00793-8
- Kirikae T. Multidrug-resistant Pseudomonas aeruginosa T. Nihon Rinsho Japan J Clin Med. 2012;70(2):231–235.
- Liu ZH, Xu Y, Duo LB, et al. Pseudomonas aeruginosa isolates of distinct sub-genotypes exhibit similar potential of antimicrobial resistance by drugs exposure. Antonie van Leeuwenhoek. 2013;103(4):797–807. doi:10.1007/s10482-012-9862-4
- Swathirajan CR, Rameshkumar MR, Solomon SS, Vignesh R, Balakrishnan P. Changing drug resistance profile in Pseudomonas aeruginosa infection among HIV patients from 2010-2017: a retrospective study. J Global Antimicrob Resist. 2019;16:274–277. doi:10.1016/j.jgar.2018.10.019
- Guo Y, Ban W, Yang L, Shi G. Analysis of drug resistance characteristics of pulmonary tuberculosis complicated with Pseudomonas aeruginosa infection. Chin Med J. 2022;57(07):801–803.
- Pang Z, Zhu Q. Traditional Chinese Medicine is an Alternative Therapeutic Option for Treatment of Pseudomonas aeruginosa Infections. Front Pharmacol. 2021;12:737252. doi:10.3389/fphar.2021.737252
- Cai Y, Zhang Q, Fu Y, et al. Effectiveness of Chinese Herbal Medicine Combined with antibiotics for extensively drug-resistant enterobacteria and nonfermentative bacteria infection: real-life experience in a retrospective cohort. Biomed Res Int. 2017;2017:2897045. doi:10.1155/2017/2897045
- Hou Y, Nie Y, Cheng B, et al. Qingfei Xiaoyan Wan, a traditional Chinese medicine formula, ameliorates Pseudomonas aeruginosa-induced acute lung inflammation by regulation of PI3K/AKT and Ras/MAPK pathways. Acta Pharmaceutica Sinica B. 2016;6(3):212–221. doi:10.1016/j.apsb.2016.03.002
- Yu M, Gu X, Qu Y, et al. Combination therapy with TCM preparation kumu injection and azithromycin against bacterial infection and inflammation: in vitro and in vivo. Evid Based Complement Alternat Med. 2022;2022:8533005. doi:10.1155/2022/8533005
- Huang P, Guo Y, Zhao J, Li B, Wu Y, Liu Q. Efficacy and safety of the Qiguiyin formula in severe pneumonia: study protocol for a randomized, double-blind, placebo-controlled clinical trial. J Traditional Chin Med. 2020;40(2):317–323.
- Kong LB, Ma Q, Gao J, et al. Effect of Qiguiyin Decoction on multidrug-resistant Pseudomonas aeruginosa infection in rats. Chin J Integr Med. 2015;21(12):916–921. doi:10.1007/s11655-015-2089-2
- Chen G, Zhang W, Kong L, et al. Qiguiyin decoction improves multidrug-resistant Pseudomonas aeruginosa infection in rats by regulating inflammatory cytokines and the TLR4/MyD88/NF-κB signaling pathway. Biomed Res Int. 2022;2022:5066434. doi:10.1155/2022/5066434
- Naik P, Singh S, Vishwakarma S, et al. Multidrug-resistant Pseudomonas aeruginosa evokes differential inflammatory responses in human microglial and retinal pigment epithelial cells. Microorg. 2020;8(5):735. doi:10.3390/microorganisms8050735
- Miyoshi-Akiyama T, Tada T, Ohmagari N, et al. Emergence and spread of epidemic multidrug-resistant Pseudomonas aeruginosa. Genome Biol Evol. 2017;9(12):3238–3245. doi:10.1093/gbe/evx243
- Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32(2):101–119. doi:10.2165/00003088-199732020-00002
- Chulavatnatol S, Chindavijak B, Vibhagool A, Wananukul W, Sriapha C, Sirisangtragul C. Pharmacokinetics of levofloxacin in healthy Thai male volunteers. J Med Assoc Thailand. 1999;82(11):1127–1135.
- Choi JS, Choi I, Choi DH. Effects of nifedipine on the pharmacokinetics of repaglinide in rats: possible role of CYP3A4 and P-glycoprotein inhibition by nifedipine. Pharmacol Rep. 2013;65(5):1422–1430. doi:10.1016/S1734-1140(13)71502-0
- Goud T, Maddi S, Nayakanti D, Thatipamula RP. Altered pharmacokinetics and pharmacodynamics of repaglinide by ritonavir in rats with healthy, diabetic and impaired hepatic function. Drug Metabol Person Ther. 2016;31(2):123–130. doi:10.1515/dmpt-2015-0046
- Kong L, Liu Q, Yang Q, Gao J, Fu Y. Effect of Qiguiyin Prescription on in vitro inhibition of multidrug-resistant Pseudomonas aeruginosa by antibiotics. World J Tradit Chin Med. 2014;9(03):288–290+295.
- Ding J, Ding X, Lu Y, An S, Cui X, Liu Q. Preliminary study on immunomodulatory effect of Qi Guiyin on pneumonia induced by Pseudomonas aeruginosa in rats. J Hebei Tradit Chin Med Pharmacol. 2019;34(02):1–5.
- Liang L, Jin X, Li J, et al. A comprehensive review of pharmacokinetics and pharmacodynamics in animals: exploration of interaction with antibiotics of shuang-huang- lian preparations. Curr Top Med Chem. 2022;22(2):83–94. doi:10.2174/1568026621666211012111442