 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
It is not known whether or not ward-specific antimicrobial use density (AUD) affects the ratio of methicillin-resistant Staphylococcus aureus (MRSA) in culture-positive S. aureus. A 60-month study was attempted to ascertain the association between inpatient MRSA ratio and ward-specific AUDs as well as the former and latter study intervals, specimen types, and ward specialty. During the study, the professionals in infection control regulated the use of broad-spectrum antimicrobials and those for MRSA. By both month and ward, the ratio of inpatients positive for MRSA to those positive for S. aureus was calculated. Factors associated with MRSA ratio included AUDs averaged for the sampling month and its previous month, outpatient MRSA ratio by age, ward specialty, specimen type, and half intervals to represent historical changes. Of a total of 4,245 strains of S. aureus isolated during the 5-year study, 2,232 strains (52.6%) were MRSA. By year, outpatient MRSA ratio at age ≥15 decreased in later years, as did inpatient MRSA ratio. Multivariate analysis for inpatient MRSA ratio revealed a positive risk in AUDs for meropenem (odds ratio [OR] 1.761; 95% confidence interval [CI] 1.761–2.637, P = 0.01), imipenem-cilastatin (OR 1.583; 95% CI 1.087–2.306, P = 0.02), ampicillin-sulbactam (OR 1.623; 95% CI 1.114–2.365, P = 0.01), and minocycline (OR 1.680; CI 1.135–2.487, P = 0.01), respiratory care ward (OR 2.292; 95% CI 1.085–4.841, P = 0.03), and outpatient MRSA ratio (OR 1.536; 95% CI 1.070–2.206, P = 0.02). Use of broad-spectrum antimicrobials, such as meropenem, imipenem-cilastatin, and ampicillin-sulbactam may increase inpatient MRSA ratio. Ward factor should be included in MRSA surveillance because of the possible effect on AUD and considering patients’ backgrounds.
Introduction
As a surveillance indicator of methicillin-resistant Staphylococcus aureus (MRSA), the inpatient ratio of MRSA in positive culture with S. aureus may be reduced by infection control within a hospital. The same ratio in outpatients, however, may show MRSA endemic in the region and thus can affect the inpatient MRSA ratio. Lee et alCitation1 described that the susceptibility of S. aureus is associated with broad-spectrum antimicrobial use, an index of which is antimicrobial use density (AUD). Cheng et alCitation2 described that AUD of broad-spectrum antimicrobials could measure the effect of an antimicrobial stewardship program. Alternatively, monthly AUD may represent an antimicrobial pressure in a unit, either within a ward or a hospital. However, the relationship among susceptibility of S. aureus, AUD, and inpatient ratio of MRSA remains unresolved.
Thus, a 60-month study was conducted retrospectively on a total of 22 antimicrobials with the objective to elucidate the association between MRSA prevalence and ward-specific AUD as well as half intervals, the annual ratios of lower respiratory tract specimens, and ward specialty represented by respiratory care.
Material and methods
Infection control
Throughout January 2006 to December 2010, the infection control professionals regulated the use of broad-spectrum antimicrobials and agents for MRSA. They also submitted culture specimens for any suspicious infection and compliance with contact precautions at weekly rounds across the wards. Surveillance culture specimens on the day of admission were regarded as those of outpatients. Hand hygiene measures were constantly observed by compliance to hand washing and the use of alcohol gel or non-sterile gloves.
Laboratory workup
In the laboratory, colonies growing on the blood agar medium were extracted onto MRSA Screen Agar (Japan Becton Dickinson, Tokyo, Japan). Colonies growing on this medium underwent the coagulase test (Rabbit Plasma Test; Eiken-kagaku, Tokyo, Japan), where positive strains were defined as S. aureus positive. Thereafter, strains of MRSA were defined using the Clinical and Laboratory Standards Institute:Citation3 (1) having a minimum inhibitory concentration (MIC) of oxacillin equal or more than 4 μg/mL; or (2) having a MIC of cefoxitin equal or more than 8 μg/mL. Also, MRSA was defined as strains which showed MIC of oxacillin equal to 2 to 4 μg/mL and were positive for the penicillin-binding protein 2′ test (MRSA-LA; Denka Seiken, Niigata, Japan).
Inpatient strains of methicillin-sensitive and -resistant S. aureus underwent analysis of 50- and 90-percentile MIC (MIC50 and MIC90, respectively) for 13 agents.
Antimicrobial use density
Types of specimens positive for S. aureus were classified regardless of whether they were from the lower respiratory tract including sputum or the tracheal aspirate. Annual ratios of each type of specimen were calculated, which were later included in the data set.
AUDs were calculated for a total of 22 agents, 60 months, and specific wards using the formula:
where DDD is the defined daily dose, as determined by the World Health Organization.Citation4 The data on the monthly and ward-specific inpatients were provided by the hospital accounting office. For the data set, we entered the ward AUDs averaged for the month when the sample was submitted and for the preceding month. For example, the average value of AUD/pediatrics ward/2006-06/ampicillin and AUD/pediatrics ward/2006-05/ampicillin was inputted into the AUD data for pediatrics ward/2006-06/ampicillin.
Antimicrobials subjected to AUD included: ampicillin, cefazolin, ceftazidime, cefmetazole, cefotiam, cefpirome, ceftriaxone, cefotaxime, cefozopran, clindamycin, flomoxef, fosfomycin, gentamicin, imipenem-cilastatin, linezolid, meropenem, minocycline, panipenem-betamipron, piperacillin, ampicillin-sulbactam, cefoperazone-sulbactam, and tobramycin.
Questionnaires to physicians
To help evaluate the presence or absence of cross infection among patients positive for MRSA, questionnaires (see supplementary material) were issued to physicians in charge of patients positive for MRSA upon first detection. At the physicians’ discretion, they reported the infectious status of their patients and estimates of propagation route, which were analyzed by dividing the 5 years into former and latter intervals. When a patient was found positive for MRSA and later admitted, the physician judged whether or not the patient was colonized with MRSA or was manifesting infection with MRSA (see supplementary material).
Statistical analysis
By the month and ward, patients with MRSA and all of those with S. aureus were counted without repetition to obtain their ratio. If no strains of S. aureus could be isolated in a month and a ward, the patient ratio was regarded as 0. The MRSA ratios were calculated separately for inpatients and outpatients; outpatients were further classified into younger than 15 years of age or older.
Factors associated with increased inpatient MRSA ratio included the averaged AUDs, half intervals, the annual ratios of the lower respiratory tract specimens, and the respiratory care and pediatrics wards. The pediatrics factor was included as an age indicator less than or equal to 15 years old for AUD. In the logistic regression analysis, AUD of any month above its median value was assigned 1 whereas AUD equal or less than its median value was assigned 0. Likewise for the remaining factors, a value of 1 was assigned when positive whereas a value of 0 was assigned when negative.
To each MRSA ratio of a particular month and a ward, the background factors above were added. Inpatient MRSA ratios were age-adjusted to their outpatient counterpart separated at 15 years of age. Variables more or less than the median values were assigned 1 or 0, respectively. The data underwent univariate logistic regression analysis where outcome was determined as inpatient MRSA ratio higher than its median, which was assigned 1. Inpatient MRSA ratio less than its median was, however, assigned 0. Factors significant in univariate analysis underwent subsequent multivariate analysis to exclude mutual confounding effect of background factors. For all statistical analyses, we used SPSS® (IBM Corporation; Armonk, NY, USA) and statistical significance was considered when P < 0.05.
At the admission of patients, informed consent was provided for their clinical and bacterial data use under the condition that their identifying information be omitted.
Results
Overview
A total of 30,536 microbes were isolated during the 5-year study period, including 23,566 from ten wards with a capacity of 419 beds and 6,970 from outpatient units. Among them, a total of 4,245 strains (13.9% of the grand total) of S. aureus were obtained, in which 2,232 strains (a ratio of 0.526 of all the S. aureus isolates) were MRSA from outpatients and inpatients (). A ratio of MRSA/S. aureus at 0.349 in outpatients was lower than in inpatients at 0.657. Of the 2,232 cases of MRSA from outpatients and inpatients, 1,601 (71.7%) were from inpatients. These included 433 patients (a ratio of 0.270) who had been positive as outpatients ().
Table 1 Monthly number of patients positive for Staphylococcus aureus, those positive for methicillin-resistant S. aureus (MRSA), and inpatients having been positive for MRSA as outpatients (Outpt+)
The median ratio of MRSA-positive cases as outpatients/MRSA-positive inpatients for the same patients was highest in the pediatrics ward at 0.589, whereas in respiratory care ward the ratio was 0.333, close to the ward’s subtotal (). Median inpatient MRSA ratio was the highest in respiratory care (a median of 0.787) but low in pediatrics (a median of 0.348) and obstetrics/gynecology (a median of 0.400).
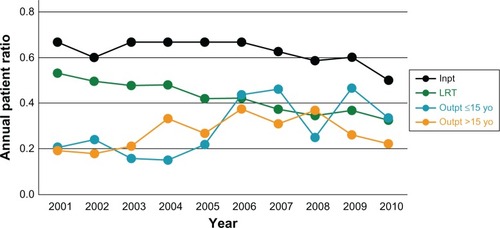
Of the 4,245 strains of S. aureus, 1,565 (36.9%) were derived from the lower respiratory tract, the ratios of which tended to decrease annually (). As for outpatients, MRSA ratio for age ≤ 15 years (a median of 0.437) fuctuated, whereas the ratio for age > 15 years (a median of 0.309) decreased (). Also, inpatient MRSA ratio showed a decreasing trend ().
Figure 1 Annual patient ratios of methicillin-resistant Staphylococcus aureus to S. aureus in outpatients ≤ 15 years old, in outpatients > 15 years old, and in inpatients. For reference, the ratios of specimens derived from the lower respiratory tract are depicted. Lower respiratory tract and inpatient ratios show decreasing trends.
Abbreviations: Inpt, inpatient; LRT, lower respiratory tract; Outpt, outpatient; yo, years old.
The MIC50 and MIC90 for 13 drugs in a total of 2,438 strains of S. aureus from inpatients showed minor time shift over former and latter study intervals (). For example, the MIC90 of vancomycin increased from 1.0 to 2.0 μg/mL.
Table 2 Minimum inhibitory concentration of 50 percentile (MIC50) and of 90 percentile (MIC90) of methicillin-sensitive and -resistant Staphylococcus aureus (n = 2,438) isolated from inpatients
Antimicrobial use density
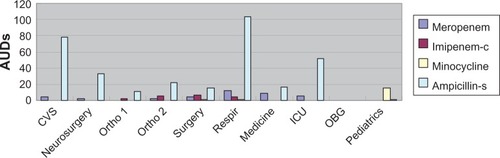
By agent, the median values of AUDs were high in cefazolin (19.25/patient), ampicillin-sulbactam (26.45/patient), and cefoperazone-sulbactam (8.39/patient). Annual trends of median values of AUDs fuctuated in AUDs with median values higher than 0 (). By ward, the median AUD values were highest in the respiratory care ward using m eropenem (12.66/patient) and ampicillin-sulbactam (103.52/patient), in the surgery ward using imipenemcilastatin (6.85/patient), and in the pediatrics ward with the use of minocycline (15.76/patient; ).
Figure 2 Annual trend of AUD in 22 agents. AUDs of ampicillin-sulbactam and cefazolin are high, but over time, like other agents, their AUDs fuctuate.
Note: Vertical axis shows the median values of AUDs.
Abbreviation: AUD, antimicrobial use density.
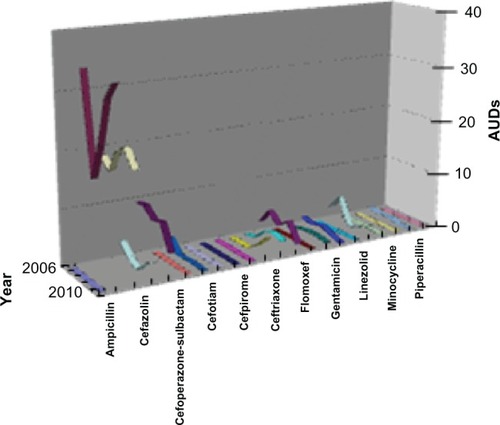
Figure 3 AUD of four agents by ten wards over 60 months (n = 600). AUDs of meropenem and ampicillin-sulbactam (Ampicillin-s) are highest in the respiratory care ward (Respir) whereas AUD of imipenem-cilastatin (Imipenem-c) is highest in the surgery ward.
Note: Vertical axis shows median values of AUDs.
Abbreviations: AUD, antimicrobial use density; CVS, cardiovascular service; Ortho1, 1st orthopedics; Ortho 2, 2nd orthopedics; ICU, intensive care unit; OBG, obstetrics and gynecology; Respir, respiratory care ward.
Statistical analysis
Of the total 1,016 questionnaires, the physicians seeing patients with MRSA responded in 935 cases (92.0%) and 899 cases (88.5%) for the infectious status and the propagation route, respectively (). Dividing the 5 years into former and latter periods, the portion determined to be overt infection by MRSA and the portion estimated to be in-hospital propagation were comparable between the two periods by Pearson’s Chi-square test (both P > 0.05).
Table 3 Questionnaires (n = 1,016) for (A) infectious status and (B) propagation route discerned by physicians in charge of patients positive for MRSA
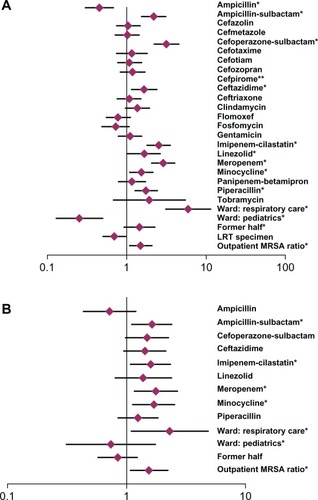
Of all the data for the 60 months and the ten wards for the analysis of AUD, univariate analysis for the higher MRSA ratio showed significance with 13 factors, nine of which were AUDs (). Subsequent multivariate analysis showed positive risk in AUDs for meropenem (odds ratio [OR] 1.761; 95% confidence interval [CI] 1.761–2.637, P = 0.01), ampicillin-sulbactam (OR 1.470; CI 1.114–2.365, P = 0.01), imipenem-cilastatin (OR 1.583; CI 1.087–2.306, P = 0.02), and minocycline (OR 1.680; CI 1.135–2.487, P = 0.01), respiratory care ward (OR 2.292; CI 1.085–4.841, P = 0.03), and outpatient MRSA ratio (OR 1.536; CI 1.070–2.206, P = 0.02; ).
Figure 4 (A) Univariate and (B) multivariate logistic regression analysis on the ward-specific antimicrobial use density (ampicillin through tobramycin) and other factors for the patient ratios of methicillin-resistant Staphylococcus aureus (MRSA)/S. aureus.
Notes: Diamonds indicate odds ratio; horizontal bar represents 95% confidence interval; *statistical significance (P < 0.05); **not available due to sample deviation.
Abbreviations: LRT, lower respiratory tract; MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
In order to investigate the possible cause of inpatient MRSA ratio, ward-specific AUD was defined to include the month of sample submission and its preceding month to represent the antimicrobial pressure in the ward. The multivariate analysis revealed significance in meropenem, imipenemcilastatin, and ampicillin-sulbactam among various AUDs, demonstrating their risk for increasing MRSA patient ratio. This effect by these antimicrobials was anticipated because of broad-spectrum activity. Indeed, Vernaz et alCitation5 demonstrated that the use of these agents augmented MRSA incidence. Lee et alCitation1 demonstrated that after hospital opening, increased AUD of broad-spectrum antimicrobials allowed rapid spread of MRSA. In our institute, however, we regulated the use of broad-spectrum antimicrobials such as carbapenems, which may have reduced the inpatient MRSA ratio. Likewise, MIC50 and MIC90 did not show noteworthy resistance for any antimicrobials. Our regulation of broad-spectrum agents may have kept the MIC constant over the study period.
Ampicillin-sulbactam, a beta-lactam with a beta-lactamase inhibitor, as well as carbapenems, were reported to retain useful activity;Citation6 however, Bantar et alCitation7 noted that rates of MRSA were inversely associated with the consumption index of ampicillin-sulbactam to the third-generation cephalosporins. The discrepancy between the literature and our finding of the positive risk of ampicillin-sulbactam may be partially derived from the current multivariate analysis involving the ward factor.
A literature review has revealed several reports on the influence of AUDs on surveillance of S. aureus. Pros for AUDs’ influence stressed that AUDs of broad-spectrum drugs were associated with their drug resistance.Citation8 Yoon and colleaguesCitation9 described that a proportion of resistance in S. aureus was correlated to penicillin use. Cons for AUDs’ influence, however, claimed that measures against cross infection more influenced the spread of S. aureus than did antimicrobial use.Citation10 However, the factor of former and latter periods did not significantly influence MRSA inpatient ratio in our study. Thus, we presume that AUD control measures played a more crucial role in the reduction of inpatient MRSA ratio than did infection control measures. Therefore, excessive use of broad-spectrum agents is discouraged in the face of MRSA prevalence.
On the other hand, the reason why AUD of minocycline showed a positive risk for the inpatient MRSA ratio remains uncertain because the pediatrics ward, having had the highest AUD of minocycline, demonstrated the lowest inpatient MRSA ratio of 0.348. Indeed, Raad and colleaguesCitation11 reported that antibiotic lock with minocycline on the intravenous line may prevent catheter-related bloodstream infection with MRSA. However, Oshiro et alCitation12 reported that minocycline antagonized the effect of vancomycin in S. aureus with heterogeneous resistance to vancomycin. Thus, further investigation may be warranted to solve this issue.
Another risk was the ward factor of respiratory care, which was selected for its highest MRSA ratio among all the wards. Borg and colleaguesCitation13 described that over-crowded general medicine wards triggered incidences of MRSA. Moreover, Kerttula and et alCitation14 noted that patients in a long-term care facility and health care ward shared similar MRSA genotypes. Likewise, our respiratory care ward admitted elderly patients referred from nursing homes, thus providing a background for increased MRSA ratio. Similarly, Kardas-Sloma and colleaguesCitation15 described that the intensive care unit plays a role of incubator in the control of community-acquired MRSA. Their intensive care unit and our respiratory care ward may commonly serve as the interface between in and out of the hospital.
Eveillard et alCitation16 described that admission at greater than 80 years of age would increase the sensitivity of MRSA surveillance. Our preliminary study using outpatient MRSA ratio for all ages, however, showed no significance. Age adjustment, on the other hand, played a crucial role because pediatrics and adult patients demonstrated different MRSA ratios. Thus, both ward and age factors are to be given credit in the analysis of MRSA surveillance.
In the multivariate analysis, we omitted the factor of contact precaution because of its constant use. The aprons for barrier precaution had been made of fabrics during the study, only to be changed to plastic ones after the study. This might account for the lack of difference between the former and the latter periods in the questionnaires discerning the rates of hospital propagation of MRSA and infection.
The ward factor of pediatrics was not significant. This factor was included as an indicator of inpatients’ age adjustment because patients in this ward were 15 years old or younger. The reason why the MRSA ratio for outpatients ≤ 15 years old was fluctuating whereas the ratio for outpatients > 15 years old decreased might derive from increased antimicrobial use and pediatrics MRSA reservoir in regional practitioners.Citation17 Future research may clarify age-specific mechanisms for MRSA surveillance in our region.
Conclusion
Broad-spectrum antimicrobials and outpatient MRSA ratio may increase inpatient MRSA ratio. Ward factor may be included in MRSA surveillance because of its confounding effect on AUD and patients’ background.
Supplementary material
Questionnaire issued to physicians in charge of patients positive for methicillin-resistant Staphylococcus aureus. A translation from the Japanese original.
A strain of MRSA was isolated from this patient. Please answer following questions.
Q1. What was the specimen positive for MRSA?
A1. Sputum/Tracheal tube/Decubitus/Drainage tube/Pus/Blood/Urine/Blood vessel catheter/Other (specify:)
Q2. When did you submit the specimen?
A2. Year: , Month: , Day:
Q3. What infectious status do you think patient was at submission?
A2. (1) Manifest infection, if so, specify: Due to MRSA/Due to MRSA and other(s)/Causality unknown either MRSA or other(s) (2) MRSA colonization
Q4. Where do you think MRSA was acquired?
A4. Imported from community/Within hospital/Unknown
Q5. Was the patient postoperative?
A5. Yes (specify operation date:)/No
Q6. What was the main diagnosis of the patient? In what ward was the patient?
A6. (specify diagnosis and ward:)
Q7. Please give comments, if any.
A7. (describe:)
Thank you for your cooperation.
Disclosure
The authors report no conflicts of interest in this work.
References
- LeeSSKimHSKangHJKimJKChungDRRapid spread of methicillin-resistant Staphylococcus aureus in a new hospital in the broad-spectrum antibiotic eraJ Infect200755435836217692383
- ChengVCToKKLiI WAntimicrobial stewardship program directed at broad-spectrum intravenous antibiotics prescription in a tertiary hospitalEur J Clin Microbiol Infect Dis200928121447145619727869
- Clinical and Laboratory Standards InstitutePerformance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement CLSI document M100-S21WayneCLSI2011
- World Health Organization Collaborating Centre [homepage on the Internet]ATC/DDD Index 2012GenevaWorld Health Organization2012 [updated December 20, 2012]. Available from: http://www.whocc.no/atc_ddd_index/Accessed March 25, 2013
- VernazNSaxHPittetDBonnabryPSchrenzelJHarbarthSTemporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficileJ Antimicrob Chemother200862360160718468995
- TalbotGHBradleyJEdwardsJEAntimicrobial Availability Task Force of the Infectious Diseases Society of AmericaBad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of AmericaClin Infect Dis200642565766816447111
- BantarCSartoriBVescoEA hospitalwide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistanceClin Infect Dis200337218018612856209
- MonnetDLArchibaldLKPhillipsLTenoverFCMcGowanJEGaynesR PAntimicrobial use and resistance in eight US hospitals: complexities of analysis and modeling. Intensive Care Antimicrobial Resistance Epidemiology Project and National Nosocomial Infections Surveillance System HospitalsInfect Control Hosp Epidemiol19981963883949669619
- YoonYKKimMJSohnJWParkDWKimJYChunBCSurveillance of antimicrobial use and antimicrobial resistanceInfect Chemother200840293101
- MatsumotoKShigemiAYajiKReduction in the incidence of MRSA with use of alcohol-based hand rub solutions and glovesJ Infect Chemother201218226927121894454
- RaadIHannaHJiangYComparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilmAntimicrob Agents Chemother20075151656166017353249
- OshiroTNagasawaZHanakiHIkeda-DantsujiYNagayamaAThe antagonistic effects of a combination of vancomycin and minocycline in Staphylococcus aureus with heterogeneous resistance to vancomycinJ Infect Chemother2008141152218297444
- BorgMASudaDSciclunaETime-series analysis of the impact of bed occupancy rates on the incidence of methicillin-resistant Staphylococcus aureus infection in overcrowded general wardsInfect Control Hosp Epidemiol200829649650218510458
- KerttulaAMLyytikäinenOVuopio-VarkilaJMolecular epidemiology of an outbreak caused by methicillin-resistant Staphylococcus aureus in a health care ward and associated nursing homeJ Clin Microbiol200543126161616316333120
- Kardas-SlomaLBoëllePYOpatowskiLBrun-BuissonCGuillemotDTemimeLImpact of antibiotic exposure patterns on selection of community-associated methicillin-resistant Staphylococcus aureus in hospital settingsAntimicrob Agents Chemother201155104888489521788461
- EveillardMMortierELancienEConsideration of age at admission for selective screening to identify methicillin-resistant Staphylococcus aureus carriers to control dissemination in a medical wardAm J Infect Control200634310811316630972
- OtsukaTYoshidaKKomiyamaKIshikawaYZaraketHOkazakiMPrevalence of methicillin-resistant Staphylococcus aureus among children in a region with controlled antimicrobial useJpn J Infect Dis201164543643821937829