Abstract
Background
Hospital wastewater (HWW) promotes the spread of carbapenem resistance genes (CRGs). Aeromonas carry a large number of CRGs in HWW, they may play a role as a suitable reservoir for CRGs, while resistomes in HWW are still poorly characterized regarding carbapenem resistant Aeromonas. Thus, the aim of the study was to evaluate the molecular epidemiological characteristics of carbapenem resistant Aeromonas in HWW.
Methods
A total of 33 carbapenem resistant Aeromonas were isolated from HWW. Antimicrobial susceptibility testing and polymerase chain reaction (PCR) were used to assess the antimicrobial resistance profiles. Molecular typing was performed using enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) and multilocus sequence typing (MLST). The horizontal transmission mode of blaKPC was explored through conjugation and transformation experiments. The stability of blaKPC-IncP-6 plasmids was assessed through plasmid stability and in vitro competition test. The PCR mapping method was used to investigate the structural diversity of blaKPC.
Results
The detection rates of blaKPC and cphA in Aeromonas were 97.0% and 39.4% respectively. Aeromonas caviae were grouped into 13 clusters by ERIC-PCR and 12 STs by MLST. Aeromonas veronii were grouped into 11 clusters by ERIC-PCR and 4 STs by MLST. 56.3% blaKPC were located on mobilizable IncP-6 plasmids. blaKPC-IncP-6 plasmid showed high stability and low cost fitness.
Conclusion
Carbapenem resistant Aeromonas from HWW mainly carried blaKPC, which exhibited great structural diversity. Aeromonas might serve as reservoirs for blaKPC and blaKPC might spread mainly through transformation in HWW.
Introduction
Carbapenems were generally considered as last resort for treating infections from multi-resistant Gram-negative pathogenic bacteria.Citation1 But the widespread prevalence of carbapenem resistant bacteria (CRBs) has seriously limited the effectiveness of carbapenems. Carrying carbapenem resistance genes (CRGs) is an important mechanism of carbapenem resistance. CRGs are generally found on mobile plasmids, exhibiting strong dissemination capabilities.Citation2 Accordingly, effective control of CRG dissemination is critical to slow the spread of resistance.
Hospital wastewater (HWW) is considered significant reservoirs of CRGs. HWW connects human activities with the ecological environment, promoting the spread of clinically important CRGs such as blaNDM, blaKPC, and blaVIM from clinical bacteria to environmental bacteria.Citation3 The slow flow rate of HWW and high-density bacterial communities promote the horizontal transfer of resistance genes within and between bacterial species. Meanwhile, a large amount of antimicrobial residues promotes the proliferation of antimicrobial resistant bacteria.Citation3 In addition to providing selective advantages, antimicrobial residues may induce the SOS-response and hence upregulate expression of conjugation associated genes and T4SS genes to enhance conjugative transfer.Citation4 Furthermore, antimicrobial residues could also promote gene transformation and transduction by increasing the fraction of competent cells and inducing prophage activity.Citation4 Hospital wastewater treatment plants could not eliminate antimicrobial resistant genes (ARGs) in wastewater and could even promote conjugations and transformations of ARGs.Citation5–7 Therefore, compared with other wastewater conditions, HWW may play a more integral role in the dissemination of CRGs.
Aeromonas is a widespread organism in aquatic environment and carry large numbers of CRGs.Citation8 Previous studies showed that cphA is prevalent in carbapenem resistant Aeromonas. Notably, more and more CRGs have been detected in Aeromonas, such as blaKPC or blaNDM.Citation9,Citation10 Especially, plasmid-associated blaKPC-2 is becoming the most prevalent CRG in Aeromonas from wastewater. Given that cphA is located on the chromosome, the spread of plasmid-associated blaKPC-2 is even more concerning.Citation11,Citation12 Bacteria of the Aeromonas genus only has a 4–5 Mbp genome, which is smaller than that of other Gram-negative bacteria. The core genome of Aeromonas comprises only 16% of the entire genome, with the remaining 84% consisting of variable represented genes, including plasmids, transposons, genomic islands or integron gene cassettes in order to compensate for its relatively small genome size. Thus, Aeromonas has a significant capacity to acquire various mobile genetic elements.Citation13,Citation14 Considering the strong ability of Aeromonas to take-up exogenous DNA, it might play a significant role in the transmission of CRGs.
To date, the wastewater resistomes have been poorly characterized regarding carbapenem resistant Aeromonas. A previous study mainly focused on prevalence and antimicrobial resistance characteristics of carbapenem resistant Aeromonas in HWW.Citation15 The transmission characteristics of CRGs from carbapenem resistant Aeromonas remain relatively unexplored. Therefore, we deciphered the molecular epidemiological characteristics of carbapenem resistant Aeromonas and the potential transmission mechanism of blaKPC in Aeromonas, helping to gain a more in-depth insight into the contribution of Aeromonas to CRGs dissemination and enhance the public consciousness about resistance-related risks.
Materials and Method
Aeromonas Isolates
The HWW samples were collected from the raw sewage influent of on-site HWW treatment plant in Fujian medical university union hospital (Fujian, China). A total of three HWW samples were taken from November 3 to November 25, 2020. Each HWW sample was independent and was cultured individually. The HWW samples were diluted and then plated on LB agar (Hopebio, China) containing Imipenem (IPM) at 2 μg/mL to select imipenem-non-susceptible Gram-negative bacteria at 35 °C. All imipenem-non-susceptible Gram-negative bacteria were cultured separately and identified through MALDI-TOF MS (Autobio, China). All isolates, identified as Aeromonas, were then reconfirmed by housekeeping gene sequencing (gyrB).
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed using the VITEK 2 Compact system (Vitek-AST-GN16) (bioMerieux, France) and the disk diffusion method (K-B method) (Oxoid, UK) according to the Clinical and Laboratory Standards Institute File.Citation16 The antimicrobials included Piperacillin/Tazobactam (TZP), Cefoxitin (FOX), Ceftriaxone (CRO), Cefepime (FEP), Aztreonam (ATM), Ertapenem (ETP), IPM, Amikacin (AN), Gentamicin (CN), Ciprofloxacin (CIP), Levofloxacin (LVX) and Trimethoprim/Sulfamethoxazole (SXT). E.coli ATCC 25922 was used as a quality control.
Detection of Carbapenemase Genes
All isolates were subjected to a polymerase chain reaction (PCR) for confirmation of carbapenemase genes, including blaKPC, blaNDM, blaVIM, blaIMP, blaOXA-48, cphA, blaSME, blaGIM, blaIMI and blaGES. The sequences and product lengths of primers are summarized in Table S1. The positive products were confirmed by sequencing (Sangon Biotech, China).
Enterobacterial Repetitive Intergenic Consensus PCR (ERIC-PCR)
The sequences of primers and PCR procedures were described previously.Citation17 The results of ERIC-PCR were analyzed using Quantity One software (version 4.6.2). The phylogenetic tree was determined using the unweighted pair-group method with arithmetic means (UPGMA) clustering method and Dice similarity.
Multilocus Sequence Typing (MLST)
Six housekeeping genes (gyrB, groL, gltA, metG, ppsA, recA) were amplified using PCR. The sequences of primers and the PCR procedures were described previously.Citation18 The products were sent to Sangon Biotech for DNA sequencing, and the results were compared with known alleles in the MLST database. The new STs were deposited in the Aeromonas PubMLST database (http://pubmlst.org/aeromonas). The phylogenetic trees were inferred using the UPGMA clustering method with MEGA11 software.
Conjugation
Conjugation experiments were performed to test the transferability of blaKPC using filter mating. Rifampin-resistant E.coli EC600 was used as the recipient. Donors and recipients were cultured to log-phase in LB broth (Hopebio, China), respectively. Donors and recipients were mixed in an initial ratio of 1:4 and then incubated on a 0.22 μM filter, which was placed on a Columbia blood agar plate, and left overnight at 35 °C. The next day, the mating mixture was washed from the filter and spread onto LB agar containing Rifampin at 500 μg/mL and IPM at 1 μg/mL. The transconjugants harboring blaKPC were identified by PCR.
Transformation and Plasmid Replicon Types Analysis
Plasmid DNA was isolated using the alkaline lysis extraction procedure and introduced to competent E.coli DH5α (Weidibio, China) through chemical transformation. The transformants were selected on LB agar supplemented with IPM at 1 μg/mL. The transformants harboring blaKPC were identified by PCR. Plasmids were classified according to their incompatibility group using the PCR-based replicon typing scheme (PBRT)Citation19 and the sequences of IncP-6 primers are listed in Table S2.
Plasmid Stability Assay and Fitness Assessment
The stability of plasmids was assessed as previously described with minor modifications.Citation20 Briefly, blaKPC-carrying E.coli DH5α was inoculated into LB broth. Subcultures were diluted daily, and each dilution was plated on LB agar every 3 days. The plates were then incubated overnight at 35 °C. A total of 50 colonies were randomly collected from the LB agar and the presence of blaKPC was identified using PCR.
The fitness costs of plasmids were evaluated through in vitro growth competition experiment. Plasmid-carrying E. coli DH5α (IPM-R) and plasmid-free E. coli DH5α (IPM-S) were cultured in an initial ratio of 1:1. The subcultures were then transferred to fresh LB broth. After 1 day, cultures were inoculated overnight on LB agar. Then colonies were collected from the LB agar and the presence of blaKPC was identified by PCR. The fitness costs were calculated using the following equation:Citation21
where Fit represents the fitness cost of the blaKPC-carrying plasmid in the host E. coli DH5α; R denotes number of the blaKPC-carrying E. coli DH5α; S represents the number of blaKPC-free E. coli DH5α; R0 indicates the initial number of blaKPC-carrying E. coli DH5α in the competition experiments, and S0 represents the initial number of blaKPC-free E.coli DH5α in the competition experiments.
blaKPC Genetic Region Analysis
The sequences and lengths of the primer products are summarized in Table S3. These primers were designed based on the sequence of the template plasmid p10265-KPC.Citation22 Plasmid DNA was extracted using the GeneJET Kit (Thermo Scientific, US). PCR products were purified using the FastPure Gel DNA Extraction Mini Kit (Vazyme, China). Then, the purified DNA samples were digested using different enzymes. Fragment I, II and III were digested by HindIII (NEB, England). Fragment IV and V were digested by BamHI (NEB, England). Fragment VI was digested by HinfI (Takara, Japan). The grouping was based on the electrophoresis results of the six fragments’ enzymatic digestion. The PCR products were sent to Sangon Biotech for Sanger sequencing, and the fragments were assembled using the CAP3 program in SnapGene software (version 5.2). One isolate from each type was sequenced and annotated using the RAST (Rapid Annotation using Subsystems Technology) annotation website to determine the blaKPC region.
Statistical Analysis
GraphPad Prism software (version 8) was used for statistical analysis. Fisher’s exact test was performed to analyze statistical significance. Only p < 0.05 was considered statistically significant.
Results
Prevalence of Carbapenem-Resistant Aeromonas
16 Aeromonas caviae and 17 Aeromonas veronii isolates from HWW were collected in this study. The percentage of carbapenem resistant Aeromonas was 26.83%. The percentage of other carbapenem resistant organisms detected have been listed in Table S4.
Antimicrobial Susceptibility Testing
16 Aeromonas caviae and 17 Aeromonas veronii isolates from HWW were collected in this study. Aeromonas caviae demonstrated high resistance to FOX (87.5%), CRO (100.0%), FEP (100.0%), ATM (100.0%), ETP (100.0%) and IPM (100.0%). The resistance to TZP (37.5%), AN (25.0%) and LVX (12.5%) was less than 40.0% in Aeromonas caviae. Meanwhile, Aeromonas veronii exhibited high resistance to CRO (100.0%), FEP (94.1%), ATM (88.2%), ETP (100.0%), IPM (94.1%), while showing low resistance to TZP (35.3%), AN (35.3%), CIP (29.4%) and SXT (35.3%). The Results are listed in .
Table 1 Antimicrobial Susceptibility Analysis of Aeromonas caviae and Aeromonas veronii
Carbapenemase Genes Profiles
All 33 Aeromonas isolates in our study contained carbapenemase genes which included blaKPC and cphA (). 16 Aeromonas caviae isolates carried blaKPC-2. Among the 17 Aeromonas veronii isolates, 1 isolate carried cphA, 4 isolates carried blaKPC-2, 4 isolates carried both cphA and blaKPC-24, 8 isolates carried cphA and blaKPC-2 simultaneously.
Table 2 The Frequency of Carbapenemase Genes Among Aeromonas caviae and Aeromonas veronii
ERIC-PCR
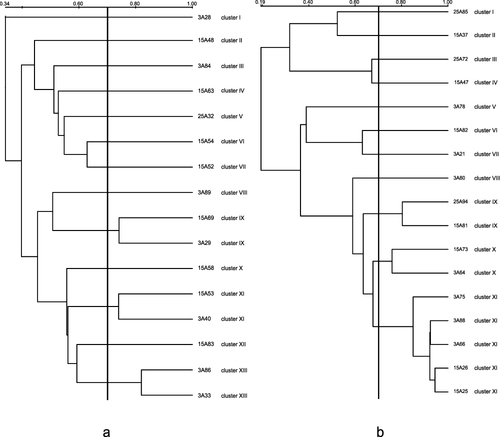
The fingerprints of Aeromonas isolates consisted of various amplification bands, ranging in size from 100 to 5000 bp. The similarity coefficient of Aeromonas caviae ranged from 0.34 to 0.82 and that of Aeromonas veronii ranged from 0.19 to 0.94 (). When the similarity coefficient was 0.70, Aeromonas caviae were divided into 13 clusters and Aeromonas veronii were divided into 11 clusters. The results of ERIC-PCR in this study divided Aeromonas into various clusters, confirming the high genetic diversity in Aeromonas.
MLST
16 Aeromonas caviae isolates from HWW showed a high level of genetic diversity. 12 STs were identified in our study, including ST2170, ST2171, ST2379, ST2172, ST2173, ST2380, ST2174, ST2178, ST2179, ST2180, ST2181 and ST747. Additionally, 11 STs were novel founded (except ST747). ST2174 (3/16, 18.8%) was the most prevalent in Aeromonas caviae. Among 17 Aeromonas veronii isolates, 4 novel STs were identified, including ST2381, ST2175, ST2176 and ST2177. ST2176 (10/17, 58.8%) was the most prevalent ST in Aeromonas veronii. The evolutionary trees () could more directly illustrate the diversity among Aeromonas caviae and Aeromonas veronii. Notably, with the identification of 15 novel STs, this study also importantly contributed to the diversity in the MLST database of Aeromonas.
Conjugation, Transformation and Plasmid Replicon Types Analysis
None of 32 blaKPC-positive Aeromonas isolates were able to transfer blaKPC into E.coli EC600 successfully. A total of 18 blaKPC-positive Aeromonas (56.3%) isolates could transfer blaKPC into E.coli DH5α through chemical transformation successfully, which included 5 Aeromonas caviae and 13 Aeromonas veronii isolates. All of the replicon types of mobilizable blaKPC-carrying plasmids were IncP-6.
Plasmid Stability Assay and Fitness Assessment
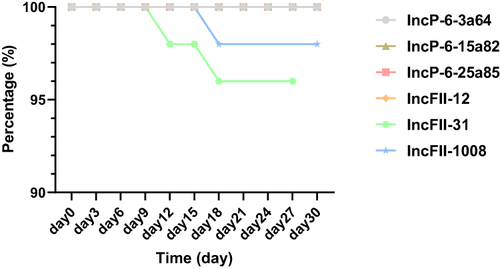
The plasmid stability assay showed blaKPC-IncP-6 plasmids could be stably maintained in E.coli DH5α for 30 days without any loss. But the blaKPC-IncFII plasmids were not as perfect as blaKPC-IncP-6 plasmids, blaKPC-IncFII-31 began to lose stability from day 12 and plasmid blaKPC-IncFII-1008 began to lose stability from day 18. However, plasmid blaKPC-IncFII still showed a high level of stability, the rate of blaKPC remaining above 96.0%. The results of plasmid stability were shown in .
The fitness assessment was shown by in vitro growth competition experiment. The relative fitness costs of blaKPC-IncP-6 plasmids were 1.024, 1.018 and 0.981. The relative fitness costs of blaKPC-IncFII plasmids were 0.962, 1.016 and 1.039. There was 1 isolate (33.3%) with IncP-6 plasmid and 1 isolate (33.3%) with IncFII plasmid that increased the fitness cost of the host in our study. The results of fitness assessment were listed in .
Table 3 Different Types of blaKPC-Carrying Plasmids and Their Fitness Cost Results
blaKPC Genetic Region Analysis
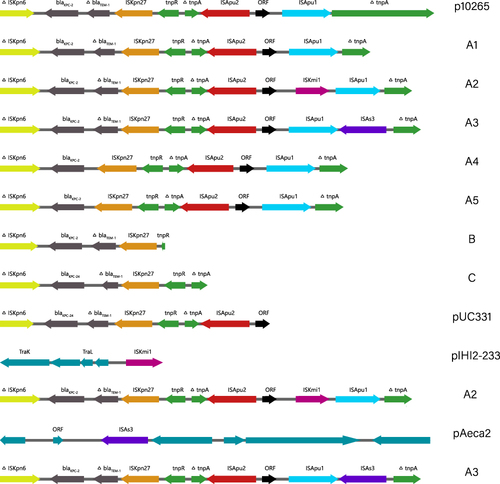
In template plasmid p10265-KPC, from the genetic content we found that it had undergone two main evolutionary events from ΔISKpn6-blaKPC-2-ISKpn27: (i) the insertion of ΔblaTEM−1 between ISKpn27 and Tn3, and (ii) the insertion of ISApu1-ORF-ISApu2 into the Tn3 tnpA gene, disrupting the fragment into two separate parts.Citation22 The genetic region of blaKPC might undergoing the events of gene acquisition and lost in our study. showed these types of blaKPC genetic regions.
Type A1 has the same genetic region with the reference sequence p10265-KPC, as ΔISKpn6-blaKPC-2-ΔblaTEM-1-ISKpn27-tnpR-ΔtnpA-ISApu2-ORF-ISApu1-ΔtnpA. Among type A2, there was a transposase element ISKmi1, which was inserted between the ORF and ISApu1. There was a transposase element ISAs3 inserted between ISApu1 and ΔtnpA in type A3. The loss of ΔblaTEM-1 was found in type A4 and A5. Deletion of downstream fragments of tnpR occurred in type B. Type C received blaKPC-24 instead of blaKPC-2 and experienced loss of the downstream fragments of ΔtnpA.
Discussion
Aeromonas is a widespread organism in aquatic environment and carry large numbers of CRGs, it might play a significant role in the transmission of CRGs. Previous studies showed that cphA and blaKPC are prevalent in carbapenem resistant Aeromonas.Citation15,Citation23 Notably, the most prevalent carbapenemase gene in Aeromonas from wastewater was the plasmid-associated blaKPC.Citation15 In this study, 97.0% Aeromonas isolates carried blaKPC indicating the potential transmission risk of blaKPC. The high prevalence of cphA and blaKPC suggests that carbapenem resistant Aeromonas may play a role in the transmission of carbapenem resistance. Although Aeromonas were not the predominant species, the high prevalence of CRGs and strong capacity to acquire various mobile genetic elements demonstrate the contribution of carbapenem resistant Aeromonas to CRGs’ dissemination. Given that cphA is located on bacterial chromosomes, plasmid-associated blaKPC-2 might exhibit stronger dissemination capabilities.Citation24 The high prevalence and transmission risk of blaKPC indicate that Aeromonas might serve as a potential reservoir for blaKPC transmission.
In this study, we found that blaKPC-carrying plasmids cannot be transferred through conjugation in Aeromonas, but can be transferred through transformation. All of the replicon types of mobilizable blaKPC-carrying plasmids were IncP-6. The failed conjugation might be attributed to the lack of necessary genetic elements for conjugation in IncP-6 plasmids (such as the traA and traB operons).Citation25 Meanwhile, Aeromonas is equipped with the type VI secretion system which might be implicated in transformation.Citation13 Previous studies found that blaKPC cannot be transferred through conjugation in Aeromonas with or without the presence of self-transmissible plasmid.Citation25,Citation26 Therefore, transformation might be an important mechanism for the transmission of blaKPC in Aeromonas.
To further investigate the transmission risk of blaKPC, we analyzed the stability of blaKPC-IncP-6 plasmids by plasmid stability and in vitro competition test. The blaKPC-IncP-6 plasmids had a strong stability and low fitness cost in this study. The reason for the high stability of blaKPC-IncP-6 plasmids might be due to them carrying some genetic elements (such as the ParA/B/C operon), which can maintain their existence in the host.Citation20 A previous study found that blaKPC-IncP-6 plasmids were broad host range plasmids and present in diverse environmental bacterial species of opportunistic, indicating that it might be a suitable vector of blaKPC in HWW.Citation9 The plasmid persistence depends on plasmid stability and their effect on the host fitness.Citation27 The high stability of IncP-6 plasmids is beneficial to the persistence of plasmids in host and the low fitness cost of IncP-6 plasmids could be an important advantage of carbapenem-resistant bacteria propagation. Therefore, the high stability of IncP-6 plasmids might promote the transmission of CRGs.
The wide spread of CRGs has posed a major public health threat. Previous research has suggested some solutions to control the spread of carbapenem resistance in clinic, such as antimicrobial restriction systems.Citation28 However, HWW, an important source of CRGs, had received increasing attention. HWW connects human activities with the ecological environment, promoting the spread of clinically important CRGs from clinical bacteria to environmental bacteria.Citation3 Compared to other wastewater environments, HWW may have a stronger promoting effect on ARGs’ dispersion. Therefore, more emphasis should be placed on the contribution of HWW on the CRGs’ dissemination and an efficient method of controlling the CRGs’ dissemination is also urgently needed to relieve the current crisis of antimicrobial resistance.
There is a limitation to our study: whole genome sequencing was not applied in our study. Therefore, we have no idea about the genetic environment and molecular evolutionary relationships of carbapenem resistant Aeromonas in HWW, which might limit the exploration of contribution of Aeromonas in transmission of blaKPC. In our further study, the phylogenomic analysis of carbapenem resistant Aeromonas will be further explored. Despite these limitations, this study deciphered the molecular epidemiological characteristics of carbapenem resistant Aeromonas and the potential transmission mechanism of blaKPC in Aeromonas, helping to gain a more in-depth insight into the contribution of Aeromonas to CRGs’ dissemination and enhance public awareness about resistance-related risks.
Conclusions
Carbapenem resistant Aeromonas in HWW showed high genetic diversity. Aeromonas mainly carried blaKPC, which exhibited structural diversity. Transformation might be an important mechanism for the transmission of blaKPC in Aeromonas. Aeromonas might serve as reservoirs for blaKPC and promote its spread in HWW.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Additional information
Funding
References
- Patel G, Bonomo RA. ”Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol. 2013;4:48. doi:10.3389/fmicb.2013.00048
- Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263–272. doi:10.1016/j.molmed.2012.03.003
- Zhu L, Shuai XY, Lin ZJ, et al. Landscape of genes in hospital wastewater breaking through the defense line of last-resort antibiotics. Water Res. 2022;209:117907. doi:10.1016/j.watres.2021.117907
- Lopatkin AJ, Sysoeva TA, You L. Dissecting the effects of antibiotics on horizontal gene transfer: analysis suggests a critical role of selection dynamics. BioEssays. 2016;38(12):1283–1292. doi:10.1002/bies.201600133
- Zhang S, Xu B, Chen M, et al. Profile and actual transmissibility of Carbapenem resistance genes: intracellular and extracellular DNA in hospital wastewater. J Environ Manage. 2023;329:117085. doi:10.1016/j.jenvman.2022.117085
- Wang Z, Fu L, Gu JD, Deng S, Huang C, Luo L. The factors controlling antibiotic resistance genes in different treatment processes of mainstream full-scale wastewater treatment plants. Sci Total Environ. 2023;900:165815. doi:10.1016/j.scitotenv.2023.165815
- Jin M, Liu L, Wang DN, et al. Chlorine disinfection promotes the exchange of antibiotic resistance genes across bacterial genera by natural transformation. ISME J. 2020;14(7):1847–1856. doi:10.1038/s41396-020-0656-9
- Conte D, Palmeiro JK, Bavaroski AA, et al. Antimicrobial resistance in Aeromonas species isolated from aquatic environments in Brazil. J Appl Microbiol. 2021;131(1):169–181. doi:10.1111/jam.14965
- Yao Y, Lazaro-Perona F, Falgenhauer L, et al. Insights into a Novel bla(KPC-2)-encoding IncP-6 plasmid reveal carbapenem-resistance circulation in several Enterobacteriaceae species from wastewater and a hospital source in Spain. Front Microbiol. 2017;8:1143. doi:10.3389/fmicb.2017.01143
- Luo X, Mu K, Zhao Y, et al. Emergence of bla (NDM- 1)-Carrying Aeromonas caviae K433 isolated from patient with community-acquired pneumonia. Front Microbiol. 2022;13:825389. doi:10.3389/fmicb.2022.825389
- Wu CJ, Chen PL, Wu JJ, et al. Distribution and phenotypic and genotypic detection of a metallo-β-lactamase, CphA, among bacteraemic Aeromonas isolates. J Med Microbiol. 2012;61(Pt 5):712–719. doi:10.1099/jmm.0.038323-0
- Sinclair HA, Heney C, Sidjabat HE, et al. Genotypic and phenotypic identification of Aeromonas species and CphA-mediated carbapenem resistance in Queensland, Australia. Diagn Microbiol Infect Dis. 2016;85(1):98–101. doi:10.1016/j.diagmicrobio.2016.02.005
- Lamy B, Baron S, Barraud O. Aeromonas: the multifaceted middleman in the One Health world. Curr Opin Microbiol. 2022;65:24–32. doi:10.1016/j.mib.2021.09.012
- Huddleston JR, Brokaw JM, Zak JC, Jeter RM. Natural transformation as a mechanism of horizontal gene transfer among environmental Aeromonas species. Syst Appl Microbiol. 2013;36(4):224–234. doi:10.1016/j.syapm.2013.01.004
- Drk S, Puljko A, Dželalija M, Udiković-Kolić N. Characterization of third generation cephalosporin- and carbapenem-resistant Aeromonas isolates from municipal and hospital wastewater. Antibiotics. 2023;12(3):513. doi:10.3390/antibiotics12030513
- CLSI. Methods for antimicrobial dilution and disc susceptibility testing of infrequently isolated or fastidious bacteria. In: CLSI Guideline M45. Wayne, PA: Clinical and Laboratory Standards Institute; 2016.
- Szczuka E, Kaznowski A. Typing of clinical and environmental Aeromonas sp. strains by random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus sequence PCR. J Clin Microbiol. 2004;42(1):220–228. doi:10.1128/jcm.42.1.220-228.2004
- Martino ME, Fasolato L, Montemurro F, et al. Determination of microbial diversity of Aeromonas strains on the basis of multilocus sequence typing, phenotype, and presence of putative virulence genes. Appl Environ Microbiol. 2011;77(14):4986–5000. doi:10.1128/AEM.00708-11
- Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219–228. doi:10.1016/j.mimet.2005.03.018
- Rahube TO, Viana LS, Koraimann G, Yost CK. Characterization and comparative analysis of antibiotic resistance plasmids isolated from a wastewater treatment plant. Front Microbiol. 2014;5:5. doi:10.3389/fmicb.2014.00558
- Li S, Yin Y, Chen H, Wang Q, Wang X, Wang H. Fitness cost of daptomycin-resistant Staphylococcus aureus obtained from in vitro daptomycin selection pressure. Front Microbiol. 2017;8:2199. doi:10.3389/fmicb.2017.02199
- Dai X, Zhou D, Xiong W, et al. The IncP-6 Plasmid p10265-KPC from Pseudomonas aeruginosa Carries a Novel ΔISEc33-Associated bla KPC-2 Gene Cluster. Front Microbiol. 2016;7:310. doi:10.3389/fmicb.2016.00310
- Wang Y, Liu H, Zhang L, Sun B. Application of modified carbapenem inactivation method and its derivative tests for the detection of carbapenemase-producing Aeromonas. Infect Drug Resist. 2021;14:3949–3960. doi:10.2147/idr.S330115
- Adams RJ, Mathys DA, Mollenkopf DF, Whittle A, Daniels JB, Wittum TE. Carbapenemase-producing aeromonas veronii disseminated in the environment of an equine specialty hospital. Vector Borne Zoonot Dis. 2017;17(6):439–442. doi:10.1089/vbz.2016.2083
- Zhu Z, Wu S, Zhu J, et al. Emergence of Aeromonas veronii strain co-harboring bla(KPC-2), mcr-3.17, and tmexC3.2-tmexD3.3-toprJ1b cluster from hospital sewage in China. Front Microbiol. 2023;14:1115740. doi:10.3389/fmicb.2023.1115740
- Hu X, Yu X, Shang Y, et al. Emergence and Characterization of a Novel IncP-6 Plasmid Harboring bla (KPC-2) and qnrS2 genes in Aeromonas taiwanensis Isolates. Front Microbiol. 2019;10:2132. doi:10.3389/fmicb.2019.02132
- Wein T, Hülter NF, Mizrahi I, Dagan T. Emergence of plasmid stability under non-selective conditions maintains antibiotic resistance. Nat Commun. 2019;10(1):2595. doi:10.1038/s41467-019-10600-7
- Wanla W, Katip W, Supakul S, Apiwatnakorn P, Khamsarn S. Effects of an antimicrobial restriction system on appropriate carbapenem use in a hospital without infectious diseases consultation. Int J Gene Med. 2017;10:443–449. doi:10.2147/ijgm.S145133