Abstract
Objective
Owing to the rising incidence of multidrug-resistant organisms (MDRO) and the high mortality rates associated with such bacterial infections post-hematopoietic stem cell transplantation (HSCT), we investigated the MDRO colonization rate prior to transplantation using an active surveillance approach and determined its impact on subsequent infection during the pre-engraftment period.
Methods
A single-center observational study was conducted, and surveillance cultures from multiple body sites, including the rectum, nasal cavity, and groin, were performed at admission to determine MDRO colonization. Serological tests were used to detect certain viruses and toxoplasmosis before HSCT.
Results
In the pre-transplant setting, 59 MDRO were recovered from the 40 HSCT recipients. Of the 59 isolates recovered from one or more body sites, 29 were positive for methicillin-resistant Staphylococcus aureus (MRSA), 7 for carbapenem-resistant Enterobacterales (CRE), and 23 were positive for extended-spectrum β-lactamase (ESBLs). Serological assessment before HSCT revealed active or reactivation of latent infection with cytomegalovirus (7.5%), Epstein–Barr virus (EBV; 5%), and Toxoplasma gondii (2.5%) among HSCT patients. In terms of factors associated with pre-engraftment infections, the type of transplant (p=0.04) was statistically significant, whereas other factors, such as age, sex, and underlying conditions, were not. In post-transplant settings, bloodstream infections (BSIs) were documented in 2 allogeneic HSCT patients (5%), and the isolated microorganisms were ESBL-producing E. coli and non-MDR Acinetobacter baumannii.
Conclusion
Active screening cultures are a helpful tool for identifying patients colonized by MDRO or relevant viruses before HSCT, and for predicting those at risk of developing subsequent pre-engraftment infections. Additionally, active screening may aid in predicting those who are likely to develop subsequent pre-engraftment infections Our findings highlight the importance of pre-transplant screening for high-priority multidrug-resistant pathogens and the application of infection control interventions after HSCT.
Introduction
To replace and repopulate the hematopoietic system, hematopoietic stem cell transplant (HSCT), previously known as bone marrow transplant, has been used as a potentially curative treatment for several life-threatening malignant diseases and immunological disorders over the last decades.Citation1 Before stem cell infusion, patients undergo a preparative regimen (myeloablative conditioning regimen) that involves high-dose chemotherapy and/or total body irradiation to eliminate the numerous malignant cells and to prevent rejection of the transplanted graft.Citation2 As a result of the interplay between weakened defense mechanisms induced by conditioning regimens and opportunistic pathogens, life-threatening infections with bacteria, especially multidrug-resistant organisms (MDRO), as well as the acquisition of respiratory viral infections, and/or reactivation of human herpes viruses remains a major determinant of morbidity and mortality after allogeneic or autologous HSCT.Citation3–6 Additionally, reactivation of the Hepatitis B virus (HBV),Citation7 Hepatitis C virus (HCV),Citation8 and latent toxoplasmosisCitation9 remain a risk exacerbated by the immunosuppressive status of HSCT recipients.
Carbapenem-resistant Enterobacterales (CRE), MDR-Pseudomonas aeruginosa, MDR-Acinetobacter baumannii, extended-spectrum β-lactamase producers (ESBLs), and methicillin-resistant Staphylococcus aureus (MRSA) currently account for the majority of hospital-acquired infections (HAIs) within 30 days after HSCT in the pre-engraftment phase,Citation10,Citation11 data on HSCT-related infections remain limited in developing countries. During the pre-engraftment neutropenic phase, the dysbiosis of endogenous gastrointestinal flora, mucosal damage with indwelling devices, and prior colonization with MDRO can contribute to different clinical manifestations, including bloodstream infections (BSIs), pneumonia and gastrointestinal infections.Citation12,Citation13 Despite major advances in the management of post-transplant outcomes, the incidence ranges from 13–46% and 5–10% among allogeneic HSCT, in which patients receive progenitor cells from the donorCitation13,Citation14 and autologous HSCT, in which the patient receives their own progenitor cells,Citation15 respectively, with a mortality rate of 32.7%.Citation16
Although active surveillance strategies to detect the colonization state of HSCT recipients are important tools for controlling and preventing the spread of MDRO within hospital settings and may be useful in guiding empirical therapy for post-transplantation neutropenia, their impact on patient outcome and cost-effectiveness remains controversial,Citation17 particularly in developing countries where MDRO is endemic. Accordingly, this study aimed to evaluate the effect of pre-transplant screening for high-priority MDRO and relevant viruses on the outcome of pre-engraftment infections. We also studied the effect of infection control policies on the rate of HAIs during the pre-engraftment period after post-HSCT.
Materials and Methods
Study Design
This prospective observational cohort study was conducted on adult patients who underwent HSCT between January 2021 and December 2021 at the International Medical Center (IMC), Cairo, Egypt. The bone marrow transplant (BMT) unit at the IMC contains four highly isolated and super-equipped units for autologous and allogeneic transplantations of hematological cancers. Data of the enrolled patients, including demographics, underlying diseases, and microbiological data, both pre- and post-HSCT, were retrieved from the hospital’s electronic medical record system. The study procedure was in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Faculty of Pharmacy, Ain Shams University (ACUC-FP-ASU RHDIRB2020110301 REC #72).
Pre-Transplant and Post-Transplant (Pre-Engraftment) Screening
Within 48 hours of admission, all patients who underwent BMT were subjected to routine CRE, ESBLs, and MRSA pre-transplant screening. Rectal swabs were used for CRE/ESBL screening, whereas swabs from the anterior nares, groin, and axilla were used for MRSA screening. Additionally, extensive screening covering different body sites, including the eye, ear, sputum, urine, and blood, adhering to international guidelines https://apps.who.int/iris/rest/bitstreams/911060/retrieve (accessed on February 5, 2024) was performed.
HB&L-carbapenemase® and ESBL-producers-AmpC® kits (Alifax, Padua, Italy) were used for active screening of CRE and ESBLs producers from rectal swabs, respectively. The patented laser beam technology with dedicated reagents (selective supplement broth that contains carbapenem and certain third-generation cephalosporins), enables us to perform rapid bacterial culture and to rapidly screen CRE and ESBLs with high sensitivity and specificity.Citation18 For MRSA screening/isolation, an oxacillin-resistant screening agar base medium was used (Oxoid, UK) and a cefoxitin disk (30 μg) was used to identify MRSA isolates as per clinical laboratory standard institute.Citation19 The automated system Vitek-2 (BioMérieux, Marcy L’Etoile; Lyon, France) was used for the further confirmation and detection of antimicrobial breakpoints, in accordance with the manufacturer’s recommendations. Polymerase chain reaction (PCR) was used to determine the type of carbapenemase enzyme and ESBLs as previously reported.Citation18
Serological tests were used to determine prior (IgG) or recent (IgM) exposure to a multitude of pathogens, including certain viruses (human herpes virus type 1 and 2, CMV, EBV, HCV, human immunodeficiency virus (HIV)), and parasites such as Toxoplasma gondii which are prevalent among HSCT recipients. HBsAg, anti-HBc IgM, and total anti-HBc serum markers were tested for HBV. An Architect Plus immunoassay analyzer (Abbott, Illinois, USA) was used to deliver reliable results without delay and to enhance the laboratory workflow, as previously mentioned. The Architect anti-HCV assay was used for qualitative detection of anti-HCV antibodies in human serum and plasma. The chemiluminescent signal in the reaction was compared to the cutoff signal obtained from Architect Anti-HCV calibration to determine the presence or absence of IgG/IgM anti-HCV in sample https://www.corelaboratory.abbott/int/en/offerings/segments/infectious disease/hepatitis.html (accessed on 5 February 2024).
The Architect HIV Ag/Ab combo assay was used for the detection of HIV, based on the simultaneous qualitative detection of HIV-p24-Ag and Ab to HIV-1/HIV-2. https://www.ilexmedical.com/files/PDF/HIVAgAbCombo.pdf (accessed on 5 February 2024).
To exclude primary infections with CMV, CMV IgG reactive samples (detected by Architect CMV IgG assay) should be tested for CMV-IgM and CMV-IgG Avidity as previously reported https://www.ilexmedical.com/files/PDF/CMVIgM_ARC.pdf; (accessed on 5 February 2024).
A rapid antigen test was used for coronavirus disease 2019 (COVID-19) screening and positive results were confirmed by PCR whenever indicated according to the Centers for Disease Control and Prevention (CDC) standard guidelines (https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html) (accessed on February 5 2024).Citation20 For monitoring post-transplant infections, patients with fever or with any other signs or symptoms of infections were monitored through the collection of two blood culture sets in addition to other diagnostic procedures based on the patient’s clinical presentation, as a part of follow-up care during the pre-engraftment period (https://apps.who.int/iris/rest/bitstreams/911060/retrieve) (accessed on February 5, 2024).
Microbiological Definition
MDR phenotype is defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories.Citation21 Colonization is defined as the presence of the respective organism in at least one surveillance culture. BSIs are defined as the isolation of the microorganism from at least one set of blood cultures associated with systemic signs of infection, such as fever, chills, and hypotension, when drawn from a transplantation recipient at any phase of transplantation.Citation22 In cases of skin commensals, BSIs are defined by the identification of at least two consecutive blood cultures of the same species isolated within 24 hours, as reported by the Centers of Disease Control and Prevention (CDC) (https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf); (accessed on February 5, 2024).
Infection Prevention and Control
Cornerstones of infection control procedures, including routine hand hygiene, environmental cleaning, contact precautions, antimicrobial stewardship programs along with using intense aseptic/antiseptic regimens were routinely applied to stop transfer of MDRO within BMT unit (https://apps.who.int/iris/rest/bitstreams/911060/retrieve) (accessed on February 8, 2024). Except for those colonized with MDRO Gram-negative bacteria, all patients received levofloxacin (500 mg/day) as a prophylaxis from the beginning of the conditioning regimen until engraftment, while colonized individuals by MDRO received a regimen based on the isolate’s susceptibility profile.Citation23 Acyclovir was used as a routine antiviral prophylactic agent for all HSCT recipients, while ganciclovir was only used for CMV-related indications among high-risk HSCT recipients.Citation24 Fluconazole was used as an antifungal prophylactic agentCitation25,Citation26 and posaconazole was used among allogeneic patients with acute myeloid leukemia or of high risk to develop graft versus host disease.Citation27,Citation28 Trimethoprim-sulfamethoxazole was used as a prophylactic agent to prevent both toxoplasmosis and Pneumocystis jirovecii pneumonia.Citation29 A decolonization protocol was used to reduce asymptomatic MRSA carriers, but it was not used in the case of ESBLs and CRE due to concerns about the emergence of resistant strains and negative effects on the gut microbiome.Citation23 For MRSA decolonization regimen, the body was washed with chlorhexidine gluconate 4% on a daily basis for 5 days. To determine the clearance of MRSA, a post-decolonization screening step was conducted by reswabbing different multibody sites to ensure the effectiveness of decolonization. https://www.cdc.gov/infectioncontrol/pdf/strive/MRSA202-508.pdf (accessed on 5 February 2024).
Association Between Colonization State and Pre-Engraftment Infections
Before transplantation, we determined the colonization state of recipients by initial screening for MDRO including MRSA,ESBLs and CRE. After HSCT, we examined the association between prior colonization with MDRO and probability of developing subsequent infections with the same MDR pathogens at pre-engraftment period.
Statistical Data Analysis
All analyses were conducted using the GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). Chi-square or Fisher’s exact test was used to compare the proportions of categorical variables, and p < 0.05 was considered statistically significant.
Results
Patient Demography and History
During the study period, forty patients received HSCT procedures, (15, 37.5%) allogeneic transplant and (25, 62.5%) autologous transplantation. The median patient age was 43 years (range: 34.5–55), 25 patients were males (62.5%) and 15 (37.5%) were females. The most common underlying condition was multiple myeloma (11, 27.5%), followed by Hodgkin Lymphoma (10, 25%) and acute myeloid leukemia (6, 15%).
Patient Surveillance Prior to HSCT
Among the 40 patients, 280 surveillance cultures (2 rectal swabs, n = 80; 1 swab from nostrils, axilla, and groin, n = 40 each) were performed per patient to detect the colonization state by CRE, ESBLs, and MRSA upon admission to the hospital prior to HSCT (). Other multi-body site colonization screening, including eye, ear, and urine, was performed. Of the 40 patients, 59 MDRO, including 29 MRSA, seven CRE, and 23 ESBLs, were isolated from one or more body sites. Most MRSA isolates were recovered from groins (n = 19), followed by axial (n = 16) and nasal swabs (n = 8). Of the 40 patients screened for MRSA, 4 had results assessable by all three sites (axilla, groin, and nostrils), 9 had results assessable by two combined sites (axilla and groin). On the other hand, 6, 4, and 3 patients were assessable using only a single site, including groin, nasal, and axillary swabs, respectively.
Table 1 Data on MDRO Recovered from Surveillance Cultures and Viral/Parasitic Serostatus of Patients Admitted to the HSCT Unit
For viral/parasitic serological assessment prior to HSCT, CMV (3, 7.5%), EBV (2, 5%), and Toxoplasma gondii (1, 2.5%) were recorded, whereas none of the other tested viruses were detected.
Distribution of MDRO Pre- and Post-HSCT and Antibiotic Resistance Profile
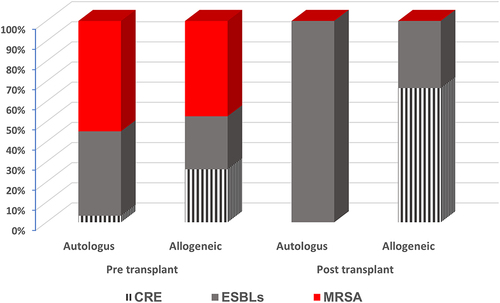
As shown in , the distribution of MDRO pre-autologous HSCT revealed that MRSA colonization accounted for approximately 55%, followed by ESBLs at 42%, and CRE at 3%. In the pre-allogeneic HSCT group, MRSA colonization accounted for approximately 47.4%, followed by ESBLs and CRE (26.3%). In post-autologous HSCT, 100% of the recovered MDROs were ESBLs, whereas in post-allogeneic HSCT, ESBLs accounted for 33% and CRE for approximately 67%.
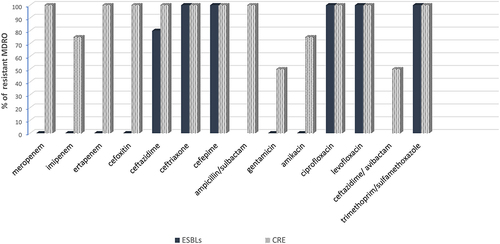
The antibiotic resistance pattern of CRE post-HSCT showed reduced susceptibility to carbapenems (75–100%), cephalosporins (100%), aminoglycosides (50–75%), quinolones (100%), and trimethoprim/sulfamethoxazole (100%). For β-lactam/β-lactamase inhibitors, 100% of the recovered CRE were resistant to ampicillin/sulbactam and 50% were resistant to ceftazidime/avibactam. On the other hand, the antibiotic resistance pattern of ESBLs post-HSCT revealed a high resistance pattern towards cephalosporins (80–100%), quinolones (100%), and trimethoprim/sulfamethoxazole (100%). In contrast, the recovered ESBLs were 100% sensitive to carbapenems and aminoglycosides ().
Statistical Association Between Colonization State and Pre-Graft Infections
As shown in , before transplantation, there was no statistical difference between colonized patients with MDRO and non-colonized patients with regard to age, sex, underlying conditions, and type of transplant. However, there was a statistically significant difference between the type of transplant and pre-engraftment infections caused by MDRO after transplantation (p = 0.04), whereas the other three factors were not statistically significant.
Table 2 Demographic Data of Enrolled Patients Pre- and Post-HSCT
Pre-Transplant Colonization Rate by MDR Pathogens, Relevant Viruses, and Parasites
The pre-transplant colonization rates of MRSA, ESBLs, and CRE were 26 (65%), 18 (45%), and 6 (15%), respectively, as shown in . Among all the colonized patients in the cohort, only one patient who was previously colonized by MDR E. coli expressing the ESBL phenotype and another patient colonized by Acinetobacter baumannii subsequently developed BSIs with the same isolate during the pre-engraftment period. Notably, the majority of patients colonized by MDRO prior to transplantation and after applying infection control interventions did not develop subsequent infections post-HSCT.
Table 3 Relationship Between Pre-Transplant Screening and Post-Transplant Infections Among HSCT Recipients
Regarding viral and parasitic serostatus, only one allogeneic HSCT recipient had active CMV infection during the pre-engraftment phase and was negative prior to transplantation. The overall mortality rate during the pre-engraftment phase was 10%, and four patients who died had undergone allogeneic HSCT (p=0.006), which could be related to central line-associated BSIs, as confirmed in one patient. The microbiological profiles of these 4 patients revealed that one patient had CLA BSI caused by MDR E. coli. In contrast, one patient was infected with MDR A. baumannii, another patient was infected with non-MDR K. pneumoniae and the last patient had coagulase-negative staphylococci. Notably, these organisms did not lead to HAIs as defined by the CDC.
Correlation Between Colonization State and Pre-Engraftment Infections
shows the correlation between colonization state by MDRO prior to transplantation and infection by the same MDRO post-transplantation, and their significant association. Statistically significant results (p = 0.037) were observed between the pre-transplant colonization state with certain MDRO (ESBLs, CRE, MRSA) and the development of subsequent infection during the pre-engraftment period. Notably, prior colonization with CRE showed a significant association (p=0.000) with CRE post-transplant infections.
Table 4 Association Between Colonization State by MDRO Prior to Transplant and Infection by Same MDRO Post-Transplant
Discussion
Infections caused by MDRO, especially gram-negative bacteria, in addition to reactivation of Herpesviridae family, have a significant impact on the prognosis of immunocompromised patients post-HSCT. For the management of such infections, understanding microbial colonization prior to transplant through surveillance programs, along with serological investigation of certain viruses, might be crucial to initiate preventive strategies among HSCT recipients.Citation30 In this study, we used an extensive screening strategy to investigate the rate of colonization by MDRO from multi-body sites as well as to analyze the association between colonization and subsequent infection by the same pathogen post-HSCT.
Pre-transplant screening revealed a high prevalence of MRSA colonization (65%) among the admitted patients, indicating that its spread within community settings should not be underestimated. To increase the sensitivity of MRSA screening, groin and axillary swabs were screened in addition to nares in our cohort. Among the 26 patients colonized by MRSA, 73%, 61.5%, and 30.7% were identified using groin, axilla, and nasal swabs, respectively. In contrast to our findings, other studies have shown that the anterior nares are the sites most frequently colonized by MRSA,Citation31,Citation32 reflecting disagreement over the ideal anatomical site for MRSA sampling. Several factors, including the nature of swabs, decolonization protocol, and laboratory testing tools, can contribute to the variation in MRSA colonization detection rate among HSCT patients.
The rising threat of infections caused by MDR Gram-negative bacteria among HSCT recipients has been documented in the centers, but data on the consequences of these infections remain limited in our region. The prevalence rates of CRE and ESBLs colonization in our study cohort were 15% and 45%, respectively. The rate of colonization by CRE was higher than that recently reported from a center in Turkey,Citation33 while rate of ESBLs was in tune with a study conducted to predict bacteremia among hematological patients.Citation34 Such variations in colonization rates among HSCT recipient assure that understanding local epidemiology particularities may have a significant impact on outcomes.
Serological testing was used as a pre-transplant screening indicator to determine the serostatus of certain viral/parasitic infections. Our results revealed that three, two, and one HSCT patients had CMV, EBV and Toxoplasmosis active or reactivation of latent infection, respectively. The presence of only one case of active CMV infection during the pre-engraftment period and the absence of other viral infections, despite the COVID-19 pandemic, indicate the effectiveness of prophylactic antiviral therapy and the enhanced pre-transplant serological clearing that covered a wide array of viruses.
In our cohort, the type of transplant was associated with an increased risk of infection during the pre-engraftment phase (p = 0.04), whereas other factors, including age, sex, and underlying conditions, were not statistically significant. This could be attributed to the rate of immune reconstitution, which is typically faster in autologous HSCT recipients than in allogeneic HSCT recipients, who continue to have measurable deficits in cell-mediated and humoral immunity, even after engraftment, as reported in other studies.Citation35,Citation36
Prospective monitoring for subsequent infections with the same MDRO post-transplant revealed the absence of MRSA and CRE and low rates of ESBLs (5.5%) in our center. Although there is limited evidence that pre-transplant MRSA or ESBLs colonization predicts subsequent infection among adult recipients post-HSCT, there is still a significant risk for infections among patients colonized by CRE.Citation37 The success of the management strategy for preventing MRSA infections in our cohort could be attributed to early screening of MRSA, contact precautions, and decolonization protocol as reported in other studies.Citation38,Citation39 In the context of infections by ESBLs and CRE, their management was even more complicated due to disruption of the gut microbiome leading to predominance of gram-negative MDROCitation40 and neutropenia that predispose to bacterial infectionsCitation41 and invasive fungal infections during the pre-engraftment period.Citation42 However, the low rate of ESBLs and absence of CRE infections post-HSCT supports the effectiveness of multimodal infection control interventions in our cohort, in addition to the tailoring of empirical antibiotic treatment depending on the previously known colonization state.
Within 30 days after HSCT, BSIs were documented in 2 patients (5%), and the isolated microorganisms were ESBL-producing E. coli and non-MDR Acinetobacter baumannii. Our findings were similar to those reported by the Tunisian BMT center (5.9%).Citation43 In total, four patients (10%) died during the pre-engraftment period after allogeneic HSCT, and mortality was related to BSIs caused by ESBL-producing E. coli in one patient. Our results are similar to those of other studies that reported infection-related mortality as a major hurdle among allogeneic HSCT recipients, particularly during the early period of transplantation.Citation44,Citation45
A recent study conducted in 2023 showed that the emergence of MDR pathogens as well as patient characteristics should be considered for better management of bacteremia in patients receiving allogeneic HSCT.Citation46 Another study revealed that a short course of antibiotics for 7 days might be sufficient in patients with Cancer and Hematopoietic Stem Cell Transplantation suffering from infections with gram-negative bacteria.Citation47 Our findings were in accordance with those of Goloshchapov et al, who demonstrated the correlations between colonization of EBV positivity in the colon and delayed hematopoietic reconstitution.Citation48
In previous studies, pre-screening and multimodal infection control approaches for the highly priority pathogens have been confirmed to avoid and combated hospital-acquired infection caused by CRE and ESBLs producers.Citation18,Citation49 Moreover, it is recommended to use advanced and accurate methods such as Biofire FilmArray panel or Vitek-system for identifying the colonized pathogens in order to define the proper preventive measure for each pathogen as previously reported.Citation49,Citation50. The relatively small sample size and analysis of single-center data might limit the generalizability of our study. However, this is one of the few studies in regions that has determined the impact of MDRO screening prior to transplantation on subsequent infections with the same isolate during the pre-engraftment period.
Conclusion
This study highlights the effectiveness of pre-transplant screening for high-priority multidrug-resistant pathogens and the application of infection control interventions after HSCT. The majority of patients colonized by MDRO before transplantation and after applying infection control interventions did not develop subsequent infections post-HSCT. Prior to transplantation, there was no statistical difference between colonized patients with MDRO and non-colonized patients with regard to age, sex, underlying conditions, or transplant type. However, there was a statistically significant difference between the types of transplant and pre-engraftment infection. Therefore, understanding the colonization state is critical for guiding infection control measures in HSCT recipients.
Abbreviations
BSIs, bloodstream infections; BMT, Bone marrow transplant; CRE, Carbapenem-resistant Enterobacterales; CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; CMV, Cytomegalovirus; EBV, Epstein–Barr virus; ESBLs, extended-spectrum β-lactamase; HBV, Hepatitis B virus; HCV, Hepatitis C virus; HIV, Human immunodeficiency virus; HAIs, Hospital-acquired infections; IMC, International medical center; MRSA, methicillin-resistant Staphylococcus aureus; MDR, multidrug-resistant; MDRO, multidrug-resistant organism; HSCT, post-hematopoietic stem cell transplant; PCR, Polymerase chain reaction.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki, and was reviewed and approved by the Faculty of Pharmacy, Ain Shams University Research Ethics Committee (ACUC-FP-ASU RHDIRB2020110301 REC #72). Informed consent was obtained from all subjects and/or their legal guardians (s).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
Acknowledgment
The authors are thankful to the Deanship of Graduate studies and Scientific Research at the University of Bisha for supporting this work through the Fast-Track Research Support Program and to the Deanship of Scientific Research King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research group Project Under grant number R.G.P.2/369/44.. We hereby acknowledge the Department of Hematology and Bone Marrow Transportation (BMT) Unit, International Medical Center (IMC), Cairo, Egypt, for providing us with the specimens, facilities, and support required to perform this practical work. The authors also acknowledge the Department of Microbiology and Immunology, Faculty of Pharmacy, Misr International University (MIU), Department of Microbiology and Immunology, Faculty of Pharmacy, Ain Shams University (ASU), Cairo, Egypt, and Department of Pharmaceutical Life Sciences, Faculty of Pharmacy, University Technology MARA (UiTM), Campus Puncak Alam, Bandar Puncak Alam, Selangor, Malaysia, for supporting this research.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article in the main manuscript.
Additional information
Funding
References
- Snowden JA, Sánchez-Ortega I, Corbacioglu S. et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57(8):1217–1239. doi:10.1038/s41409-022-01691-w
- Khaddour K, Hana CK, Mewawalla P. Hematopoietic stem cell transplantation. StatPearls [Internet]. StatPearls Publishing. Treasure Island (FL), USA: StatPearls Publishing. 2022. 1–25. PMID: 30725636. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536951/. Accessed May 28 2024.
- Christopeit M, Schmidt-Hieber M, Sprute R, et al. Prophylaxis, diagnosis and therapy of infections in patients undergoing high-dose chemotherapy and autologous haematopoietic stem cell transplantation. 2020 update of the recommendations of the infectious diseases working party (AGIHO) of the German society of hematology and medical oncology (DGHO). Ann Hematol. 2021;100(2):321–336. doi:10.1007/s00277-020-04297-8
- Nucci M, Anaissie E. Infections after high-dose chemotherapy and autologous hematopoietic stem cell transplantation. Infect Hematol. 2014;27:49–61.
- Rojas-Rechy MH, Gaytán-Morales F, Sánchez-Ponce Y, et al. Herpesvirus screening in childhood hematopoietic transplant reveals high systemic inflammation in episodes of multiple viral detection and an EBV association with elevated IL-1β, IL-8 and graft-versus-host disease. Microorganisms. 2022;10(8):1685. doi:10.3390/microorganisms10081685
- Kim YJ, Waghmare A, Xie H, et al. Respiratory viruses in hematopoietic cell transplant candidates: impact of preexisting lower tract disease on outcomes. Blood Adv. 2022;6(18):5307–5316. doi:10.1182/bloodadvances.2021004915
- Gentile G, Andreoni M, Antonelli G, et al. Screening, monitoring, prevention, prophylaxis and therapy for hepatitis B virus reactivation in patients with haematologic malignancies and patients who underwent haematologic stem cell transplantation: a systematic review. Clin Microbiol Infect. 2017;23(12):916–923. doi:10.1016/j.cmi.2017.06.024
- Oliver NT, Nieto YL, Blechacz B, et al. Severe hepatitis C reactivation as an early complication of hematopoietic cell transplantation. Bone Marrow Transplant. 2017;52(1):138–140. doi:10.1038/bmt.2016.196
- Štajner T, Vujić D, Srbljanović J, et al. Risk of reactivated toxoplasmosis in haematopoietic stem cell transplant recipients: a prospective cohort study in a setting withholding prophylaxis. Clin Microbiol Infect. 2022;28(5):733.e1–733.e5. doi:10.1016/j.cmi.2021.09.012
- Sahitya DSK, Jandiyal A, Jain A, et al. Prevention and management of carbapenem-resistant Enterobacteriaceae in haematopoietic cell transplantation. Ther Adv Infect Dis. 2021;27(8):20499361211053480. doi:10.1177/20499361211053480
- Yan CH, Wang Y, Mo XD, et al. Incidence, risk factors, microbiology and outcomes of pre-engraftment bloodstream infection after haploidentical hematopoietic stem cell transplantation and comparison with HLA-identical sibling transplantation. Clin Infect Dis. 2018;67(2):S162–S173. doi:10.1093/cid/ciy658.
- Balletto E, Mikulska M. Bacterial infections in hematopoietic stem cell transplant recipients. Mediterr J Hematol Infect Dis. 2015;7(1):e2015045. doi:10.4084/mjhid.2015.045
- Moghnieh R, Tamim H, Abyad A, et al. Pre-engraftment infectious complications and patient outcomes after allogeneic hematopoietic cell transplantation: a single-center experience from Lebanon. Infection. 2020;48(3):385–401. doi:10.1007/s15010-020-01407-6.
- Esquirol A, Pascual MJ, Kwon M, et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transplant. 2021;56(10):2432–2444. doi:10.1038/s41409-021-01328-4
- Girmenia C, Bertaina A, Piciocchi A, et al. Incidence, risk factors and outcome of pre-engraftment gram-negative bacteremia after allogeneic and autologous hematopoietic stem cell transplantation: an Italian prospective multicenter survey. Clin Infect Dis. 2017;65(11):1884–1896. doi:10.1093/cid/cix690.
- Chen W, Zhao Y, Luo Y, et al. Clinical characteristics, microbiology, and risk factors for mortality of pre-engraftment and post-engraftment bloodstream infection in hematopoietic stem cell transplantation recipients. Infect Drug Resist. 2022;15:6893–6905. doi:10.2147/idr.s392804
- Mendes ET, Salomão MC, Tomichi LM, et al. Effectiveness of surveillance cultures for high priority multidrug-resistant bacteria in hematopoietic stem cell transplant units. Rev Inst Med Trop Sao Paulo. 2021;63:e77. 10.1590/s1678-9946202163077
- Kamel NA, Elsayed KM, Awad MF, et al. Multimodal interventions to prevent and control carbapenem-resistant Enterobacteriaceae and extended-spectrum β-lactamase producer-associated infections at a tertiary care hospital in Egypt. Antibiotics. 2021;10(5):509. doi:10.3390/antibiotics10050509
- CLSI: Performance standards for antimicrobial susceptibility testing M 100 In: 31st Edition. vol. 31; 2021. Available from: https://clsi.org/standards/products/microbiology/documents/m100/. Accessed February 22, 2023.
- CDC. Centers for disease control and prevention: guidance for antigen testing for SARS-CoV-2 for healthcare providers. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html>. Accessed February 5, 2024.
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x.
- Carreira AS, Salas MQ, Remberger M, et al. Bloodstream infections and outcomes following allogeneic hematopoietic cell transplantation: a single-center study. Transplant Cell Ther. 2022;28(1):50.e1–50.e8. doi:10.1016/j.jtct.2021.10.008
- Satlin MJ, Weissman SJ, Carpenter PA, et al. American society of transplantation and cellular therapy series, 1: enterobacterales infection prevention and management after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(2):108–114. doi:10.1016/j.jtct.2020.10.001
- Reed DR, Petroni GR, West M, et al. Prophylactic pretransplant ganciclovir to reduce cytomegalovirus infection after hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2023;16(1):61–69. doi:10.1016/j.hemonc.2021.05.001
- Akhmedov M. Infectious complications in allogeneic hematopoietic cell transplant recipients: review of transplant-related risk factors and current state of prophylaxis. Clin Transplant. 2021;35(2):e14172. doi:10.1111/ctr.14172
- Rahi MS, Jindal V, Pednekar P, et al. Fungal infections in hematopoietic stem-cell transplant patients: a review of epidemiology, diagnosis, and management. Ther Adv Infect Dis. 2021;8:20499361211039050. doi:10.1177/20499361211039050
- Wang J, Zhou M, Xu JY, et al. Comparison of antifungal prophylaxis drugs in patients with hematological disease or undergoing hematopoietic stem cell transplantation: a systematic review and network meta-analysis. JAMA Network Open. 2020;3(10):e2017652. doi:10.1001/jamanetworkopen.2020.17652.
- Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–347. doi:10.1056/nejmoa061098.
- Rauwolf KK, Floeth M, Kerl K, et al. Toxoplasmosis after allogeneic haematopoietic cell transplantation-disease burden and approaches to diagnosis, prevention and management in adults and children. Clin Microbiol Infect. 2021;27(3):378–388. doi:10.1016/j.cmi.2020.10.009.
- Nellore A, Lee RA. Multidrug-Resistant Organisms: pre-transplant Evaluation and Management. In: Morris MI, Kotton CN, Wolfe CR, editors. Emerging Transplant Infections. USA, Cham: Springer; 2021:287–312. doi:10.1007/978-3-030-25869-6_8
- Grmek Kosnik I, Storman A, Petrovic Z, et al. The evaluation of MRSA surveillance cultures by the number and combinations of anatomical sites. Zdr Varst. 2016;56(1):24–30. doi:10.1515/sjph-2017-0004.
- Al Musawi S, Alkhaleefa Q, Alnassri S, et al. Predictive role of targeted, active surveillance cultures for detection of methicillin-resistant. Staphylococcus aureus Infect Drug Resist. 2021;14:4757–4764. doi:10.2147/idr.s340871
- Öksüz L, Hindilerden İY, Erciyestepe M, et al. The association of CMV infection with bacterial and fungal infections in hematopoietic stem cell transplant recipients: a retrospective single-center study. New Microbiol. 2022;45(1):40–50. PMID: 35403846.
- Torres I T, Huntley D, Tormo M, et al. Multi-body-site colonization screening cultures for predicting multi-drug resistant Gram-negative and Gram-positive bacteremia in hematological patients. BMC Infect Dis. 2022;22(1):172. doi:10.1186/s12879-022-07154-3
- Pereira MR, Pouch SM, Scully B. Infections in allogeneic stem cell transplantation. Princ Pract Transplant Infect Dis. 2018;8:209–226.
- Fiorenza S, Turtle CJ. Associations between the gut microbiota, immune reconstitution, and outcomes of allogeneic hematopoietic stem cell transplantation. Immunometabolism. 2021;3(1):e210004. doi:10.20900/immunometab20210004
- Gea-Banacloche J. Risks and epidemiology of infections after hematopoietic stem cell transplantation. Transplant Infections: Fourth Edition. editors. Ljungman P, Snydman D, Boeckh M. Cham. Springer International Publishing, USA. 2016. 81–99. 10.1007/978-3-319-28797-3_6
- George B, Bhattacharya S. Infections in hematopoietic stem cell transplantation (HSCT) Patients. In: Chandy M, Radhakrishnan VS, Sukumaran RK, editors. Contemp Bone Marrow Transplant. Organ and Tissue Transplantation. Cham: Springer USA; 2021:545–560. doi:10.1007/978-3-030-36358-1_7
- Füller MA, Kampmeier S, Wübbolding AM, et al. Prospective surveillance of colonization and disease by methicillin-resistant Staphylococcus aureus (MRSA) at a European pediatric cancer center. Support Care Cancer. 2022;30(9):7231–7239. doi:10.1007/s00520-022-07140-0
- Alexander T, Snowden JA, Burman J, et al. Intestinal microbiome in hematopoietic stem cell transplantation for autoimmune diseases: considerations and perspectives on behalf of autoimmune diseases working party (ADWP) of the EBMT. Front Oncol. 2021;11:722436. doi:10.3389/fonc.2021.722436
- Cho SY, Lee HJ, Lee DG. Infectious complications after hematopoietic stem cell transplantation: current status and future perspectives in Korea. Korean J Intern Med. 2018;33(2):256–276. doi:10.3904/kjim.2018.036
- Puerta-Alcalde P, Garcia-Vidal C. Changing epidemiology of invasive fungal disease in allogeneic hematopoietic stem cell transplantation. J Fung. 2021;7(10):848. doi:10.3390/jof7100848
- Mellouli A, Chebbi Y, El Fatmi R, et al. Multidrug resistant bacteremia in hematopoietic stem cell transplant recipients. Tunis Med. 2021;99(2):269–276. English. PMID: 33899198.
- Styczyński J, Tridello G, Koster L, et al. Infectious diseases working party EBMT. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020;55(1):126–136. doi:10.1038/s41409-019-0624-z.
- Kong SG, Jeong S, Lee S, et al. Early transplantation-related mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia. BMC Cancer. 2021;21(1):177. doi:10.1186/s12885-021-07897-3.
- Gill J, Busca A, Cinatti N, et al. Bacterial bloodstream infections after allogeneic hematopoietic stem cell transplantation: etiology, Risk factors and outcome in a single-center study. Microorganisms. 2023;11(3):742. doi:10.3390/microorganisms11030742
- Herrera F, Torres D, Carena A, et al. Short course of antibiotic therapy for gram-negative bacilli bacteremia in patients with cancer and hematopoietic stem cell transplantation: less is possible. Microorganisms. 2023;11(2):511. doi:10.3390/microorganisms11020511
- Goloshchapov OV, Shvetsov AN, Chukhlovin AB, et al. Incidence of common herpesviruses in colonic mucosal biopsies following hematopoietic stem cell transplantation. Microorganisms. 2022;10(11):2128. doi:10.3390/microorganisms10112128
- Kamel NA, Alshahrani MY, Aboshanab KM, El Borhamy MI. Evaluation of the biofire filmarray pneumonia panel plus to the conventional diagnostic methods in determining the microbiological etiology of hospital-acquired pneumonia. Biology. 2022;11(3):377. doi:10.3390/biology11030377
- El Sherif HM, Elsayed M, El-Ansary MR, Aboshanab KM, El Borhamy MI, Elsayed KM. BioFire filmArray BCID2 versus VITEK-2 System in determining microbial etiology and antibiotic-resistant genes of pathogens recovered from central line-associated bloodstream infections. Biology. 2022;11(11):1573. doi:10.3390/biology11111573