Abstract
Background
This retrospective cohort study explores a practical approach to acquiring pathogenic microorganisms in patients with bone and joint infections.
Methods
From Aug 2018 to Mar 2022, 68 consecutive patients (87 cultures) with bone and joint infection were recruited in this study. All cultures followed the Peking University First Hospital Procedure of Culturing Pathogenic microorganisms for bone and joint infection. Tissue samples were obtained through fluoroscopy-guided biopsy or open debridement. Tissue samples were divided into manual homogenization (MH), manual mixture (MM), and pathological examination. The baseline, antibiotic exposure, laboratory, surgical, and microbial data were reviewed. Independent sample T-test, Mann–Whitney U-test, and Chi-square test were used to detect the difference between patients who received different processing measures.
Results
The average age was 55.8±2.4 years old. Thirty-nine patients were male. The total positive culture rate of the manual homogenization group was 80.5% (70/87). Thirty-five patients had mixed infections with more than one microorganism cultured. Staphylococci accounted for 60.23% of all microorganisms. Staphylococcus aureus (18.2%) and Staphylococcus epidermidis (15.9%) were the two most common bacteria cultured in this study. Patients with positive culture in the manual mixture group had significantly higher WBC (p = 0.006), NE% (p = 0.024), ESR (p = 0.003), CRP (p = 0.020) and IL6 (0.050) compared to patients with negative culture. After tissue homogenization, only ESR is still statistically different. Patients without SIRS had a low positive culture rate (59.4%). Tissue homogenization could significantly increase the positive culture rate of patients without SIRS. Pre-culture antibiotic exposure was not an independent risk factor for culture results.
Conclusion
Peking University First Hospital Procedure for Culturing Pathogenic microorganisms for Bone and Joint Infections was a practical approach for obtaining pathogenic microorganisms.
Background
Bone and joint infections are one of the most challenging diseases in the musculoskeletal system.Citation1 Although the incidence rate of hematogenous bone and joint infections is low in developed countries, it remains high in developing countries.Citation2 Bone and joint infections require long-term treatment and are prone to recurrence.Citation3 Bone and joint infections have a high disability rate, resulting in a vast social and medical burden.
Identifying the pathogenic microorganism and using sensitive antibiotics are the cornerstones of treating patients with bone and joint infections. Several factors could affect the culture result, including the level of c-reactive protein (CRP) and erythrocyte sedimentation rate (ESR),Citation4,Citation5 the tissue type,Citation6,Citation7 and antibiotic exposure.Citation8,Citation9 Previous literature reported that the culture-positive rate was lower than 50%.Citation10–12
Tissue homogenization, DL-dithiothreitol, and sonication can increase the quantity and viability of pathogens released from diseased tissue. These methods have been applied to improve the rate of pathogenic microorganism identification.Citation13–15 However, there is currently no standardized procedure for pathogen identification in patients with bone and joint infections. Therefore, this retrospective study aims to explore a practical approach to acquiring pathogenic microorganisms in patients with bone and joint infection.
Methods
Study Design, Inclusion, and Exclusion Criteria
This study was a single-centered retrospective cohort study. From Aug 2018 to Mar 2022, 68 consecutive patients with bone and joint infection (except patients with tuberculosis) hospitalized in our department were recruited. All diagnoses were confirmed by pathology.
Data Collection
We reviewed the medical record to assess baseline, antibiotic exposure, laboratory, surgical, and microbial data among the clinical variables. Baseline data included age, gender, infection site, complications, and systemic inflammatory response syndrome (SIRS). Laboratory data included the total number of white blood cells (WBC), neutrophils (NE%), c-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), and interleukin-6 (IL-6).
Patients who meet two or more of the following clinical manifestations are diagnosed with SIRS. 1) Body temperature > 38°C or < 36°C; 2) Heart rate > 90 beats/minute; 3) Breathing > 20 times/minute or PaCO2 < 32 mmHg; 4) The total number of WBC > 12 × 109/L or < 4 × 109/L, or the proportion of immature (rod-shaped) neutrophils > 10%.
Treatment Procedure in This Study
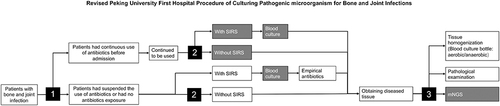
The detailed procedure of pathogenic microorganism culture for bone and joint infection in the Peking Hospital is shown in .
Figure 1 Peking University First Hospital Procedure of Culturing Pathogenic microorganism for Bone and Joint Infections.
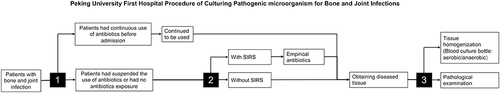
Patients were first admitted to the hospital to determine whether antibiotics were consistently applied. If antibiotics were consistently applied, they were continued after hospitalization; if antibiotics were not consistently applied, the decision to apply antibiotics was based on the patient’s condition. Antibiotics were applied if SIRS was present in the patient and not if SIRS was not present in the patient.
All patients underwent a physical examination, X-ray, Computer Tomography (CT), and Magnetic Resonance Imaging (MRI) to confirm the site of the infection. Their blood samples were taken for laboratory tests. Tissue specimen was obtained through fluoroscopy-guided biopsy or open debridement surgery in the operating room. Surgical methods were selected by a multidisciplinary team composed of an experienced spine surgeon and an infectious diseases specialist.
As described in our previous study, the tissues were divided into three groups: manual homogenization (MH), manual mixture (MM), and pathology. For the MH group, tissues were placed in the disposable sterile tissue grinder and grind clockwise; the homogenate was diluted to 20 mL with normal saline. For the MM group, tissues were placed in the disposable sterile bottle with 20 mL of normal saline and stirred clockwise. The infection was confirmed by pathological examination results, which showed acute and chronic inflammatory cell infiltration and no caseous necrosis or granuloma formation. All cultures are conducted in blood culture bottles (Becton, Dickinson, and Company Spark, MD 21152 USA). Blood cultures were sent to the bacteriological laboratory for subsequent culture and bacterial identification. The identification methods were consistent across all patients studied. The criteria for culture-positive were bacterial growth in blood culture bottles within two weeks. The criteria for culture-negative were no bacterial growth in blood culture bottles within two weeks. The criteria for multi-organism positive were more than one Genus/species growth in blood culture bottles within two weeks. Detailed processing methods were described in our previous study.Citation16
Statistical Analysis
When continuous variables conform to the standard distribution, they were expressed as mean (mean ± SE), and when they did not conform, they were expressed as median (range). Categorical variables were expressed as numbers. Independent sample T-test or Mann–Whitney U-test was used to detect the difference among continuous variables. The differences among the categorical variables were analyzed using the chi-square test. All the tests were on two sides. A p-value < 0.05 was considered statistically significant. We deleted cases with missing values during the statistical process. Data were analyzed with SPSS 25.0 statistical software (IBM Corporation, Armonk, NY).
Results
A total of 68 consecutive patients with 87 times pathogen cultures were recruited in this study. All patients follow the Peking University First Hospital Procedure of Culturing Pathogenic Microorganisms for Bone and Joint Infection. The baseline data of 68 patients are shown in . The average age was 55.8 ± 2.4 years old. Twenty-nine patients (42.6%) were female. Thirty-nine patients (57.4%) were involved in the spine. Thirty-six patients (52.9%) had no risk factors such as diabetes. Sixty-two patients (92.1%) had no primary infectious disease. Five patients (7.3%) performed a blood culture.
Table 1 The Baseline Information of 68 Patients
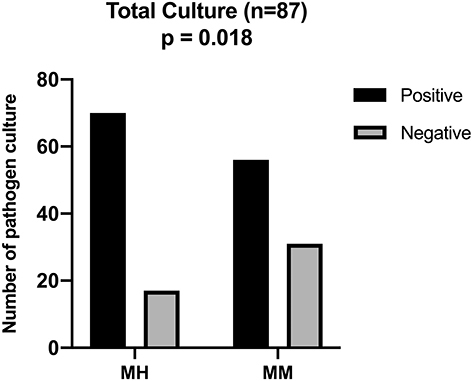
Culture results are shown in . The positive rate of pathogen culture in the Manual homogenization and manual mixture groups was 80.5% (70/87) and 64.4% (56/87), respectively. The manual homogenization group’s positive rate was significantly higher than the manual mixture group (p = 0.018).
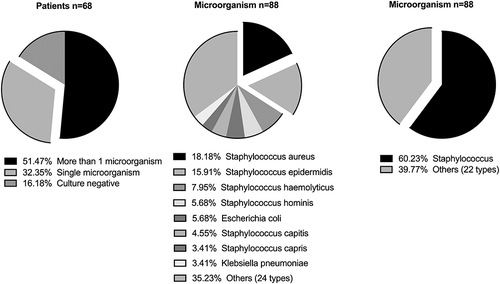
About 51.47% of the patients had mixed infection with more than one microorganism cultured. About 32.35% of the patients had a single pathogen infection. Pathogenic microorganisms are predominantly Gram-positive cocci. Staphylococci accounted for 60.23% of all microorganisms. Gram-negative bacilli are predominantly Escherichia coli and Klebsiella pneumoniae. The top five microorganisms were Staphylococcus aureus (18.2%), Staphylococcus epidermidis (15.9%), Staphylococcus haemolyticus (8.0%), Staphylococcus hominis (5.7%), Escherichia coli (5.7%). .
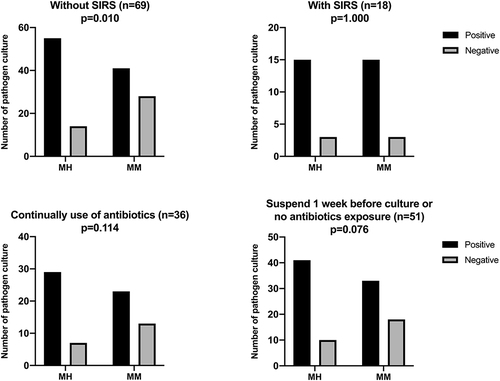
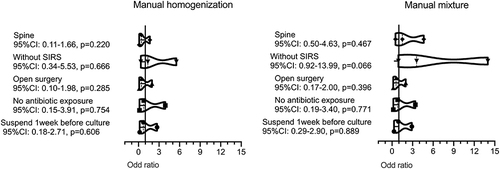
The characteristics of patients with different cultures, as a result of manual homogenization and manual mixture groups, are shown in . There were no significant differences in pre-culture antibiotic exposure, surgical intervention, and infection site of patients with different culture results. Patients with positive culture results in the manual mixture group had significantly higher inflammatory indicators compared to patients with negative culture results, WBC (p = 0.006), NE% (p = 0.024), ESR (p = 0.003), CRP (p = 0.020), IL6 (0.050). These differences did not reach statistical levels in manual homogenization, except ESR (p = 0.046). Patients with SIRS had a higher rate of positive cultures in the manual mixture group (p = 0.059). Thirteen culture-negative patients with spinal infection (24.5%) and 14 culture-negative patients without SIRS (20.3%) in the manual mixture group had final positive culture results after tissue homogenization. Multivariate analysis also showed that tissue homogenization would increase the positive culture rate of patients without SIRS. .
Table 2 Characteristics of Patients with Different Culture Results of Manual Homogenization and Manual Mixture Group
Figure 4 Logistic regression analysis of association between culture result and clinical factors (Multivariate analysis).
Fourteen culture-negative patients in the manual mixture group (16.1%) had final positive culture results after tissue homogenization. The characteristics of these patients are shown in . Most patients in the moderate-risk group had spinal infections and were without SIRS. The inflammatory indicators of the moderate-risk group (WBC, NE%, and ESR) were lower than the low-risk group.
Table 3 Characteristics of Patients with Different Risk Level
Patients were stratified depending on the SIRS and pre-culture antibiotic exposure. . The results showed that tissue homogenization could significantly increase the positive culture rate of patients without SIRS. For patients with continual use of antibiotics, the positive culture rate of the manual homogenization group did not reach a statistical difference when compared with that of the manual mixture.
Discussion
This study showed that strictly following the Peking University First Hospital Procedure could increase the positive rate of microorganism identification in patients with bone and joint infection. The positive rate of microorganism identification in this study was 80.5%, which was higher than in previous studies (28.0%,Citation10 42.0%,Citation11 73.6%)Citation17 More than half of the patients have mixed infections (51.5%), and the pathogenic microorganisms were mainly Gram-positive cocci (staphylococcus aureus: 18.2%; staphylococcus epidermidis: 15.91%).
Previous studies showed a correlation between the expression levels of inflammatory indicators and positive culture results. Patients with higher levels of inflammatory indicators were more likely to identify pathogenic microorganisms.Citation4,Citation5 In this study, patients with positive culture results in the manual mixture group had significantly higher inflammatory indicators compared to patients with negative culture results, WBC (p = 0.006), NE% (p = 0.024), ESR (p = 0.003), CRP (p = 0.020), IL6 (0.050). Fourteen patients with lower inflammatory indicators had final positive culture results after tissue homogenization. However, the ESR (p = 0.046) was still significantly higher in patients with positive culture results in the manual homogenization group. Kim CJ et al showed that soft tissue was better than bone or microbiological diagnosis. Further analysis showed that the CRP of the soft tissue group was significantly higher compared to the bone group.Citation6
Sepsis is a clinical syndrome caused by a dysregulation of the host response due to infection, often accompanied by systemic inflammatory response syndrome (SIRS) and life-threatening organ dysfunction. Mohammad Anas et al study showed that PCT might be a reliable indicator of sepsis in spinal cord injury patients. Meanwhile, they found that there were positive correlations between PCT and inflammatory indicators, such as CRP (R2=0.673, p<0.05) and WBC (R2=0.110, p < 0.05).Citation18 M Sauer et al also showed that serum PCT correlates with the severity of sepsis among profoundly immunocompromised patients.Citation19 The present study showed that, like patients with high expression of inflammatory factors, patients with SIRS were more likely to identify pathogenic microorganisms. SIRS was the most significant factor affecting patient culture results. Tissue homogenization could improve the culture positivity rate of patients without SIRS and , and . Inflammatory indicators and SIRS were essential factors that predict culture results of patients with bone and joint infection.Citation20 Compared with inflammatory indicators, SIRS is more straightforward, practical, and accessible to judge.
Several studies have shown that tissue homogenization could increase the culture-positive rate by breaking down biofilm composed of bacteria, proteins, and matrices and releasing more pathogenic microorganisms into the culture medium.Citation13,Citation16,Citation21 Consistent with previous research, our study showed that the manual homogenization group’s positive rate was significantly higher than the manual mixture group (80.5% vs 64.4%). Tissue homogenization could significantly increase the positive culture rate of patients without SIRS, .
The 2015 Infectious Diseases Society of America Clinical Practice Guidelines suggest antibiotics should be suspended for 1–2 weeks unless the patient has a hemodynamic compromise or is accompanied by neurologic symptoms.Citation22 The impact of antibiotics on culture results was still controversial.Citation8–10,Citation17 Several studies showed that antibiotics can reduce the positive rate of culture.Citation9,Citation17 Russo et al’s study showed that strictly following the UDIPROVE procedure could improve the positive rate of pathogenic culture. However, stopping antibiotics might lead to disease aggravation for patients with SIRS. Consistent with Lavery LA et alCitation8 and Hirschfeld CB et al,Citation10 there was no significant difference in the positive culture rate between patients with different pre-culture antibiotic exposures. This study treated patients with the following conditions with antibiotics before culture. Those who had been using antibiotics before hospitalization and those who had not used antibiotics before hospitalization but had SIRS.
Blood culture was influential for patients with bone and joint infections, especially for patients. Mylona et al’s study showed that blood culture had an average positive culture rate of 58% for patients with pyogenic vertebral osteomyelitis.Citation23 Blood cultures play the most crucial role in pathogen identification in pyogenic vertebral osteomyelitis.Citation24
Metagenomic next-generation sequencing (NGS) was more sensitive to identifying pathogenic microorganisms. Hayder Hamad et al’s study showed that polymerase chain reaction could identify pathogenic microorganisms more than blood culture.Citation25 Metagenomic next-generation sequencing was sensitive and accurate in detecting pathogenic microorganisms in prosthetic joint infectionCitation26 and spinal infection.Citation27
There are limitations to this study. First, it is limited by its retrospective nature. However, our study provides valuable insight into establishing an effective procedure for identifying pathogenic microorganisms for bone and joint infection. Second, this was a single-center study with a relatively small sample size. A large-scale case should be carried out to validate these results. Third, there needs to be more in our present procedure. Some details need to be handled more rigorously, such as managing antibiotic elution in patients without SIRS and ignoring blood culture and metagenomic next-generation sequencing. We propose a revision of the present procedure and validate its clinical efficacy in future studies .
Conclusions
Peking University First Hospital Procedure for Culturing Pathogenic microorganisms for Bone and Joint Infections was a practical approach for obtaining pathogenic microorganisms.
Summary
1) Staphylococcus aureus (18.2%) and Staphylococcus epidermidis (15.9%) were the two most common bacteria cultured in patients with bone and joint infection.
2) Patients with positive culture in the manual mixture group had significantly higher WBC (p = 0.006), NE% (p = 0.024), ESR (p = 0.003), CRP (p = 0.020) and IL6 (0.050) compared to patients with negative culture.
3) Patients without SIRS had a low positive culture rate (59.4%).
4) Tissue homogenization could significantly increase the positive culture rate of patients without SIRS.
Abbreviations
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell count.
Ethics Approval and Consent to Participate
This study was a retrospective study. There was no follow-up requirement in this study. It was unnecessary to collect blood samples and other samples of the patients, and no additional examination was required. The research ethics committee of Peking University First Hospital approved the study protocol (2022 scientific research 081). The research ethics committee of Peking University First Hospital required neither patient approval nor informed consent to review patients’ routine medical records in this study. Researchers would strictly keep patients’ personal information confidential. Identifiable information would not be disclosed to persons other than research members unless permission was obtained from the patient. All research members were required to keep the identity of patients confidential. No patients’ personal information would be disclosed when the research results were published. This study was conducted following the ethical standards in the 1964 Declaration of Helsinki.
Disclosure
The authors declare that they have no conflict of interest. Each author certifies that no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
Data Sharing Statement
The data supporting this study’s findings are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Additional information
Funding
References
- Bejon P, Robinson E. Bone and joint infection. Medicine. 2021;41(12):719–722. doi:10.1016/j.mpmed.2013.09.008
- Moon MS. Management of Bone and Joint Infections. J Musculoskel Res. 2022;25(1):2230001. doi:10.1142/s0218957722300010
- Sigmund IK, McNally MA. Diagnosis of bone and joint infections. Orthop Trauma. 2019;33(3):144–152. doi:10.1016/j.mporth.2019.03.001
- Heyer CM, Brus LJ, Peters SA, et al. Efficacy of CT-guided biopsies of the spine in patients with spondylitis – an analysis of 164 procedures. Eur J Radiol. 2012;81(3):e244–e249.
- Dai G, Li S, Yin C, et al. Culture-negative versus culture-positive in pyogenic spondylitis and analysis of risk factors for relapse. Br J Neurosurg. 2021;8:1–5. doi:10.1080/02688697.2021.18966779
- Kim CJ, Kang SJ, Choe PG, et al. Which tissues are best for microbiological diagnosis in patients with pyogenic vertebral osteomyelitis undergoing needle biopsy? Clin Microbiol Infect. 2015;21(10):931–935. PMID: 26119720. doi:10.1016/j.cmi.2015.06.021
- Chang CY, Simeone FJ, Nelson SB, Taneja AK, Huang AJ. Is biopsying the paravertebral soft tissue as effective as biopsying the disk or vertebral endplate? 10-Year retrospective review of ct-guided biopsy of diskitis-osteomyelitis. AJR Am J Roentgenol. 2015;205(1):123–129. PMID: 26102390. doi:10.2214/AJR.14.13545
- Lavery LA, Crisologo PA, Ryan E, et al. Does antibiotic treatment before bone biopsy affect the identification of bacterial pathogens from bone culture? Wounds. 2023;35(6):E186–E188. PMID: 37347593. doi:10.25270/wnds/22084
- Gramberg MCTT, Van Hattem JM, Dijkstra JA, et al. Effect of prior antibiotic use on culture results in people with diabetes and foot osteomyelitis. Antibiotics. 2023;12(4):684. PMID: 37107046; PMCID: PMC10135220. doi:10.3390/antibiotics12040684
- Hirschfeld CB, Kapadia SN, Bryan J, et al. Impact of diagnostic bone biopsies on the management of non-vertebral osteomyelitis: a retrospective cohort study. Medicine. 2019;98(34):e16954. PMID: 31441894; PMCID: PMC6716736. doi:10.1097/MD.0000000000016954
- Hoang D, Fisher S, Oz OK, La Fontaine J, Chhabra A. Percutaneous CT guided bone biopsy for suspected osteomyelitis: diagnostic yield and impact on patient’s treatment change and recovery. Eur J Radiol. 2019;114:85–91. PMID: 31005182. doi:10.1016/j.ejrad.2019.01.032
- Ang MT, Wong GR, Wong DR, Clements W, Joseph T. Diagnostic yield of computed tomography-guided biopsy and aspiration for vertebral osteomyelitis. J Med Imaging Radiat Oncol. 2019;63(5):589–595. PMID: 31301094. doi:10.1111/1754-9485.12923
- Fang X, Zhang L, Cai Y, et al. Effects of different tissue specimen pretreatment methods on microbial culture results in the diagnosis of periprosthetic joint infection. Bone Joint Res. 2021;10:96–104. doi:10.1302/2046-3758.102.BJR-2020-0104.R3
- Sambri A, Cadossi M, Giannini S, et al. Is treatment with dithiothreitol more effective than sonication for the diagnosis of prosthetic joint infection? Clin Orthop Relat Res. 2018;476:137–145. doi:10.1007/s11999.0000000000000060
- Carlson BC, Hines JT, Robinson WA, et al. Implant sonication versus tissue culture for the diagnosis of spinal implant infection. Spine. 2020;45:E525–E532. doi:10.1097/BRS.0000000000003311
- Cui Y, Mi C, Wang B, et al. Manual homogenization improves the sensitivity of microbiological culture for patients with pyogenic spondylitis. Infect Drug Resist. 2022;15:6485–6493. PMID: 36386415; PMCID: PMC9642858. doi:10.2147/IDR.S386148
- Russo A, Graziano E, Carnelutti A, et al. Management of vertebral osteomyelitis over an eight-year period: the UDIPROVE (UDIne PROtocol on VErtebral osteomyelitis). Int J Infect Dis. 2019;89:116–121. PMID: 31629078. doi:10.1016/j.ijid.2019.10.010
- Anas M, Hasan T, Raja U, Raza WA. Is procalcitonin a reliable indicator of sepsis in spinal cord injury patients: an observational cohort study. Eur Spine J. 2023;32(5):1591–1597. PMID: 36966256. doi:10.1007/s00586-023-07609-4
- Sauer M, Tiede K, Fuchs D, Gruhn B, Berger D, Zintl F. Procalcitonin, C-reactive protein, and endotoxin after bone marrow transplantation: identification of children at high risk of morbidity and mortality from sepsis. Bone Marrow Transplant. 2003;31(12):1137–1142. PMID: 12796793. doi:10.1038/sj.bmt.1704045
- Xiao H, Zhang H, Wang G, et al. Comparison among presepsin, procalcitonin, and C-reactive protein in predicting blood culture positivity and pathogen in sepsis patients. Shock. 2023. PMID: 37878488. doi:10.1097/SHK.0000000000002243
- Pradal M, Saint-Sardos P, Faïs T, Bonnet R, Delmas J. Comparison of homogenization with the GentleMacs Dissociator vs conventional methods for routine tissue processing. Lett Appl Microbiol. 2022;74(5):666–670. PMID: 35007361. doi:10.1111/lam.13651
- Berbari EF, Kanj SS, Kowalski TJ, et al.; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–46. PMID: 26229122. doi:10.1093/cid/civ482
- Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10–17. PMID: 18550153. doi:10.1016/j.semarthrit.2008.03.002
- Hijazi MM, Siepmann T, Disch AC, et al. Diagnostic Sensitivity of Blood Culture, Intraoperative Specimen, and Computed Tomography-Guided Biopsy in Patients with Spondylodiscitis and Isolated Spinal Epidural Empyema Requiring Surgical Treatment. J Clin Med. 2023;12(11):3693. PMID: 37297888; PMCID: PMC10253496. doi:10.3390/jcm12113693
- Hayder Hamad M, Eidan Hadi M, Ajam IK. Comparison between polymerase chain reaction and blood culture for diagnosis of neonatal sepsis. Arch Razi Inst. 2023;78(1):221–226. PMID: 37312696; PMCID: PMC10258275. doi:10.22092/ARI.2022.358608.2259
- Huang Z, Li W, Lee GC, et al. Metagenomic next-generation sequencing of synovial fluid demonstrates high accuracy in prosthetic joint infection diagnostics: mNGS for diagnosing PJI. Bone Joint Res. 2020;9(7):440–449. PMID: 32864114; PMCID: PMC7437524. doi:10.1302/2046-3758.97.BJR-2019-0325.R2
- Wang G, Long J, Zhuang Y, et al. Application of metagenomic next-generation sequencing in the detection of pathogens in spinal infections. Spine J. 2023;23(6):859–867. PMID: 36773890. doi:10.1016/j.spinee.2023.02.001