Abstract
Introduction
Current immunologic methods cannot distinguish Mycobacterium tuberculosis (Mtb) infection statuses, especially to discriminate active tuberculosis (ATB) from latent tuberculosis infection (LTBI). This study explored the potential of latency-associated antigens (Rv1733cSLP and Rv2028c) and multifactorial cytokine detection to distinguish tuberculosis infection states.
Methods
ATB patients (20), LTBI healthcare workers (25), fever patients (11), and healthy controls (10) were enrolled. Cytokine levels (IFN-γ, TNF-α, IL-2, IL-6, IP-10, IL-1Ra, CXCL-1, and MCP-1) were measured using Luminex with/without MTB-specific virulence factor and latency-associated antigens stimulation.
Results
Without antigen stimulation, IL-6, IP-10, MCP-1, and IL-1Ra were higher in the ATB group than in the LTBI group (p<0.05), but no significant differences between the ATB group and the fever group. Stimulated with the four antigens, respectively, the cytokines, including IP-10Esat−6, IP-10CFP−10, IFN-γRv1733cSLP, IFN-γRv2028c, IL-6Esat−6, IL-6Rv1733cSLP, IL-6Rv2028c, IL-2Rv1733cSLP, IL-2 Rv2028c, IL-1RaEsat−6, IL-1RaCFP−10, IL-1RaRv2028c, CXCL-1Esat−6, CXCL-1CFP−10, CXCL-1Rv1733cSLP, CXCL-1Rv2028c, MCP-1Esat−6 and MCP-1CFP−10, demonstrated accurate discrimination between ATB and LTBI (p<0.05). Additive concentrations demonstrated significant secretion differences of IFN-γ, IP-10 and IL-2, primarily by virulence factors in ATB and latency-associated antigens in LTBI. Latency-associated antigens synergized with virulence factors, enhancing TH1-type cytokine diagnostic efficacy for discriminating ATB from LTBI, the AUC for TNF-α increased from 0.696 to 0.820 (p=0.038), IFN-γ increased from 0.806 to 0.962 (p=0.025), and IL-2 increased from 0.565 to 0.868 (p=0.007). Model selected by forward likelihood method indicated combined detection of IFN-γCFP−10, IFN-γRv1733cSLP, IP-10Rv1733cSLP, and CXCL-1Rv1733cSLP achieved ATB diagnosis (AUC=0.996) and ATB-LTBI differentiation (AUC=0.992). Combined detection of IFN-γCFP−10 and IFN-γRv1733cSLP achieved tuberculosis infection diagnosis (AUC=0.943).
Conclusion
Latency-associated antigens enhance multiple cytokine discriminatory ability, particularly TH1-type cytokines, for differentiating Mtb infection statuses.
Background
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb), which can invade multiple tissues and organs within the human body. According to World Health Organization, there were an estimated 10.6 million new cases of active tuberculosis (ATB) worldwide in 2021, marking a 3.6% increase in incidence—the first increase in nearly two decades. In China, there were 780,000 new cases of TB, ranking it third among the 30 countries with high burden of TB.Citation1
It is of utmost importance to differentiate between different Mtb infection statuses and accurately diagnose ATB to guide appropriate treatment and control the disease. However, due to the slow growth of Mtb, obtaining direct pathogenic evidence may not always be feasible, making immunological methods a valuable option for timely diagnosis. Interferon-gamma release assays (IGRAs) can detect Mtb infection but fail to distinguish ATB from latent tuberculosis infection (LTBI). The limited diagnostic efficacy of IGRAs poses a significant challenge, particularly in high TB burden regions,Citation2,Citation3 therefore, it is necessary to improve and enhance immunological methods.
Cytokines/chemokines are a group of small soluble proteins secreted by cells, which play a crucial role in mediating immune responses during Mtb infection in humans. These proteins have been shown in recent studies to have the potential to differentiate between different Mtb infection statuses. Specifically, interferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin-2 (IL-2), IL-6, interferon-inducible protein-10 (IP-10), IL-1 receptor antagonist (IL-1Ra), chemokine (C-X-C) ligand-1 (CXCL-1), and macrophage chemoattractant protein 1 (MCP-1) have demonstrated promising discriminatory capabilities. These cytokines/chemokines serve as key immune mediators and provide valuable insights into the immune response during Mtb infection, holding potential for their application as biomarkers to distinguish different Mtb infection statuses.
Early secreting antigen target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10) are well-known virulence factors of Mtb and have been widely utilized in traditional diagnostic methods such as IGRAs. However, additional antigens associated with tuberculosis latency have been identified, including Rv2028c and Rv1733c, which are regulatory proteins encoded by the dormancy survival regulon (DosR) of Mtb. These antigens have shown a preferential recognition by T cells in individuals with latent tuberculosis infection (LTBI). Studies have demonstrated that the secretion of cytokines in whole blood, particularly in response to stimulation with Rv1733c and Rv2028c, is significantly higher in the LTBI population compared to ATB patients,Citation4,Citation5 These findings suggest that Rv1733c and Rv2028c may serve as associated antigens specifically related to LTBI.
Furthermore, our research group has investigated the immunogenicity of Rv1733c SLP, a synthetic long peptide derived from Rv1733c, and found that it elicits a stronger immune response than Rv1733c alone in individuals with LTBI.Citation6 Specifically, we observed higher secretion of IL-2 upon stimulation with Rv1733c SLP.Citation7 Combining Rv1733c SLP with virulence factors may enhance the ability to discriminate between ATB and LTBI.Citation6 In the case of the antigen Rv2028c, limited studies have examined the difference in IFN-γ secretion levels between ATB and LTBI populations,Citation5 however, there is a lack of research evaluating the differential multi-cytokine immune response between ATB and LTBI populations upon stimulation with Mtb-latency-associated antigens.
Early identification and accurate diagnosis of ATB is crucial for clinicians. However, current immunological methods based on virulence factors and cytokines are insufficient to differentiate ATB from LTBI. In this study, we aim to address this limitation by combining virulence factors (ESAT-6 and CFP-10) and Mtb-latency-associated antigens (Rv2028c and Rv1733c SLP) to stimulate whole blood samples from LTBI individuals, ATB patients, febrile patients, and healthy individuals. We are going to explore potential biomarkers to distinguish different Mtb infection statuses.
Method
Participant Enrollment
We conducted our study from February to April 2022 and enrolled participants from various groups, including ATB patients, LTBI population, febrile patients, and healthy individuals. ATB patients and LTBI healthcare individuals were recruited from Beijing Chest Hospital, while febrile patients were recruited from Peking Union Medical College Hospital. The healthy individuals included in the study were freshmen from Peking Union Medical College. The detailed inclusion and exclusion criteria are provided in Supplementary Table 1. This study was approved by the Ethics Committee of the Peking Union medical College Hospital (ZS-3415). All eligible ATB patients, febrile patients, healthy controls and LTBI healthcare workers provided written informed consent according to institutional requirements. The study completely followed the guiding principles in the Declaration of Helsinki.
Cytokine/Chemokine Detection with/without Antigen Stimulation
Freshly collected sodium heparin-anticoagulated peripheral blood (0.5 mL) was added to individual wells of a 24-well cell culture plate. RPMI1640 medium, containing 10% FBS and 2% penicillin-streptomycin, was added in a 1:2 ratio with whole blood. The blood samples were then stimulated with ESAT-6, CFP-10, Rv1733c SLP, and Rv2028c antigens, respectively, at a final concentration of 10 μg/mL. A black control was included, where an equal volume of RPMI1640 medium was added. The cell culture plate was incubated at 37°C for 18 hours. After incubation, the supernatant was collected by aspirating it into a 1.5 mL centrifuge tube and centrifuged at 10,000 rpm for 2 minutes. The final supernatant was collected, and the secretion levels of TNF-α, IL-2, IL-6, IP-10, IL-1Ra, CXCL-1, MCP-1, and IFN-γ were measured using the Human Premixed Multi-Analyte Kit (Luminex® Discovery Assay) following the manufacturer’s protocol. The blank control was used as the result without stimulation, and the other results were obtained by subtracting the blank control from the stimulated results.
Data Analysis
The Kolmogorov–Smirnov test was used to check for normal distribution of variable data. Normally distributed variables were presented as mean ± standard deviation, while non-normally distributed variables were shown as median and interquartile range. To account for baseline secretion levels, cytokine secretion levels with antigen stimulation were subtracted from those without stimulation. Statistical tests such as Chi-square tests, Fisher’s exact test, t-test, or Mann–Whitney U-test were employed for comparing cytokine secretion levels among the four groups based on data characteristics. ROC curves were used to evaluate the diagnostic performance of selected cytokines/chemokines. Statistical analyses were conducted using GraphPad Prism v.7.0 (GraphPad Software, California, USA) and SPSS 26.0 (IBM, Armonk, NY, USA). A significance level of p < 0.05 was considered statistically significant for all analyses.
Results
Characteristics of Study Participants
A total of 66 subjects were included, consisting of 20 pathogenically confirmed ATB patients, 25 individuals with LTBI, 11 febrile patients, and 10 healthy individuals. The mean age was 44.00±13.70 years, with 27 cases being male (40.90%). Detailed information is presented in .
Table 1 Basic Characteristics of the Subjects in Our Study
Cytokines/Chemokines Secretion with/without Stimulation
The secretion of IL-6, IP-10, MCP-1, and IL-1Ra was significantly higher in the ATB group compared to the LTBI group (p<0.05) without stimulation (). However, the secretion of these cytokines/chemokines was also elevated in the fever group and did not exhibit significant differences compared to the ATB group (p>0.05).
Figure 1 Cytokine/chemokines secretion in four groups with/without stimulation. (A) cytokine/chemokines secretion in four groups without stimulation. (B) Cytokine/chemokines secretion with virulence factors (ESAT-6 and CFP-10) and Mtb-latency-associated antigens (Rv2028c and Rv1733c SLP) in ATB and LTBI groups. (C) pie charts of individual concentrations of cytokines induced by virulence factor and latency-associated antigens in ATB and LTBI (the sum is set to 1). (*: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001).
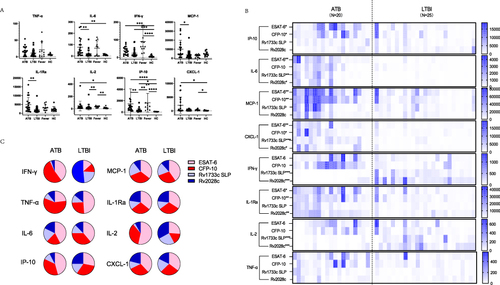
As shown in , with the stimulation of ESAT-6, the secretion of IP-10Esat−6, IL-6Esat−6, IL-1RaEsat−6, CXCL-1Esat−6, and MCP-1Esat−6 was significantly higher in the ATB group compared to the LTBI group (p<0.05); with the stimulation of CFP-10, the secretion of IP-10CFP−10, IL-1RaCFP−10, CXCL-1CFP−10 and MCP-1CFP−10 was significantly higher in the ATB group compared to the LTBI group (p<0.05); with the stimulation of Rv1733c SLP, the secretion of IFN-γRv1733cSLP, IL-6Rv1733cSLP, IL-2Rv1733cSLP, and CXCL-1Rv1733c SLP was significantly higher in the ATB group compared to the LTBI group (p<0.05), and with the stimulation of Rv2028c, the secretion of IFN-γRv2028c, IL-6Rv2028c, IL-2 Rv2028c, IL-1RaRv2028c and CXCL-1Rv2028c was significantly higher in the ATB group compared to the LTBI group (p<0.05).
Differential Cytokine Pattern Between ATB and LTBI in Response to Mtb Risk Factor and Latency-Associated Antigens
Antigen specificity and expressed cytokines varied among individuals and study groups (Supplementary Figures 1 and 2). Therefore, we performed a combined analysis of virulence factor (ESAT-6 and CFP-10) and latency-associated (Rv1733c SLP and Rv2028c) antigen-induced cytokine expression by cumulating individual concentrations for both study groups. The comparison of pie charts indicated differential patterns for individual cytokines and between the study groups (). Between the ATB and LTBI groups, significant differences were observed in the responses of IFN-γ, IL-2, and IP-10 to stimulation with virulence factors and latency-associated antigens. Specifically, in the ATB group, 93.0% of IFN-γ secretion was stimulated by virulence factors, whereas in the LTBI group, only 25.8% was stimulated by virulence factors. Similarly, 88.9% of IL-2 in the ATB group was secreted after stimulation with virulence factors, compared to 44.2% in the LTBI group. Moreover, 82.1% of IP-10 in the ATB group was secreted after stimulation with virulence factors, while 59.6% was secreted in the LTBI group. The remaining cytokines exhibited relatively stable patterns of secretion after specific stimulation with virulence factors and latency-associated antigens between the two groups.
Enhanced Diagnostic Potential of Mtb-Latency-Associated Antigens
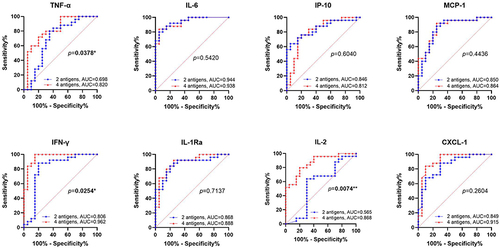
The diagnostic ability of Mtb-latency-associated antigens in differentiating ATB from LTBI was explored in our study. illustrated the AUC values of eight cytokines, comparing their discrimination ability when stimulated with virulence factors alone versus when combined with Mtb-latency-associated antigens. Notably, the stimulation of Mtb-latency-associated antigens and virulence factors resulted in increased secretion of IL-2, IL-1Ra, IFN-γ, MCP-1, TNF-α, and CXCL1 to varying degrees, and the AUC values for distinguishing ATB from LTBI also improved, with significant enhancements observed for the cytokines secreted by Th1 cells, the AUC for TNF-α increased from 0.696 to 0.820 (p=0.038), IFN-γ increased from 0.806 to 0.962 (p=0.025) and IL-2 increased from 0.565 to 0.868 (p=0.007).
Figure 2 ROC curves of cytokine/chemokines secretion with stimulation by virulence factors (2 antigens: ESAT-6 and CFP-10) versus virulence factors combined with Mtb-latency-associated antigens (4 antigens: ESAT-6, CFP-10, Rv2028c and Rv1733c SLP) in ATB VS LTBI. AUC: The area under the curve. (*p < 0.05, **p < 0.01).
As a mature commercial tuberculosis infection test kit, the addition of latent tuberculosis-related antigens to IGRA will increase its detection efficiency for various tuberculosis infection states (Supplementary Figure 3), including tuberculosis infection (ATB vs Fever & HC, AUC=1), ATB (ATB vs LTBI & Fever & HC, AUC=0.923), and the discrimination between ATB and LTBI (AUC=0.962).
The Combination of Multiple Cytokines Further Improves the Diagnosis of Tuberculosis Infection States
Incorporating cytokines stimulated by the four antigens in this study, a binary logistic regression model was constructed using forward likelihood ratio method to differentiate between ATB and LTBI. After four steps of selection, IFN-γCFP−10, IFN-γRv1733c SLP, IP-10Rv1733c SLP, and CXCL-1Rv1733c SLP were identified, achieving an AUC of 0.992 for distinguishing ATB from LTBI, and for diagnosing ATB, the AUC was 0.996, and for Mtb infection, the AUC was 0.698 (). Using IFN-γCFP−10 and IFN-γRv1733c SLP to build another logistic regression model for diagnosing Mtb infection, the AUC reached to 0.943 (), indicating that the detection of IFN-γCFP−10, IFN-γRv1733c SLP, IP-10Rv1733c SLP, and CXCL-1Rv1733c SLP allow for accurately diagnosing different tuberculosis infection states.
Figure 3 Diagnostic efficacy of cytokines selected by logistic regression for different tuberculosis states. (A) Diagnostic efficacy of IFN-γCFP−10, IFN-γRv1733c SLP, IP-10Rv1733c SLP, and CXCL-1Rv1733c SLP for different tuberculosis states; (B) Diagnostic efficacy of IFN-γCFP−10 and IFN-γRv1733c SLP for Mtb infection versus Mtb non-infection.

Discussion
Mtb infection triggers the activation of antigen-specific T lymphocytes, which play a crucial role in controlling and limiting Mtb growth. Previous studies have shown elevated secretion of cytokines/chemokines by T lymphocytes in the peripheral blood of ATB patients, providing valuable insights into TB diagnosis.Citation6,Citation8 However, these cytokines/chemokines are not specific to Mtb, other immune cells, including macrophages, dendritic cells, lymphocytes, and NK cells, can also produce these cytokines/chemokines during bacterial infections, as supported by our study findings. The stimulation of tuberculosis-specific antigens will enhance the reliability of immunological test for diagnosing tuberculosis infections.
The ability to differentiate between ATB and LTBI is crucial for guiding appropriate medical management decisions for patients, especially in settings where access to Mtb culture facilities is limited. In our study, when stimulated with four different antigens individually, 18 Mtb-specific cytokines showed differences across all four antigens in ATB, and LTBI groups, which highlighted the potential utility of cytokines/chemokines as biomarkers for differentiating ATB from LTBI. However, these cytokines/chemokines are not routinely tested in clinical practice. There is long distance from reporting to application, providing insights for improving the diagnostic efficiency of existing immune test kits and developing new diagnostic reagents.
Stimulation with Mtb-latency-associated antigens holds promise as a complementary approach to clinically detect different Mtb infection statuses. Previous studies have demonstrated that the secretion of IFN-γ is significantly higher in the LTBI population compared to ATB patients when stimulated with Mtb-latency-associated antigens such as Rv1733c and Rv2028c.Citation4,Citation5 These findings suggest that Mtb-latency-associated antigens may have immunoprotective effects on LTBI individuals. Additionally, a separate study reported that combining the detection of IFN-γ stimulated by Mtb-latency-associated antigens with IL-6 stimulated by virulence factors yielded higher diagnostic efficacy in differentiating ATB.Citation9 In our study, we observed significant differences in the secretion of IL-6, CXCL-1, IFN-γ and IL-2 when stimulated with Rv1733cSLP or Rv2028c between the ATB and LTBI groups. These findings further support the potential of Mtb-latency-associated antigens in the detection of ATB.
The combined stimulation of Mtb-latency-associated antigens with virulence factors has also shown promising results in enhancing the diagnostic ability to discriminate ATB from LTBI. Notably, we observed a significant increase in TNF-α, IFN-γ, and IL-2 secretion. All three cytokines belong to Th1-type cytokines, indicating that Mtb-latency-associated antigens can effectively activate Th1-dominated immune responses, as shown in previous studies.Citation10,Citation11 Additionally, the Mtb-latency-associated proteins, acting as Toll-like receptor (TLR) agonist-adjuvantsCitation10 have been shown to stimulate the expression of TLRs, co-stimulatory molecules, and pro-inflammatory cytokines, thereby further enhancing the production of Th1-type cytokines. These findings support the notion that the specific secretion of IL-2, TNF-α, and IFN-γ holds potential as biomarkers for distinguishing between ATB and LTBI.
However, the diagnostic value of IL-2 in distinguishing between ATB and LTBI remains controversial. Some studies, such as Mamishi et alCitation12 and Wang et alCitation13 have supported the use of IL-2 as markers for differentiating ATB from LTBI, while Suzukawa et alCitation14 have a different opinion. In our study, we found that without stimulation, only IFN-γ and IL-2 could identify Mtb infection when comparing it to the HC group. The inclusion of Mtb-latency-associated antigens resulted in a trend of increased AUC of IL-2 to discriminate between ATB and LTBI, although the difference was not statistically significant, which may be attributed to the limitations in our sample size. Moreover, when stimulated with the four specific antigens, IL-2 could not only distinguished Mtb infection from the HC group but also, surprisingly, accurately differentiated ATB from LTBI.
Chemokines play important roles in the immune response to Mtb infection. They promote T-cell aggregation, recruit monocytes and lymphocytes to the site of infection, and form nodules that limit the spread of Mtb.Citation15,Citation16 Previous studies have highlighted the diagnostic value of chemokines IP-10, MCP-1, and CXCL-1 in detecting Mtb infection.Citation17–19 In our study, we found that IP-10 and MCP-1 could distinguish ATB from LTBI without stimulation, indicating their potential as predictive markers for ATB when other diseases are ruled out. However, they could not differentiate ATB from Fever (). These findings support the notion that increased secretion of IP-10 and MCP-1 in the absence of stimulation is highly indicative of ATB. With the stimulation of ESAT-6 or CFP-10 antigens, IP-10, MCP-1, and CXCL-1 demonstrated the ability to discriminate between ATB and LTBI (). Furthermore, with the stimulation of Rv1733c SLP or Rv2028c antigens, only CXCL-1 showed significant differences between ATB and LTBI (). Among the three chemokines, CXCL-1 had the highest discriminatory ability, with an AUC of 0.915, followed by MCP-1 (AUC=0.864) and IP-10 (AUC=0.812), in distinguishing ATB from LTBI. These findings underscore the potential clinical utility of chemokines in discriminating between different Mtb infection statuses. Overall, our study demonstrates the valuable diagnostic potential of chemokines in distinguishing between different Mtb infection statuses. Chemokines, particularly CXCL-1, hold promise for clinical applications in the discrimination of ATB from LTBI.
IL-1Ra plays a crucial role in the regulation of TB by maintaining a balanced internal environment. Previous studies have demonstrated significantly increased IL-1Ra in plasma of ATB patients without stimulation, which subsequently decrease at the end of anti-TB treatment.Citation20 Moreover, it has been observed that the risk of TB reactivation is significantly higher in patients with autoimmune diseases treated with monoclonal IL-1Ra antibodies. In recent years, Akashi et alCitation21 identified IL-1Ra as a potential biomarker for discriminating between ATB and LTBI. They found that IL-1Ra levels in cultured supernatants of QFT-GIT and QFT-Plus assays could serve as useful indicators. Building upon these findings, our study incorporated Mtb-latency-associated antigens in the stimulation protocol, resulting in significantly different IL-1Ra secretion among the ATB, LTBI, and Fever groups. Notably, the combined stimulation with the four antigens yielded an AUC of 0.888 (95% CI: 0.790–0.986) for discriminating ATB from LTBI.
The immune mechanisms involved in TB are highly complex, and the secretion of cytokines can reflect the immune homeostasis of the infected host. However, relying on single cytokines or stimulation with virulence factors alone has limited diagnostic value in discriminating between different stages of Mtb infection.Citation22 To improve diagnostic efficacy, studies have explored the combination of multiple cytokines or antigens.Citation23 For instance, a multi-cytokine diagnostic model incorporating IL-18, IL-37, and IP-10 has been utilized to differentiate between ATB and LTBI, demonstrating the validity and feasibility of this approach.Citation24 In our study, we selected cytokines and built a binary logistic regression model using forward likelihood ratio method to discriminate different tuberculosis infection status. The final model achieved an impressive AUC, further confirming the feasibility and effectiveness of this multi-cytokine approach. These findings highlight the potential of utilizing a combination of cytokines to improve the accuracy and reliability of TB diagnosis.
Our study has some limitations. Firstly, it was exploratory with a relatively small sample size. Secondly, there might be potential selection bias as we recruited newly diagnosed, unmedicated ATB patients. Drug interventions can influence cytokine/chemokine secretion in TB patients,Citation20,Citation25 and, ethnic heterogeneity may affect cytokine secretion patterns during Mtb infection.Citation26,Citation27 Therefore, the generalizability of our findings to diverse populations and patients on different treatment regimens may be limited. Moreover, healthy controls were all young subjects and were not matched to the other subjects, which may affect the cytokine production. Additionally, we only focused on Mtb infection and did not evaluate cytokine/chemokine secretion in NTM patients. Including NTM patients as a control group in future investigations can provide a comprehensive analysis, considering the potential overlap in immune responses between Mtb and NTM infections.
Conclusion
Non-specific cytokine secretion in tuberculosis cannot distinguish between febrile and ATB patients. Latency-associated antigens can enhance the diagnostic capability of multiple cytokines for tuberculosis infection, and their combined use with tuberculosis virulence factors can significantly improve the ability of Th1-type cytokines to differentiate between ATB and LTBI. Under stimulation with tuberculosis virulence factors and latency-associated antigens, the detection of cytokines can achieve a comprehensive diagnosis of multiple tuberculosis infection states.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Peking Union medical College Hospital (ZS-3415). All eligible ATB patients, febrile patients, healthy controls and LTBI healthcare workers provided written informed consent according to institutional requirements. The study completely followed the guiding principles in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- World Health Organization. Global Tuberculosis Report 2022. World Health Organization; 2022.
- Petruccioli E, Vanini V, Chiacchio T, et al. Analytical evaluation of QuantiFERON- Plus and QuantiFERON- Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis. 2017;106:38–43. doi:10.1016/j.tube.2017.06.002
- Kang WL, Wang G-R, Wu M-Y, et al. Interferon-gamma release assay is not appropriate for the diagnosis of active tuberculosis in high-burden tuberculosis settings: a retrospective multicenter investigation. Chin Med J. 2018;131(3):268–275. doi:10.4103/0366-6999.223860
- Meier NR, Jacobsen M, Ottenhoff THM, et al. A systematic review on novel mycobacterium tuberculosis antigens and their discriminatory potential for the diagnosis of latent and active tuberculosis. Front Immunol. 2018;9:2476. doi:10.3389/fimmu.2018.02476
- Zhao HM, Du R, Li C-L, et al. Differential T cell responses against DosR-associated antigen Rv2028c in BCG-vaccinated populations with tuberculosis infection. J Infect. 2019;78(4):275–280. doi:10.1016/j.jinf.2018.10.016
- Zellweger JP, Sotgiu G, Corradi M, et al. The diagnosis of latent tuberculosis infection (LTBI): currently available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med Lav. 2020;111(3):170–183. doi:10.23749/mdl.v111i3.9983
- Zhang L, Ma H, Wan S, et al. Mycobacterium tuberculosis latency-associated antigen Rv1733c SLP improves the accuracy of differential diagnosis of active tuberculosis and latent tuberculosis infection. Chin Med J. 2022;135(1):63–69. doi:10.1097/CM9.0000000000001858
- Vasil’eva EV, Verbov VN, Ivanovskiĭ VB, et al. [Combined determination of spontaneous and antigen-induced production of cytokines for differential diagnostics of active tuberculosis of lungs and latent tuberculosis infection]. Zh Mikrobiol Epidemiol Immunobiol. 2013;4:77–85.
- Adankwah E, Nausch N, Minadzi D, et al. Interleukin-6 and Mycobacterium tuberculosis dormancy antigens improve diagnosis of tuberculosis. J Infect. 2021;82(2):245–252. doi:10.1016/j.jinf.2020.11.032
- Bhatt P, Sharma M, Sharma PP, et al. Mycobacterium tuberculosis dormancy regulon proteins Rv2627c and Rv2628 as Toll like receptor agonist and as potential adjuvant. Int Immunopharmacol. 2022;112:109238.
- Kanaparthi KJ, Afroz S, Minhas G, et al. Immunogenic profiling of Mycobacterium tuberculosis DosR protein Rv0569 reveals its ability to switch on Th1 based immunity. Immunol Lett. 2022;242:27–36. doi:10.1016/j.imlet.2022.01.001
- Takeda K, Nagai H, Suzukawa M, et al. Comparison of QuantiFERON-TB Gold Plus, QuantiFERON-TB Gold In-Tube, and T-SPOT.TB among patients with tuberculosis. J Infect Chemother. 2020;26(11):1205–1212. doi:10.1016/j.jiac.2020.06.019
- Wang F, Hou H, Xu L, et al. Mycobacterium tuberculosis-specific TNF-α is a potential biomarker for the rapid diagnosis of active tuberculosis disease in Chinese population. PLoS One. 2013;8(11):e79431. doi:10.1371/journal.pone.0079431
- Whitworth HS, Badhan A, Boakye AA, et al. Clinical utility of existing and second-generation interferon-γ release assays for diagnostic evaluation of tuberculosis: an observational cohort study. Lancet Infect Dis. 2019;19(2):193–202. doi:10.1016/S1473-3099(18)30613-3
- Hasan Z, Cliff JM, Dockrell HM, et al. CCL2 responses to Mycobacterium tuberculosis are associated with disease severity in tuberculosis. PLoS One. 2009;4(12):e8459. doi:10.1371/journal.pone.0008459
- Domingo-Gonzalez R, Prince O, Cooper A, et al. Cytokines and chemokines in mycobacterium tuberculosis infection. Microbiol Spectr. 2016;4(5). doi:10.1128/microbiolspec.TBTB2-0018-2016
- Ruhwald M, Bjerregaard-Andersen M, Rabna P, et al. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res Notes. 2009;2:19. doi:10.1186/1756-0500-2-19
- Hong JY, Jung GS, Kim H, et al. Efficacy of inducible protein 10 as a biomarker for the diagnosis of tuberculosis. Int J Infect Dis. 2012;16(12):e855–9. doi:10.1016/j.ijid.2012.07.013
- Liang Y, Wang Y, Li H, et al. Evaluation of a whole-blood chemiluminescent immunoassay of IFN -γ, IP −10, and MCP −1 for diagnosis of active pulmonary tuberculosis and tuberculous pleurisy patients. Apmis. 2016;124(10):856–864. doi:10.1111/apm.12583
- Clifford V, Tebruegge M, Zufferey C, et al. Mycobacteria-specific cytokine responses as correlates of treatment response in active and latent tuberculosis. J Infect. 2017;75(2):132–145. doi:10.1016/j.jinf.2017.04.011
- Akashi S, Suzukawa M, Takeda K, et al. IL-1RA in the supernatant of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus is useful for discriminating active tuberculosis from latent infection. J Infect Chemother. 2021;27(4):617–624. doi:10.1016/j.jiac.2020.11.023
- Balcells ME, Ruiz-Tagle C, Tiznado C, et al. Diagnostic performance of GM-CSF and IL-2 in response to long-term specific-antigen cell stimulation in patients with active and latent tuberculosis infection. Tuberculosis. 2018;112:110–119. doi:10.1016/j.tube.2018.08.006
- Dirix V, Collart P, Van Praet A, et al. Immuno-diagnosis of active tuberculosis by a combination of cytokines/chemokines induced by two stage-specific mycobacterial antigens: a pilot study in a low TB incidence country. Front Immunol. 2022;13:842604. doi:10.3389/fimmu.2022.842604
- Wawrocki S, Seweryn M, Kielnierowski G, et al. IL-18/IL-37/IP-10 signalling complex as a potential biomarker for discriminating active and latent TB. PLoS One. 2019;14(12):e0225556. doi:10.1371/journal.pone.0225556
- Djoba Siawaya JF, Chegou NN, Heuvel MMVD, et al. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine. 2009;47(2):132–136. doi:10.1016/j.cyto.2009.05.016
- Coussens AK, Wilkinson RJ, Nikolayevskyy V, et al. Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathog. 2013;9(7):e1003468. doi:10.1371/journal.ppat.1003468
- Chegou NN, Sutherland JS, Malherbe S, et al. Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax. 2016;71(9):785–794. doi:10.1136/thoraxjnl-2015-207999
