Abstract
Purpose
We investigated the Epidemiology, risk factors and outcomes of Candida bloodstream infection.
Methods
The electronic laboratory records data of patients with candidemia (2015–2022) were collected. We used univariate and multivariate logistic regression to determine the risk factors of candidemia.
Results
Of the 134 patients with candidemia, the most prevalent species were Candida albicans (37.2%), followed by Candida glabrata (27.7%), Candida parapsilosis (18.9%), and others. The mean annual incidence was 0.33/1000 admissions. The overall resistance rate of Candida spp. against fluconazole and voriconazole were 4.9% (7/142) and 5.9% (6/101), while Candida tropicalis showed high resistance to fluconazole (38.8%) and voriconazole (27.8%). The 30-day mortality rate was 32.8%. On multivariate analysis, age ≥ 65 (odds ratio [OR] = 3.874, 95% confidence interval [CI]: 1.146, 13.092; P = 0.029), high Acute Physiology and Chronic Health Evaluation II (APACHE II) score (OR = 12.384, 95% CI: 2.963, 51.762; P = 0.001), shock (OR = 3.428, 95% CI: 1.097, 10.719; P = 0.034), initial antifungal therapy (OR = 0.057, 95% CI: 0.011, 0.306; P = 0.001) and White blood cells (OR = 1.129, 95% CI: 1.016, 1.255; P = 0.024) were the independent risk factors with mortality within 30 day in patients with candidemia.
Conclusion
The incidence rate and the mortality rate of candidemia are high, and lower azole susceptibility was found in Candida tropicalis. Age≥65 years, Shock, high APACHE II score, Antifungal therapy and White blood cells count were independently associated with 30-day mortality.
Introduction
Candidemia is a common healthcare-associated bloodstream infection that leads to longer hospital stays, increased healthcare costs, and high mortality rate.Citation1–3 The hospital days increased from 4.1 to 5.5 days, and the medical costs increased from $10,755 to $14,479 per patient attributed to Candidemia, when compared with Gram-negative bacterial bloodstream infection.Citation4 The mortality rate within 30 days among patients with Candida bloodstream infection was 25.6–56.7%.Citation5–7
Among Candida spp, Candida albicans is reported in a high proportion worldwide, but a shift towards non-albicans Candida spp. has been observed.Citation8 A recent systematic study in China reported the similar conclusions.Citation9
Some literatures had shown that the complicated surgery, hemodialysis, prolonged use of central venous catheters (CVC), administration of total parenteral nutrition (TPN), undergoing mechanical ventilation, immunosuppressive therapies (eg chemotherapy, corticosteroids), and exposure to broad-spectrum antibacterial agents, neutropenia, prior fungal colonization and intensive care unit (ICU) admission were the risk factors for candidemia.Citation10–13 Several retrospective studies suggested that ICU admission, underlying diseases and comorbidities, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, multiple invasive interventions and treatments were associated with increased mortality.Citation11,Citation14–16
The forming of biofilms for Candida spp. is a crucial virulence factor, which can significantly reduce its susceptibility to antifungal drugs and weakens the host immune response, which makes candidemia a great threat to the Public Health System with serious outcomes.Citation17,Citation18 Due to the lack of specific clinical manifestations, early diagnosis and treatment are usually absent. However, the species distribution and susceptibility patterns of candidemia can vary with geographic region and time. Therefore, the local epidemiology, variable antifungal susceptibility and the potential risk factors of mortality are critical for appropriate antifungal therapy and treatment measures.
In this study, we retrospectively analyzed the epidemiology, clinical characteristics, species distribution of infection and antifungal drug susceptibility from 148 patients with candidemia at our hospital between 2015 and 2022. And we aimed to identify the potential risk factors associated with 30-day mortality in candidemia patients.
Material and Methods
Patients and Study Design
A single-center, retrospective observational study was undertaken from 1 January 2015 to 31 December 2022 at the Beijing Shijitan Hospital with over 1000 beds currently. The study was done in patients with culture-confirmed candidemia, the clinical data were collected from the electronic records. And the study was approved by the ethics committees of the Beijing Shijitan Hospital (Grant No. 2019–29). Since all the data were obtained from the hospital laboratory as routine work and not for this study, the informed consent was waived by the ethics committee. Patient details were anonymized before data analysis. And the research was conducted in accordance with the Declaration of Helsinki.
Definitions
An episode of candidemia was defined as the isolation of a Candida spp. from at least one blood culture in a patient presenting with clinical signs and symptoms. The onset of candidemia was defined as the day, when the first positive blood culture for Candida spp. was drawn from the patient. With the exception of recent surgery (surgery within 3 months), the predisposing factors occurred within 30 days prior to the onset of candidemia. Antifungal therapy is defined as empiric treatment (preferred azole agents) that is initiated before the patient has obtained the results of a drug susceptibility test. The severity of illness was assessed by APACHE II score. The outcome was registered after 30 days from the onset of candidemia.
Microbiological Procedures and Antifungal Susceptibility Testing
Blood samples were cultured with Bactec-9120 and Bactec-FX200 systems (Becton Dickinson, Sparks, MD). Positive blood cultures were plated on the Columbia blood and Sabouraud Dextrose agar, and then incubated aerobically at 37°C for up to 48 h. Candida species were identified by VITEK-2 Compact system (bioMerieux, Marcyl’Etoile, France) or matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Microflex LT, Bruker Daltonics, Bremen, Germany). And the antifungal susceptibility test for fluconazole and voriconazole was determined by the ATB FUNGUS 3 (BioMérieux) strip following the manufacturer’s instructions. The susceptibility to fluconazole and voriconazole was evaluated according to the clinical breakpoints of the Clinical Laboratory Standards Institute M60.Citation19
Statistical Analysis
The SPSS statistical software (ver. 24.0, SPSS Inc., Chicago, IL, USA) was used to analyze the data. The continuous variables are presented as the median ± standard deviation (SD); categorical variables are expressed as a number and percentages. For continuous variables, we used Student’s t-test, and Chi-square test or Fisher exact test was used for categorical variables. Using the Kaplan–Meier survival analysis, we compared the patients receiving and not receiving antifungal therapy. Multiple logistic regressions were performed to determine independent risk factors associated with mortality within 30 days. P≤0.05 was considered statistically significant.
Results
Study Participants and Incidence Rates
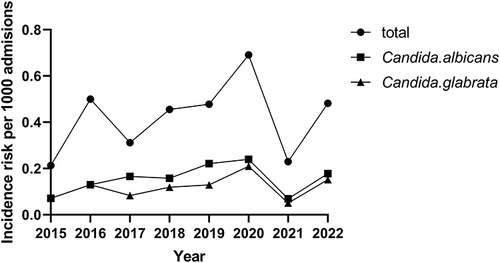
During the entire study period, a total of 148 episodes of candidemia were identified. 14 episodes were excluded, because of insufficient clinical information. Thus, 134 episodes were used to estimate the risk of candidemia in the hospital. The total candidemia incidence rate was 0.33 cases per 1000 admissions. We found a rise in candidemia incidence from 2015 to 2022 (from 0.21 to 0.48), except for 2021 ().
Species Distribution
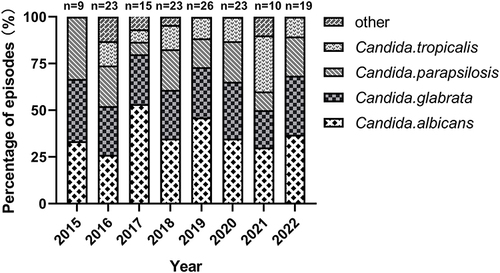
The Candida albicans (55/148; 37.2%) was the most common species causing candidemia, followed by Candida glabrata (41/148, 27.7%), Candida parapsilosis (28/148, 18.9%), Candida tropicalis (18/148, 12.2%), and other Candida spp. (6/148, 4.1%). More than half of all the isolates were Candida non-albicans species. And Candida glabrata accounted for 44.1% of non-albicans species. The trend in the Candida spp. distribution of candidemia from 2015 to 2022 is shown in .
Antifungal Susceptibility Testing
A total of 142 isolates were tested for the susceptibility to fluconazole and voriconazole (). Isolates identified as Candida famata (n = 2), Candida lusitaniae (n = 2), Candida guilliermondii (n=1), and Candida dubliniensis (n = 1) were excluded from this analysis, because of the lack of specific clinical breakpoints. The resistance rates of Candida spp. strains against fluconazole and voriconazole were 4.9% (7/142) and 5.9% (6/101), respectively. Candida tropicalis strains showed high resistance to fluconazole (7/18, 38.8%) and voriconazole (5/18, 27.8%). The susceptibility to fluconazole for Candida albicans, Candida glabrata and Candida parapsilosis strains was high, and no fluconazole resistant isolate was found. Candida albicans and Candida parapsilosisstrains showed the most susceptible to voriconazole, and only one Candida albicans isolate showed voriconazole resistance.
Table 1 Antifungal Susceptibility of Candida Spp. Isolated
Clinical Characteristics of Patients with Candidemia
A total of 134 patients were included in the analysis. And the 30-day mortality rate was 32.8% (44/134). The basic characteristics of patients and their outcomes are shown in . The mean age of all patients was 66.8 years (range, 1–100 years) and elderly patients (age ≥ 65) accounted for 56.0% of the sample. And 58.2% (78/134) of these patients were male. The mean hospital staying time of these patients before the occurrence of candidemia was 41.9 ± 54.4 days. For the underlying diseases, a high proportion was enumerated for solid tumor (65.7%), followed by pulmonary disease (53.7%), cardiovascular disease (49.3%), diabetes mellitus (35.1%), neurologic diseases (23.1%) and shock (23.1%). The median APACHE II score was 16.9 ± 8.3. CVC (132/134, 98.5%) was the most common invasive procedure, followed by TPN (100/134, 74.6%) and urinary catheter (89/134, 66.4%). Before the candidemia was detected,133 (99.3%) of these patients had broad-spectrum antibiotic use. When candidemia is detected, 114 (85.1%) of these patients started to take the appropriate antifungal therapy.
Table 2 Overall Characteristics and Univariate Analysis of Mortality in Patients with Candidemia
Risk Factors for Mortality from Candidemia
We used the univariate analysis to screen the risk factors of candidemia (). Age (P < 0.001), pulmonary disease (P = 0.019), diabetes mellitus (P = 0.004), shock (P < 0.001), APACHE II score (P < 0.001), antifungal therapy (P = 0.001), C-reactive protein (P = 0.021) and White blood cells (P < 0.001) were significantly related to mortality.
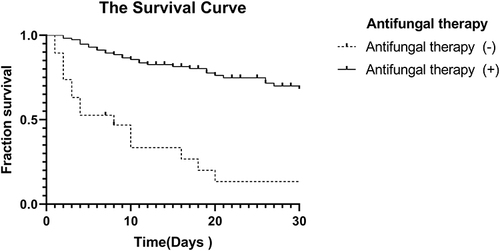
Furthermore, patients receiving and not receiving antifungal therapy was compared by Kaplan–Meier survival analysis (P < 0.001) ().
Figure 3 Kaplan–Meier survival analysis for candidemia. Patients treated with antifungal drugs and patients not treated with antifungal drugs were included (p<0.001).
By multivariate logistic regressions analysis, five independent risk factors for candidemia related 30-day mortality were identified: age ≥ 65 (odds ratio [OR] = 3.874; 95% confidence interval [CI]: 1.146, 13.092; P = 0.029), high APACHE II score (OR = 12.384; 95% CI: 2.963, 51.762; P = 0.001), shock (OR = 3.428; 95% CI:1.097, 10.719; P = 0.034), antifungal therapy (OR = 0.057; 95% CI: 0.011, 0.306; P = 0.001) and White blood cells (OR = 1.129; 95% CI: 1.016, 1.255; P = 0.024) ().
Table 3 Multivariate Analysis of Factors Associated with 30-Day Mortality in Patients with Candidemia
Discussion
In this single-center retrospective study, the clinical characteristics of candidemia episodes of a tertiary-care hospital in the past 8 years were analyzed. The incidence rate was 0.33 cases per 1000 admissions, which was similar to a study,Citation20 higher than another Chinese report.Citation21 We also found that the incidence rate has increased in these years, consistent with the reported studies.Citation6,Citation22
In addition, the species distribution pattern of candidemia varies greatly in different regions of the world. 37.2% of isolated species in our study period were Candida albicans, followed by Candida glabrata, Candida parapsilosis, Candida tropicalis, similar with reports from other regions.Citation21,Citation23,Citation24 However, some Chinese studies showed different results, the incidence of Candida tropicalisCitation6 rather than Candida albicans ranked first in leading candidemia according to their observation.
During the past 20 years, increased drug resistance of Candida spp. to azoles has attracted the attention from the world. In our hospital, only three Candida albicans isolates (5.5%) and one Candida parapsilosis (3.6%) showed intermediate susceptibility. Candida albicans and Candida parapsilosis exhibited excellent susceptibility (>98.0%) to voriconazole, and only one Candida albicans isolate showed voriconazole resistance. These results were similar with previously reports.Citation6,Citation24 But high resistance to fluconazole of Candida glabrata have been reported from the studies conducted in the European and American countries.Citation25 The Candida tropicalis isolates in our study had 38.8% resistance to fluconazole and 27.8% resistance to voriconazole. The frequency of fluconazole resistance was only 20.5% in an Australian study.Citation26 But our result is consistent with the findings of other Chinese studies.Citation5,Citation6,Citation27 The rapid emergence of azole-resistant Candida tropicalis strains was also revealed in China CHIF-NET surveillance.Citation28 So, azoles may be not the appropriate antifungal drugs for empirical treatment of Candida tropicalis, which is consistent with other reports.Citation29,Citation30
High mortality are associated with candidemia. In our study, the 30-day mortality rate of candidemia was 32.8%, which is consistent with some previous reports,Citation31–33 but lower than these studiesCitation22,Citation34 and higher than other Chinese reports.Citation5,Citation6
Univariate analysis revealed that age ≥ 65 years, pulmonary disease, diabetes mellitus, shock, high APACHE II score, antifungal therapy, C-reactive protein and White blood cells count were the factors that affected the mortality within 30 days. However, age ≥ 65 years, shock, high APACHE II score, antifungal therapy and White blood cells count were the independent risk factors of 30-day mortality from candidemia in our study. Consistent with our results, previous studies showed that age ≥ 65 years could be the predictors of high mortality.Citation32,Citation35 Because of low immunity, chronic diseases, and multi-organ failure, elderly patients were susceptible to candidemia. Given rapidly aging societies, age ≥ 65 maybe a more predictive risk factor for mortality in cases with candidemia. Moreover, our study showed that shock was significantly associated with 30-day mortality after candidemia diagnosis. And shock may be considered to be significant predictors for mortality by affecting immunity.Citation22,Citation36 Several previous studies support our findings that a high APACHE II score also can be used as a predictor of high mortality in patients with candidemia.Citation37–39 Our results revealed that the use of appropriate antifungal agents in patients with candidemia is a protective factor, and some studies showed the similar result.Citation40,Citation41 In addition, the finding that White blood cells count is an independent significant predictor of mortality in patients with candidemia is consistent with some studies.Citation33,Citation42 But some studies have had different results: they found that TPN, exposure to broad-spectrum antibacterial agents, Hemodialysis, and arteriovenous catheter were associated with an increased risk of mortality.Citation33,Citation43
Several limitations have showed in our study. First, it was a retrospective study, which might have lead to selection and observational bias and affected the results. Second, it was a single center study, which might have a small sample size and limited information, and then might impact the analysis results. Third, we did not perform the in vitro echinocandin susceptibility test of Candida isolates.
Conclusion
In conclusion, in this 8-year study, the incidence rate of candidemia seemed to have increased, and Candida albicans was the most frequently isolated species. We also found that Candida tropicalis had significantly lower azole susceptibility, so empirical treatment would not recommend using azoles. Moreover, patients with candidemia showed high mortality rate. In our study, we identified five independent risk factors for 30-day mortality in patients with candidemia: age ≥ 65 years, shock, high APACHE II score, antifungal therapy and White blood cells count. In order to further assess the changing epidemiology of candidemia, multi-center prospective studies may be required in future.
Disclosure
The authors declare that there are no conflicts of interest in this article.
Additional information
Funding
References
- Zaoutis TE, Argon J, Chu J, et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: A propensity analysis. Clin Infect Dis. 2005;41(9):1232–1239. doi:10.1086/496922
- Morgan J, Meltzer MI, Plikaytis BD, et al. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol. 2005;26(6):540–547. doi:10.1086/502581
- Hassan I, Powell G, Sidhu M, et al. Excess mortality, length of stay and cost attributable to candidaemia. J Infect. 2009;59(5):360–365. doi:10.1099/0022-1317-51-2-110
- Shorr AF, Gupta V, Sun X, et al. Burden of early-onset candidemia: analysis of culture-positive bloodstream infections from a large U.S. database. Crit Care Med. 2009;37(9):2519–2535. doi:10.1097/CCM.0b013e3181a0f95d
- Zhang W, Song X, Wu H, Zheng R. Epidemiology, risk factors and outcomes of Candida albicans vs. non-albicans candidaemia in adult patients in Northeast China. Epidemiol Infect. 2019;147:e277. doi:10.1017/S0950268819001638
- Ye N, Liu Z, Tang W, et al. Systematic Characterization of Epidemiology, Antifungal Susceptibility, Risk Factors and Outcomes of Candidaemia: A Six-Year Chinese Study. Infect Drug Resist. 2022;15:4887–4888. doi:10.2147/IDR.S378629
- Kutlu M, Sayın-Kutlu S, Alp-çavuş S, et al. Mortality-associated factors of candidemia: a multi-center prospective cohort in Turkey. Eur J Clin Microbiol Infect Dis. 2022;41(4):597–607. doi:10.1007/s10096-021-04394-0
- Schroeder M, Weber T, Denker T, et al. Epidemiology, clinical characteristics, and outcome of candidemia in critically ill patients in Germany: a single-center retrospective 10-year analysis. Ann Intensive Care. 2020;10(1):142. doi:10.1186/s13613-020-00755-8
- Xiao M, Chen SC, Kong F, et al. Distribution and antifungal susceptibility of candida species causing candidemia in china: An Update From the CHIF-NET Study. J Infect Dis. 2020;221(2):S139–S147. doi:10.1007/s00134-014-3400-y
- Ko JH, Jung DS, Lee JY, et al. Poor prognosis of Candida tropicalis among non-albicans candidemia: a retrospective multicenter cohort study, Korea. Diagn Microbiol Infect Dis. 2019;95(2):195–200. doi:10.1016/j.diagmicrobio.2019.05.017
- Doğan Ö, Yeşilkaya A, Menekşe Ş, et al. Effect of initial antifungal therapy on mortality among patients with bloodstream infections with different Candida species and resistance to antifungal agents: a multicentre observational study by the Turkish Fungal Infections Study Group. Int J Antimicrob Agents. 2020;56(1):105992. doi:10.1016/j.ijantimicag.2020.105992
- Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother. 2005;56(1):i5–i11. doi:10.1093/jac/dki218
- Krcmery V, Laho L, Huttova M, et al. Aetiology, antifungal susceptibility, risk factors and outcome in 201 fungaemic children: data from a 12-year prospective national study from Slovakia. J Med Microbiol. 2002;51(2):110–116. doi:10.1099/0022-1317-51-2-110
- Lee YM, Kim DY, Kim YJ, Park KH, Lee MS. Clinical impacts of delayed central venous catheter removal according to the severity of comorbidities in patients with candidaemia. J Hosp Infect. 2019;103(4):420–427. doi:10.1016/j.jhin.2019.08.018
- Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–1122. doi:10.1093/cid/cis021
- Colombo AL, Guimarães T, Sukienik T, et al. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med. 2014;40(10):1489–1498. doi:10.1007/s00134-014-3400-y
- Mitchell KF, Zarnowski R, Sanchez H, et al. Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci U S A. 2015;112(13):4092–4097. doi:10.1073/pnas.1421437112
- Atiencia-Carrera MB, Cabezas-Mera FS, Tejera E, Machado A, Saokaew S. Prevalence of biofilms in Candida spp. bloodstream infections: a meta-analysis. PLoS One. 2022;17(2):e0263522. doi:10.1371/journal.pone.0263522
- Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, M60. Clinical and Laboratory Standards Institute; 2020.
- Lausch KR, Søgaard M, Rosenvinge FS, et al. High incidence of candidaemia in a nationwide cohort: underlying diseases, risk factors and mortality. Int J Infect Dis. 2018;76:58–63. doi:10.1016/j.ijid.2018.08.010
- Li Y, Gu C, Yang Y, et al. Epidemiology, antifungal susceptibility, risk factors, and mortality of persistent candidemia in adult patients in China: a 6-year multicenter retrospective study. BMC Infect Dis. 2023;23(1):369. doi:10.1186/s12879-023-08241-9
- Kim JH, Suh JW, Kim MJ. Epidemiological Trends of Candidemia and the Impact of Adherence to the Candidemia Guideline: six-Year Single-Center Experience. J Fungi. 2021;7(4):275. doi:10.3390/jof7040275
- Toda M, Williams SR, Berkow EL, et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia - Four Sites, United States, 2012-2016. MMWR Surveill Summ. 2019;68(8):1–15. doi:10.15585/mmwr.ss6808a1
- Tsukamoto H, Higashi T, Kodawara T, et al. A longitudinal study of Candida bloodstream infections in a Japanese university hospital: species distribution, drug susceptibility, clinical features, and mortality predictors. Eur J Clin Microbiol Infect Dis. 2022;41(11):1315–1325. doi:10.1007/s10096-022-04499-0
- Castanheira M, Deshpande LM, Messer SA, Rhomberg PR, Pfaller MA. Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination. Int J Antimicrob Agents. 2020;55(1):105799. doi:10.1016/j.ijantimicag.2019.09.003
- Keighley C, Gall M, van Hal SJ, et al. Whole genome sequencing shows genetic diversity, as well as clonal complex and gene polymorphisms associated with fluconazole non-susceptible isolates of candida tropicalis. J Fungi. 2022;8(9):896. doi:10.3390/jof8090896
- Liu WL, Huang YT, Hsieh MH, et al. Clinical characteristics of Candida tropicalis fungaemia with reduced triazole susceptibility in Taiwan: a multicentre study. Int J Antimicrob Agents. 2019;53(2):185–189. doi:10.1016/j.ijantimicag.2018.10.015
- Fan X, Xiao M, Liao K, et al. Notable Increasing Trend in Azole Non-susceptible Candida tropicalis Causing Invasive Candidiasis in China Molecular Epidemiology and Clinical Azole Consumption. Front Microbiol. 2017;8:464. doi:10.3389/fmicb.2017.00464
- Liu F, Zhong L, Zhou F, et al. Clinical Features, Strain Distribution, Antifungal Resistance and Prognosis of Patients with Non-albicans Candidemia: a Retrospective Observational Study. Infect Drug Resist. 2021;14:3233–3246. doi:10.2147/IDR.S323583
- Ngamchokwathana C, Chongtrakool P, Waesamaae A, Chayakulkeeree M. Risk Factors and Outcomes of Non-albicans Candida Bloodstream Infection in Patients with Candidemia at Siriraj Hospital-Thailand’s Largest National Tertiary Referral Hospital. J Fungi. 2021;7(4):269. doi:10.3390/jof7040269
- Bourassa-Blanchette S, Biesheuvel MM, Lam JC, et al. Incidence, susceptibility and outcomes of candidemia in adults living in Calgary. BMC Infect Dis. 2023;23(1):100. doi:10.1186/s12879-023-08050-0
- Meyahnwi D, Siraw BB, Reingold A. Epidemiologic features, clinical characteristics, and predictors of mortality in patients with candidemia in Alameda County, California; a 2017-2020 retrospective analysis. BMC Infect Dis. 2022;22(1):843. doi:10.1186/s12879-022-07848-8
- Qiao Y, Tao Z, Hao F, et al. Epidemiological Characteristics, Antifungal Susceptibility, Risk Factors, and Outcomes of Candida Bloodstream Infection: a Ten-Year Surveillance in a Teaching Hospital in China. Infect Drug Resist. 2023;16:4769–4778. doi:10.2147/IDR.S411283
- El Zakhem A, El Eid R, Istambouli R, Tamim H, Kanj SS. The Utility of EQUAL Candida Score in Predicting Mortality in Patients with Candidemia. J Fungi. 2022;8(3):238. doi:10.3390/jof8030238
- Kato H, Yoshimura Y, Suido Y, et al. Mortality and risk factor analysis for Candida blood stream infection: a multicenter study. J Infect Chemother. 2019;25(5):341–345. doi:10.1016/j.jhin.2019.08.018
- Poissy J, Damonti L, Bignon A, et al. Risk factors for candidemia: a prospective matched case-control study. Crit Care. 2020;24(1):109. doi:10.1186/s13054-020-2766-1
- Nucci M, Anaissie E, Betts RF, et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis. 2010;51(3):295–303. doi:10.1086/653935
- Grim SA, Berger K, Teng C, et al. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother. 2012;67(3):707–714. doi:10.1093/jac/dkr511
- Nucci M, Braga PR, Nouér SA, Anaissie E. Time of catheter removal in candidemia and mortality. Braz J Infect Dis. 2018;22:455–461. doi:10.1016/j.bjid.2018.10.278
- Tedeschi S, Tumietto F, Giannella M, et al. Epidemiology and outcome of candidemia in internal medicine wards: a regional study in Italy. Eur J Intern Med. 2016;34:39–44. doi:10.1016/j.ejim.2016.08.020
- Zheng YJ, Xie T, Wu L, et al. Epidemiology, species distribution, and outcome of nosocomial Candida spp. bloodstream infection in Shanghai: an 11-year retrospective analysis in a tertiary care hospital. Ann Clin Microbiol Antimicrob. 2021;20(1):34. doi:10.1186/s12941-021-00441-y
- Gao Y, Tang M, Li Y, et al. Machine-learning based prediction and analysis of prognostic risk factors in patients with candidemia and bacteraemia: a 5-year analysis. PeerJ. 2022;10:e13594. doi:10.7717/peerj.13594
- Fernández-Ruiz M, Puig-Asensio M, Guinea J, et al. Candida tropicalis bloodstream infection: incidence, risk factors and outcome in a population-based surveillance. J Infect. 2015;71(3):385–394. doi:10.1016/j.jinf.2015.05.009