Abstract
Purpose
To analyze the factors affecting patients’ prognoses based on the community acquired-bloodstream infection patient data from 2017 to 2021.
Patients and Methods
The data of 940 patients were retrieved, having at least one positive bilateral blood culture within 48 hours of hospitalization, and grouped into survivor and non-survivor groups. The clinical characteristics, laboratory results, causative pathogen and other indicators were collected and compared, and risk factors were identified by applying Cox proportional hazard regression model to the data.
Results
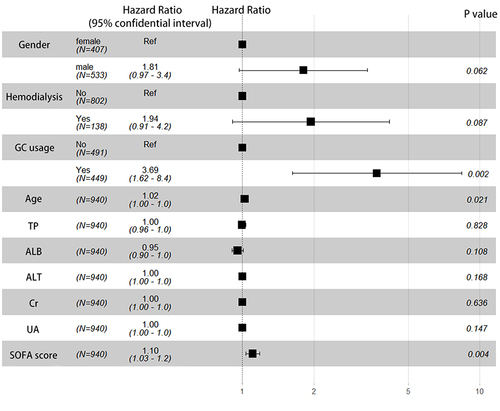
Community acquired-bloodstream infection is most commonly caused by Escherichia coli, Klebsiella species and Staphylococcus hominis. Among the total of 940 selected patients, 52 (5.5%) died during hospitalization. The demographic parameters like age and gender, clinical protocols like maintenance hemodialysis, glucocorticoid use during hospitalization, catheter placement, procaicitonin, total protein, albumin, creatinine, uric acid contents and Sequential Organ Failure Assessment scores were significantly different between the survivor and non-survivor groups. The survival analysis results revealed that age (HR=1.02, 95% CI: 1.00–1.05, P=0.002), glucocorticoid use during hospitalization (HR=3.69, 95% CI: 1.62–8.37, P=0.021) and Sequential Organ Failure Assessment score (HR=1.10, 95% CI: 1.03–1.18, P=0.004) might be the risk factors affecting 30-day mortality in patients with community acquired-bloodstream infection.
Conclusion
The identified risk factors may help guide clinical treatment protocol for patients with community acquired-bloodstream infection, providing more effective treatment strategy selection with improved clinical outcomes.
Introduction
Bloodstream infection (BSI) is a systemic disease caused by viable bacteria and fungi reaching the systemic circulation and is associated with a worse prognosis than any other infectious disease and is considered a challenging economic burden.Citation1 The BSI is also a common factor contributing to hospitalization and death in geriatrics.Citation2,Citation3 Community acquired-bloodstream infection (CA-BSI) is a widespread global disease, with an average annual incidence rate of 0.82%Citation4,Citation5 and an incidence rate increase from 16.7 to 38.1/100,000 people/year in South and Southeast Asian countries from 2004 to 2010 according to reports.Citation6 Since such a trend has proliferated, the CA-BSI has become a serious global public health concern,Citation7 yet it has received scarce attention from researchers. Although community healthcare services, medical technology, and public awareness have improved, and patients with chronic diseases now have better survival rates, the use of maintenance hemodialysis and invasive surgical procedures has increased.Citation8 Furthermore, geriatrics are more prone to BSI owing to compromised immune systems, where irrational use of empirical antibiotics and delayed treatment interventions deteriorate the poor prognosis of BSI.Citation9 In contrast, rational use of antibiotics can improve the prognosis and significantly reduce morbidity and mortality rates in BSI patients.Citation10–14 However, most of the published literature on BSI has been limited to the distribution of pathogenic bacteria in BSI, while studies focusing on the influencing factors on patient prognosis are rare. Therefore, this study aimed to analyze the factors affecting patients’ prognoses based on the CA-BSI patient data from 2017 to 2021.
Materials and Methods
Study Design
The data of 940 CA-BSI patients were retrieved from the electronic medical record system of the University of South China-affiliated Changsha Central Hospital, Hunan, China, who were admitted between July 2017 to March 2021, with a 30-days following hospitalization marked as a study endpoint. University of South China-affiliated Changsha Central Hospital is a comprehensive hospital integrating medical treatment, emergency rescue, health care, rehabilitation, scientific research, and teaching. It has 2363 beds and receives an average of over 2300 inpatients and nearly 4000 outpatients per day. Therefore, CA-BSI cases in University of South China-affiliated Changsha Central Hospital reflect the epidemiological trends and disease characteristics of CA-BSI throughout Changsha City. Because of the retrospective nature of the study, patient consent for inclusion was waived by Changsha Central Hospital Institutional Review Board (IRB# 201919).
Inclusion Criteria
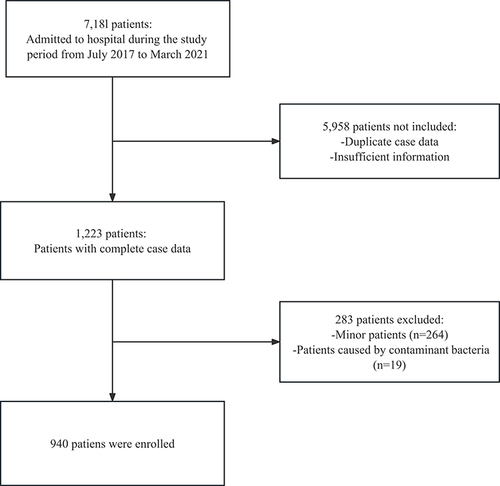
The study only included patients who either had CA-BSI or acquired it within 48 hours of hospitalization, had their first blood culture come back positive, and were diagnosed with BSI according to standardized diagnostic criteria.Citation15 Patients who were identified as infected with Diphtheria bacillus, Propionibacterium, and Micrococcus, patients under 18 years of age, patients suffering from polymicrobial bacteremia, and patients who were directly transferred from other hospitals for more than 48 hours were excluded from the study. The inclusion flow chart of this study is shown in .
Data Collection
The general clinical data of the patients were collected and recorded, including age, gender, lifestyle (smoking history and drinking), and comorbidities (hypertension (HBP), diabetes mellitus, tumor), while the laboratory data included white blood cell (WBC) count, red blood cell (RBC) count, platelets (PLT), neutrophilic granulocyte (NE) count, lymphocyte (LYM) count, hemoglobin (Hb), eosinophil (EOS) count, basophil count (BAS), procalcitonin (PCT), total protein (TP), albumin (ALB), globulin (GLB), glutamic pyruvic transaminase (ALT), glutamic oxaloacetic transaminase (AST), uric acid contents (UA), creatinine (Cr) after admission.
Similarly, other indicators comprised blood transfusion, maintenance hemodialysis, glucocorticosteroid (GC) usage during hospitalization, catheters placement (peripherally inserted central venous catheters), antibiotic combination, invasive operations, immunosuppressive drugs usage within two months, history of BSI within three months, other systemic infections within three months, skin injury within one month, use of antibiotics within three months, Sequential Organ Failure Assessment (SOFA) scores.
The patient’s prognosis was based on the status at the time of discharge from the hospital, and 30 days after hospitalization was considered an endpoint of the study. All patients were divided into two groups, non-survivors who died during hospitalization, and survivors who showed improvement after treatment and had good clinical outcomes following hospital discharge.
Bacteria and Drug Sensitivity Test
Venous blood samples was collected after the patients were admitted to the hospital and before the anti-infection treatment. Bacterial growth was monitored by automatic blood culture instrument (type BACTECTMFX40, BD, USA) and the identification of pathogenic bacteria was carried out by WalkAway96Plus automatic microbial identifier (BioMérieux, France). Drug sensitivity test was performed by Kirby-Bauerdiscagar diffusion method (OXOID, UK). Quality control strains (ATCC Strain Conservation Center) ATCC700603 Klebsiella pneumoniae, ATCC25923 Staphylococcus aureus, ATCC25922 Escherichia coli.
Statistical Analysis
All data were statistically analyzed using R software (version 4.2.2). Samples with insufficient data or lacking outcome information were disregarded. Variables with missing values constituting 20% or less of the total were addressed through multiple imputations; however, if the percentage of missing data exceeded this threshold, the variable was eliminated from the analysis. Descriptive statistics were used to show the baseline demographic information of patients. Categorical variables are presented as the number of patients and the percentage and were compared using Chi-square or Fisher’s exact test when appropriate. The numerical results are presented as mean ± standard deviation or medians (25%; 75% interquartile) and compared using Mann–Whitney U or Wilcoxon rank-sum test. Univariate analysis was performed with the Kaplan-Meier and Log rank tests. Factors with P<0.05 at univariate analysis were adopted for multivariable analysis to identify factors associated with poor prognosis of CA-BSI patients. The P<0.05 was considered statistically significant.
Results
Causative Pathogen
CA-BSI is most commonly caused by Gram-negative bacteria, 21.38% of CA-BSI were caused by Escherichia coli (n=201), 12.12% by Klebsiella pneumoniae (n=114). For Gram-positive bacteria, 9.88% of CA-BSI were caused by Staphylococcus hominis (n=93), 9.88% by Staphylococcus epidermidis (n=93). Fungi were responsible for 4.26% of CA-BSI cases (n=40), as shown in .
Table 1 Causative Pathogen of CA-BSI Patients
Patient Characteristics of Survivors and Non-Survivors
Among the selected 940 patients, 407 were females while 533 were males. Among all, 52 patients had poor prognoses comprising 14 female and 38 male patients. The male-to-female proportion was significantly higher compared to the good prognosis group. The median patient age in the good prognosis group was 65 (54, 75), which was significantly higher (73.00 (62.00–81.25)) in the poor prognosis group, respectively. Similarly, a significant difference was observed between the two groups for treatment during hospitalization regarding on maintenance hemodialysis, higher rate of glucocorticoid use, and catheter placement ().
Table 2 Demographic Characteristics Between Good Prognosis Group and Poor Prognosis Group
The poor prognosis group patients showed a higher PCT, lower ALB and TP, higher Cr and UA for laboratory parameters. The SOFA scores were significantly higher in the poor prognosis compared to the good prognosis group ().
Table 3 Laboratory Indicators Between the Good Prognosis Group and Poor Prognosis Group
Univariate and Multivariate Cox Regression Analyses of 30-Day Mortality in CA-BSI Patients
The results of univariate and multivariate Cox regression analysis are presented in . Univariate analysis result showed that gender, age, maintenance hemodialysis, GC use during hospitalization, SOFA score, TP, ALB, ALT, Cr and UA may have influence on CA-BSI patients’ outcomes. Survival analysis showed that three parameters remained significant after including variables in the multivariate analysis (P<0.05), these parameters were found to be associated with a higher risk of poor prognosis in patients with CA-BSI, ie, age (HR=1.02, 95% CI: 1.00–1.05), glucocorticoid use during hospitalization (HR=3.69, 95% CI: 1.62–8.37) and SOFA score (HR=1.10, 95% CI: 1.03–1.18), as shown in .
Table 4 Univariate and Multivariate Cox Regression Analyses of 30-Day Mortality in CA-BSI Patients
Discussion
The BSI is a life-threatening infection frequently appearing during clinical treatment in the hospital, is characterized by a high mortality rate due to delayed or irrational use of antibiotics which often negatively contributes to patients’ prognosis.Citation16,Citation17 Coagulase negative staphylococci such as Staphylococcus epidermidis and Staphylococcus haemolyticus are usually considered as skin commensal bacteria. Besides their play role in maintaining homeostasis, pathogenicity appears when the body’s protective barriers are broken down, like infections and skin injuries. Coagulase negative staphylococci account for an increasing proportion of hospital-acquired infections.Citation18 CA-BSI patients who were infected by them in this study was not insignificant either. Among the 940 patients in this study, 52 died at the 30-day outcome stage, accounting for 5.5% of the whole, significantly lower than the mortality rate reported by Kanoksil and Dat.Citation6,Citation19 Many factors influence the prognosis of patients with BSI, not simply the medial or regional differences but also the population habits in different areas and comorbidities during hospitalization, such as poor appetite, heart failure, ambulatory status, and patients’ negative psychological status.Citation2 There was a significant difference in gender ratio between the good and poor prognosis groups, with a higher proportion of male patients in the poor prognosis group, which was consistent with the finding of Li.Citation20 Similarly, patients on maintenance hemodialysis and had catheter placement had a higher risk of poor prognosis, consistent with previous studies.Citation21,Citation22 Additionally, the patients in the poor prognosis group had a lower ALB and TP, while PCT, Cr and UA were higher.
The results of multivariate Cox regression showed that use of GC during hospitalization, SOFA score and age were contributing factors affecting CA-BSI patients’ prognosis, which is also in line with previous studies.Citation2,Citation23,Citation24 The patient’s mean age in the poor prognosis group was higher, indicating geriatrics are more prone to die of BSI compared to the young population,Citation25 which could be due to chronic comorbidities like hypertension, diabetes, cardiac disease, compromised immunity, digestion ability, poor nutritional status, and healthcare-associated BSI related to complex medical care of aged patients in the community setting.Citation8,Citation26–28 BSI in geriatrics is more likely to be infected by MDR bacteria, making its treatment challenging and costly with prolonged treatment cycles, which can severely damage the patients’ physical, psychological, and economic status of the patients. It is advocated that CA-BSI be intervened through primary, secondary, and tertiary preventive measures. The primary measures include public awareness campaigns for improving personal and family hygiene and cleaning and disinfecting the environment. The secondary preventive measures include establishing geriatrics health records, conducting regular health examinations and assessments for prompt identification and intervention in patients’ infection risk factors, such as malnutrition and chronic diseases, strengthening pathogenic surveillance to ensure timely disease detection, and establishing a system for rational use of antibiotics. While the tertiary preventive measures include improving diagnosis, treatment, and management of BSI in geriatrics and ensuring timely and effective treatment. Modify the medication regimen based on the individual patient’s circumstances to prevent unnecessary use may potentially decrease the dosage and duration of glucocorticoid therapy. Enhance clinical surveillance of patients at high risk and promptly modify treatment plans as needed. Additionally, enhance the proficiency and expertise of healthcare professionals in managing high-risk patients to ensure timely and efficient interventions, as well as to enhance understanding of high-risk factors and preventive measures in order to reduce mortality rates.
Nonetheless, SOFA score is a scale used for evaluating multi-organ failure in critically ill patients, aiming to identify the degree and development of organ damage and dysfunction in patients; often used for assessing critically ill ICU patients, disease prognosis prediction, and as a primary endpoint in drug clinical trials. Patients admitted to ICU always suffer worse clinical presentation with complex conditions, Angioni’s study revealed that a higher SOFA score is a significant factor in the poor prognosis of patients with CA-BSI,Citation2 suggesting patients’ condition at admission has a significant effect on the prognosis since higher SOFA score predicts an increased likelihood of critical illness, morbidity and mortality, or prolonged hospital stay.
However, this study encountered limitations, like it fails to provide a more detailed comparison of MDR and non-MDR pathogens, which might have enriched the findings. It was a retrospective study, only identifying risk factors, but failed to provide strong evidence to demonstrate the impact of these factors on patients’ outcomes in the same way prospective studies do. Secondly, this study was restricted to one center in China, which requires further validation for results generalization to other countries.
Conclusion
The study concluded that age, usage of GC during hospitalization and SOFA score significantly influenced CA-BSI patients’ 30-day outcomes. The findings may prove helpful for the clinicians treating CA-BSI patients, identifying the clinical status of patients, providing more effective treatment for high-risk patients, and improving treatment outcomes. However, these factors still require determination by high quality clinical studies.
Abbreviations
BSI, bloodstream infection; CA-BSI, community acquired-bloodstream infection; HBP, hypertension; COPD, chronic obstructive pulmonary disease; MDR, multi-drug resistance; GC, glucocorticoid; SOFA, Sequential Organ Failure Assessment; WBC, white blood cell; RBC, red blood cell count; PLT, platelets; NE, neutrophilic granulocyte count; LYM, lymphocyte count; Hb, hemoglobin; EOS, eosinophil count; PLT, platelets; BAS, basophil count; Cr, creatinine; BAS, basophil count; PCT, procalcitonin; TP, total protein; ALB, albumin; GLB, globulin; ALT, glutamic pyruvic transaminase; AST, glutamic oxaloacetic transaminase; UA, uric acid contents.
Ethics Approval
This study was conducted in accordance with the principles of the Declaration of Helsinki, and was approved by Changsha Central Hospital Institutional Review Board (IRB# 201919). Because of the retrospective nature of the study, patient consent for inclusion was waived. The privacy and rights of the patients included in the study will be adequately protected. The data generated from the medical record data used in this study are anonymized, and the paper data will be destroyed 5 years after the end of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to University of South China-affiliated Changsha Central Hospital for its approval to search its clinical database.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article are not publicly available due to ethical restriction, but are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Hung YP, Lee CC, Ko WC. Effects of inappropriate administration of empirical antibiotics on mortality in adults with bacteraemia: systematic review and meta-analysis. Front Med. 2022;9:869822. doi:10.3389/fmed.2022.869822
- Angioni D, Hites M, Jacobs F, De Breucker S. Predictive factors of in-hospital mortality in older adults with community-acquired bloodstream infection. J Frailty Aging. 2020;9(4):232–237. doi:10.14283/jfa.2019.45
- Yahav D, Eliakim-Raz N, Leibovici L, Paul M. Bloodstream infections in older patients. Virulence. 2016;7(3):341–352. doi:10.1080/21505594.2015.1132142
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501–509. doi:10.1111/1469-0691.12195
- Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7). doi:10.1128/AAC.00355-19
- Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D, Reid SD. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One. 2013;8(1):e54714. doi:10.1371/journal.pone.0054714
- Hubner NO, Dittmann K, Begunk R, Kramer A; Action Group Infection P. Infection control measures and prevalence of multidrug-resistant organisms in non-hospital care settings in northeastern Germany: results from a one-day point prevalence study. J Hosp Infect. 2017;97(3):234–240. doi:10.1016/j.jhin.2017.08.002
- Holmbom M, Giske CG, Fredrikson M, et al. 14-Year survey in a Swedish County reveals a pronounced increase in Bloodstream Infections (BSI). Comorbidity - An independent risk factor for both BSI and mortality. PLoS One. 2016;11(11):e0166527. doi:10.1371/journal.pone.0166527
- Holmbom M, Andersson M, Berg S, et al. Prehospital delay is an important risk factor for mortality in community-acquired bloodstream infection (CA-BSI): a matched case-control study. BMJ Open. 2021;11(11):e052582. doi:10.1136/bmjopen-2021-052582
- Lee CC, Hsieh CC, Yang CY, et al. Short versus long duration antimicrobial treatment for community-onset bacteraemia: a propensity score matching study. Int J Antimicrob Agents. 2019;54(2):176–183. doi:10.1016/j.ijantimicag.2019.05.014
- Pop-Vicas A, Tacconelli E, Gravenstein S, Lu B, D’Agata EM. Influx of multidrug-resistant, gram-negative bacteria in the hospital setting and the role of elderly patients with bacterial bloodstream infection. Infect Control Hosp Epidemiol. 2009;30(4):325–331. doi:10.1086/596608
- Turnidge JD, Kotsanas D, Munckhof W, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2009;191(7):368–373. doi:10.5694/j.1326-5377.2009.tb02841.x
- Zilberberg MD, Nathanson BH, Puzniak LA, Zilberberg NWD, Shorr AF. Inappropriate empiric therapy impacts complications and hospital resource utilization differentially among different types of bacterial nosocomial pneumonia: a cohort study, United States, 2014–2019. Crit Care Explor. 2022;4(4):e0667. doi:10.1097/CCE.0000000000000667
- Al-Ani A, Othman N, Hassali MA, Ibrahim B. Adequacy of empiric antibiotics therapy and its impact on outcomes in adult critically ill sepsis patients: a review. Malays J Med Sci. 2022;29(5):17–23. doi:10.21315/mjms2022.29.5.3
- Ministry of Health P. Hospital infection diagnostic criteria (Trial). Nat Med J China. 2001;05(05):61–67.
- Shilo S, Assous MV, Lachish T, et al. Risk factors for bacteriuria with carbapenem-resistant Klebsiella pneumoniae and its impact on mortality: a case-control study. Infection. 2013;41(2):503–509. doi:10.1007/s15010-012-0380-0
- Aryee A, Rockenschaub P, Gill MJ, Hayward A, Shallcross L. The relationship between clinical outcomes and empirical antibiotic therapy in patients with community-onset Gram-negative bloodstream infections: a cohort study from a large teaching hospital. Epidemiol Infect. 2020;148:e225. doi:10.1017/S0950268820002083
- Argemi X, Hansmann Y, Prola K, Prévost G. Coagulase-negative staphylococci pathogenomics. Int J Mol Sci. 2019;20(5). doi:10.3390/ijms20051215
- Dat VQ, Long NT, Hieu VN, et al. Clinical characteristics, organ failure, inflammatory markers and prediction of mortality in patients with community acquired bloodstream infection. BMC Infect Dis. 2018;18(1):535. doi:10.1186/s12879-018-3448-3
- Li Y, Wu Y, Gao Y, et al. Machine-learning based prediction of prognostic risk factors in patients with invasive candidiasis infection and bacterial bloodstream infection: a singled centered retrospective study. BMC Infect Dis. 2022;22(1):150. doi:10.1186/s12879-022-07125-8
- Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31(8):1811–1817. doi:10.1007/s10096-011-1506-5
- Kalluru S, Eggers S, Barker A, et al. Risk factors for infection with multidrug-resistant organisms in Haryana, India. Am J Infect Control. 2018;46(3):341–345. doi:10.1016/j.ajic.2017.08.021
- Chen Y, Ying S, Jiang L, et al. A novel nomogram for predicting risk factors and outcomes in bloodstream infections caused by Klebsiella pneumoniae. Infect Drug Resist. 2022;15:1317–1328. doi:10.2147/IDR.S349236
- Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664. doi:10.1128/CMR.00002-14
- Sheikh F, Douglas W, Catenacci V, Machon C, Fox-Robichaud AE. Social determinants of health associated with the development of sepsis in adults: a scoping review. Crit Care Explor. 2022;4(7):e0731. doi:10.1097/CCE.0000000000000731
- McKane CK, Marmarelis M, Mendu ML, Moromizato T, Gibbons FK, Christopher KB. Diabetes mellitus and community-acquired bloodstream infections in the critically ill. J Crit Care. 2014;29(1):70–76. doi:10.1016/j.jcrc.2013.08.019
- Gavazzi G, Mallaret MR, Couturier P, Iffenecker A, Franco A. Bloodstream infection: differences between young-old, old, and old-old patients. J Am Geriatr Soc. 2002;50(10):1667–1673. doi:10.1046/j.1532-5415.2002.50458.x
- El Chakhtoura NG, Bonomo RA, Jump RLP. Influence of Aging and Environment on Presentation of Infection in Older Adults. Infect Dis Clin North Am. 2017;31(4):593–608. doi:10.1016/j.idc.2017.07.017