Abstract
Ventilator-associated pneumonia is the most common infection in intensive care unit patients associated with high morbidity rates and elevated economic costs; Pseudomonas aeruginosa is one of the most frequent bacteria linked with this entity, with a high attributable mortality despite adequate treatment that is increased in the presence of multiresistant strains, a situation that is becoming more common in intensive care units. In this manuscript, we review the current management of ventilator-associated pneumonia due to P. aeruginosa, the most recent antipseudomonal agents, and new adjunctive therapies that are shifting the way we treat these infections. We support early initiation of broad-spectrum antipseudomonal antibiotics in present, followed by culture-guided monotherapy de-escalation when susceptibilities are available. Future management should be directed at blocking virulence; the role of alternative strategies such as new antibiotics, nebulized treatments, and vaccines is promising.
Background
Ventilator-associated pneumonia (VAP) is the most common infection among the critically ill and the first cause of antibiotic prescription in intensive care units (ICUs), with an incidence of five to 20 cases per 1,000 mechanical ventilation (MV)-days and a global prevalence of 15.6%Citation1–Citation5 that has not changed significantly despite the implementation of care bundles. Episodes caused by multidrug-resistant (MDR) organisms, such as Pseudomonas aeruginosa are associated with significant attributable mortality;Citation3,Citation6 VAP represents a major clinical and economical problem in critically ill patients due to its associated morbidity, prolonged MV-days, and ICU length of stay (LOS), which translates to elevated health care costs as high as US$40,000 per episode.Citation7,Citation8
P. aeruginosa (with Staphylococcus aureus) is one of the most common bacteria causing VAP,Citation5,Citation9 with a prevalence of approximately 4%,Citation2 and its attributable mortality is as high as 13.5%, even with adequate antibiotic treatment.Citation3 In MDR strains, mortality rises up to 35.8%, and the presence of MDR strains has been identified as an independent predictor of hospital death (adjusted odds ratio [AOR] 1.634, 95% confidence interval [CI]: 1.124–2.374) and is the single strongest predictor of initial inadequate antibiotic therapy (AOR 5.706, 95% CI: 3.587–9.077).Citation5,Citation9 A recent study by Micek et al demonstrated that P. aeruginosa VAP mortality has increased to 41.9%, with increased age and Charlson comorbidity score, inappropriate initial antibiotic therapy, and vasopressor use as independent predictors of mortality.Citation10 Antibiotic resistance has been on the rise in the last decade,Citation5,Citation11–Citation13 which is worrisome since P. aeruginosa is one of the three top microorganisms causing health care respiratory infection and is resistant to carbapenem,Citation14 and, even in patients with early-onset VAP and no risk factors, MDR P. aeruginosa is frequent.Citation15,Citation16 Among known risk factors for MDR P. aeruginosa in MV patients, the most frequent are antimicrobial therapy within 90 days (51.9%) and current hospitalization of more than or equal to 5 days (45.3%).Citation2 Infection by MDR P. aeruginosa is associated with worse outcomes with an excess mortality rate of 12 with a more than twofold increased risk of mortality (relative risk [RR] 2.34, 95% CI: 1.53–3.57) and ICU LOS, compared to susceptible strains.Citation11 In VAP caused by MDR P. aeruginosa,Citation10,Citation17 both prior antibiotic use and delayed effective antibiotic therapy in infection also negatively affect mortality and cost.Citation5,Citation18,Citation19
P. aeruginosa serotypes causing VAP have different behavior; O6 and O11, the most common, are associated with a clinical resolution of 60%, and serotypes O1 and O2, represent less common strains, with higher mortality.Citation16 Vallés et al performed an analysis of pulsed-field electrophoresis on more than 1,700 isolates of P. aeruginosa in ICU patients, identifying different genotypes. Clones that were responsible for colonization (skin, gut, and respiratory) least frequently caused pneumonia, and VAP’s resolution was frequent and uncomplicated. However, clones that were not related to prior colonization were associated with very high mortality rates.Citation20 This observation may be associated with the expression of virulence factors in P. aeruginosa, such as type III secretory proteins.Citation21
Most clonally related isolates caused gastric colonization before skin or respiratory tract colonization, suggesting an association with instillation of tap water used for medication by the oral route. A similar study conducted in two different ICUs in a single hospital in FranceCitation4 identified an MDR clone of P. aeruginosa in the sinks of 12 rooms. As a whole, from 26 cases of colonization/infection by P. aeruginosa, five were related to an exogenous colonization (environmental colonization in four patients and cross-infection in one). These findings emphasize the fact that different risk factors may be implicated depending on whether the clone is from exogenous contamination or carried as endogenous colonization. Therefore, different infection control strategies should be applied to prevent colonization of patients with P. aeruginosa, including strategies to limit the potential of sinks to act as potential reservoirs.
Risk factors
Risk factors for P. aeruginosa in VAP are mainly prior antibiotic exposure and MV longer than 5 days.Citation22–Citation24 Patients with chronic obstructive pulmonary disease and other chronic respiratory diseases may carry endogenous colonization and can develop a severe respiratory infection following intubation and MV. Interestingly, risk factors in patients with P. aeruginosa and prior antibiotic exposure are different.Citation25 P. aeruginosa is the first cause of pneumonia in the post operative period of lung transplantCitation26 and in intubated patients with a prior episode of pneumonia.Citation27 P. aeruginosa is also the most common pathogen in patients with health care-associated pneumonia who required ICU admission and further MV.Citation28
Current management
Latest guidelines for the antibiotic treatment of P. aeruginosa VAP are the 2005 American Thoracic Society/Infectious Diseases Society of America guidelines, which recommend combination therapy with antipseudomonal cephalosporin (cefepime, ceftazidime) or carbapenem (imipenem, meropenem, or β-lactam/β-lactamase inhibitor [piperacillin–tazobactam]) plus antipseudomonal fluoroquinolone (ciprofloxacin or levofloxacin) or aminoglycoside.Citation29 However, since their publication a decade ago, many findings have been made in the field of antibiotic management in the critically ill, highlighting inappropriate treatment due to insufficient dosing and suboptimal antibiotic exposure, which are associated with increased mortality and worse outcomes.Citation30–Citation33 Furthermore, the rise of MDR strains in nosocomial pneumonia renders this approach outdated.Citation12,Citation34 It is important to bear in mind that it is critical to avoid antibiotics to which the patient has been exposed over the last 30 days, since the new episodes usually are relapses of a strain with phenotypic variations and not reinfection. Also, recently, a multicenter study has shed some light regarding treatment failure in P. aeruginosa VAP. With an occurrence rate of approximately 30% of episodes, the study identified risk factors for failure, including age, chronic illness, limitation of life support, severity of illness, previous use of a fluoroquinolone, and bacteremia. Interestingly, neither antibiotic susceptibility patterns nor combination therapy influenced failure rates; on the other hand, treatment with a fluoroquinolone did decrease it.Citation35 outlines initial P. aeruginosa VAP management.
Figure 1 Management of PA VAP.
Notes: Carbapenems are usually reserved for MDR or polymicrobial infections. Aminoglycosides should be avoided as monotherapy despite antimicrobial susceptibility given its poor performance in lung tissue. High-dose inhaled colistin: 5 million units every 8 hours.
Abbreviations: COPD, chronic obstructive pulmonary disease; MDR, multidrug-resistant; PA, Pseudomonas aeruginosa; PK/PD, pharmacokinetic/pharmacodynamic; VAP, ventilator-associated pneumonia; XDR, extensively drug resistant; ICU, intensive care unit.
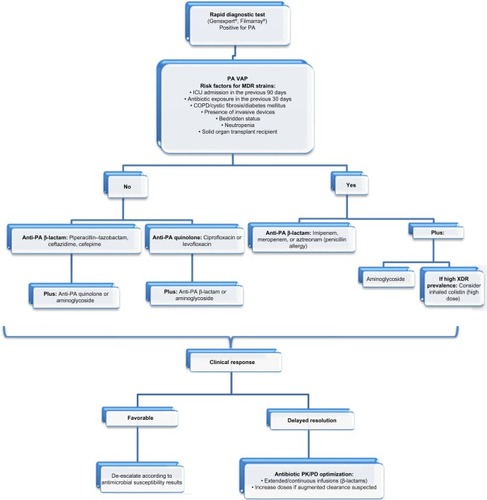
To avoid suboptimal antibiotic management, we believe that a composite approach has to be made, taking into account variables other than the classic microbiological paradigm of appropriate antibiotic therapy based only in minimum inhibitory concentration (MIC)’s susceptibility patterns and tailoring treatment to each patient, assessing specific risk factors especially for MDR ().Citation36 The cornerstone for improving outcomes is timing; early effective therapy as soon as possible might be the difference between death and successful treatment, especially when shock is present.Citation37,Citation38 Appropriate empirical choice of agent is fundamental, as is the use of a broad-spectrum antibiotic based on local ecology followed by reassessment of clinical response and microbiological data at 48–72 hours.Citation39,Citation40 In P. aeruginosa VAP, empiric combination therapy with a β-lactam plus an amino-glycoside has proved to be superior to monotherapy, reducing mortality up to 50% in many studies and meta- analyses, mainly due to appropriate initial therapy.Citation40–Citation42 However, there is no difference between one or two effective antibiotics, which is the rationale for de-escalating to monotherapy once microbiological results are available.Citation42 De-escalation is a safe strategy and has to be done when possible, even in neutropenic patients.Citation43 Regarding duration of therapy, many studies have demonstrated that 8 days of antibiotic for VAP is safe, reduces emergence of MDR and costs, and avoids unnecessary toxicity to the patient.Citation44–Citation47 However, in VAP caused by gram-negative bacilli, 8 vs 15 days of antibiotic is associated with increased pulmonary infection recurrence.Citation45 Since the aim of antibiotic therapy is pneumonia resolution and not P. aeruginosa eradication from the endotracheal tube/tracheostomy biofilm, antibiotic courses longer than 10 days in patients with clinical cure only add MDR-strain selection. In P. aeruginosa VAP, patients with inappropriate empirical antibiotic therapy, clinical resolution (fever and hypoxemia) is delayed 8 days (median), as happens with other MDR bacteria.Citation46 Furthermore, longer antibiotic courses may be recommended for immunosupressed patients with initial inappropriate empirical therapy VAP caused by MDR/extensively drug-resistant strains without clinical resolution.Citation47 Recently, biomarkers’ roles in antibiotic duration guidance have been the subject of multiple studies, with procalcitonin being the only one that has proved to be safe and reduce antibiotic days in VAP. When procalcitonin concentration is <0.5 ng/mL or has decreased by ≥80% (compared with the first peak concentration), antibiotics can be discontinued even in very short-course therapy (3 days), irrespective of the severity of the infectious episode; however, in bacteremic patients, at least 5 days of therapy is recommended.Citation48–Citation50
Another point to consider is optimizing the choice of antimicrobial according to pharmacokinetic (PK)/pharmacodynamic parameters. It is important to bear in mind that the antibiotic we choose has to reach therapeutic concentrations at the site of infection, where the bacteria–antibiotic interaction takes place, in order to obtain bacterial clearance as soon as possible.Citation51 Also, administration of a loading dose and administration of β-lactams in extended and continuous infusions increases antibiotic exposure and the probability of PK target attainment, which is essential in cases of septic shock, obesity, burn patients, and intermediate-resistant P. aeruginosa strains,Citation32 and it is associated with decreased 14-day mortality, faster recovery, and shorter ICU LOS and duration of treatment.Citation52–Citation64 With this in mind, nebulized antibiotic administration in MV may increase alveolar penetration compared with IV administration.Citation47 Nebulized colistin (high dose) in monotherapy has been studied in a small-randomized trial and a retrospective study, and noninferiority to IV combination therapy has been reported.Citation65–Citation67 This approach is very interesting since it enables delivery of high concentrations of the antibiotic with minimal absorption and marginal systemic levels, which could be a turning point in cases of MDR strains where available drugs are highly toxic. Effective treatment of VAP caused by MDR organisms such as P. aeruginosa and Acinetobacter baumannii has been reported with high-dose nebulized colistin, even achieving airway eradication.Citation65 Currently, a few agents are available for nebulization (colistin, tobramycin, aztreonam, ceftazidime, and amikacin) but are required to be tested in randomized clinical trials to know the safety and what adds to standard therapy. Further research and evidence-based guidelines are required. Other nebulized agents such as hypertonic saline and N-acetylcysteine, sometimes used as coadjutant therapy in the treatment of P. aeruginosa lung infection in cystic fibrosis patients, are still controversial, without strong evidence supporting or advice against its use in VAP treatment.Citation68–Citation72
New antibiotic treatments
Cephalosporins
Proven efficacy, broad spectrum (some of them including P. aeruginosa), and a well-characterized PK/pharmacodynamic profile, in addition to a favorable safety profile, make this antimicrobial class play an important role in nosocomial infection treatment, including VAP.Citation73 In response to the emergence of nosocomial infections due to β-lactam-resistant gram-negative bacteria in recent years, two strategies have been developed to improve their coverage: the development of new β-lactam molecules with the capacity to evade some mechanisms expressed by resistant bacteria and the addition of novel compounds capable of inactivating β-lactamases.Citation74
Ceftobiprole (BAL9141)
Ceftobiprole medocaril has enhanced activity against gram-negative pathogens, including Escherichia coli, Klebsiella pneumoniae, A. baumannii, and other Enterobacteriaceae; its antipseudomonal in vitro activity is similar to that of cefepime, and P. aeruginosa cross-resistance between ceftobiprole and other antipseudomonal cephalosporins has been reported.Citation75,Citation76 Also, it is inactive against bacteria expressing extended-spectrum β-lactamase (ESBL).Citation75,Citation76 Its bactericidal activity also acts against gram-positive bacteria, including resistant Streptococcus pneumoniae, methicillin-resistant S. aureus, and Enterococcus faecalis, but not against Enterococcus faecium.Citation78 Its activity against some of the ESKAPE pathogens and its stability against a wide range of β-lactamases (not KPC) make it an attractive option for hospital-acquired pneumonia treatment. A total of 781 patients were included in a Phase III study, 210 of whom had VAP. Clinical cure rates overall were 49.9% and 52.8% for ceftobiprole and ceftazidime/linezolid, respectively. However, while the cure rates were not different in nosocomial pneumonia, ceftobiprole performed worse on VAP (23.1% vs 36.5 cure rate). In contrast, those patients who had to be ventilated because of worsening of the pneumonia had a better outcome with ceftobiprole than with ceftazidime/linezolid ().Citation77 These findings might be associated with increases in distribution volume in septic patients receiving sedation to start MV, which cannot be anticipated using Monte Carlo simulation.
Table 1 Studies regarding the effect of new antibiotics on Pseudomonas aeruginosa infection
Ceftazidime–avibactam
Ceftazidime is a well-known antipseudomonal cephalosporin, also active against other gram-negative bacilli and gram-positive cocci and playing an important role in the treatment of nosocomial infections; however, it is susceptible to degradation due to β-lactamases, especially those of Ambler class A and C. Avibactam (NXL 104), recently added to the three approved β-lactamase inhibitors, is a molecule capable of avoiding the activity from A-, B-, and some D-class β-lactamases, including AmpC, KPC (Klebsiella pneumoniae carbapenemase), and ESBL.Citation73,Citation74,Citation78,Citation79 Despite not having antibacterial activity, its union with ceftazidime protects it from degradation from β-lactamases, enhancing its activity against Enterobacteriaceae producing β-lactamases, including P. aeruginosa.Citation79,Citation80 In a murine model, ceftazidime–avibactam has shown good penetration of epithelial lining fluid and effectiveness against P. aeruginosa with an MIC up to 32 μg/mL.Citation81 Ceftazidime–avibactam exhibits a great in vitro MIC50/90 reduction against P. aeruginosa producing β-lactamases compared with ceftazidime alone and also shows activity against some meropenem-non-susceptible strains in catheter-associated urinary tract infection.Citation74,Citation82,Citation83 Phase II trials with ceftazidime avibactam have shown favorable results, a good safety profile, and have been well tolerated when used alone for complicated urinary infections, and when used with metronidazol for intra-abdominal infections.Citation84,Citation85 Its role in nosocomial pneumonia is actually being analyzed in a Phase III study ().Citation86 Caution should be taken into account in countries/institutions where the main resistance problem is OXA-48, and consideration given to the need for initial loading dose, to avoid the potential risk of initial underdosing, particularly in those patients with decreased creatinine clearance.
Ceftolozane–tazobactam (CXA-201)
Like other cephalosporins, ceftolozane develops its bactericidal activity by inhibiting the cell wall synthesis via penicillin-binding proteins; particularly, ceftolozane has shown an enhanced affinity for these proteins in comparison with β-lactams.Citation87 In vitro studies suggest it is not affected by some β-lactam resistance mechanisms expressed by P. aeruginosa, such as efflux pumps or reduced wall permeability due to porin channel mutations,Citation88,Citation89 making it the most active antipseudomonal β-lactam.Citation90,Citation91 However, by itself it does not have activity against β-lactamase-producing strains. Tazobactam’s activity against β-lactamases bring to ceftolozane the potential to eliminate many resistant strains of P. aeruginosa and other β-lactamase-producing gram-negative bacteria.Citation92 A Phase III trial has shown ceftolozane–tazobactam’s efficacy in complicated intra-abdominal infections in combination with metronidazole, including those caused by MDR pathogens,Citation93 and a Phase II trial also demonstrated its efficacy in complicated urinary tract infection treatment.Citation94 Currently, a Phase III study is evaluating its safety and efficacy in VAP ().Citation95
Arbekacin
Arbekacin is an aminoglycoside discovered in the 1970s and has been used in many countries for more than 2 decades. Usually indicated in the treatment of infections caused by methicillin-resistant S. aureus, it has also shown activity against gram-negative pathogens, including Pseudomonas spp. Its capacity to be unaltered by many of the aminoglycoside-modifying enzymes, one of the most frequent ways by which aminoglycosides are inactivated, confers to arbekacin enhanced activity against P. aeruginosa resistant to amikacin, gentamicin, and tobramycin.Citation93,Citation96 In vitro analysis suggests that arbekacin in combination with aztreonam is an effective regimen against MDR P. aeruginosa, including metallo-β-lactamase-producing strains;Citation93 however, further studies are needed to show its applicability and safety in clinical practice. In PK studies, arbekacin has shown acceptable pulmonary tissue distribution and an adequate safety profile;Citation97 however, therapeutic plasma level monitoring is recommended to optimize its efficacy and minimize adverse effects, mainly nephrotoxicity.Citation93
POL7080
POL7080 is a novel peptidomimetic antibiotic with proven activity against P. aeruginosa in murine models.Citation98 Its mechanism of action is not totally clear, but it is known that it modifies the lipopolysaccharide-assembling of the bacterial outer membrane via the lipopolysaccharide-assembling protein LptD.Citation98 A Phase I study has shown POL7080 to be safe and well tolerated,Citation99 and actually a Phase II study is evaluating its safety and efficacy in patients with VAP due to P. aeruginosa ().Citation100 Nephrotoxicity is a major concern with this drug.
Pathogenicity and newer adjunctive therapies
Pathogenicity
P. aeruginosa’s pathogenicity is very complex,Citation101–Citation103 and a detailed analysis is far from the objective of this report. During a host’s infection process, P. aeruginosa uses pili, flagella, and fimbriae, a series of functional elements, to move and adhere on living and nonliving surfaces, such as different tissues and medical devices,Citation104,Citation105 and also employs these mobile elements to form bacterial communities based on an intricate intercellular communication mechanism (ie, quorum sensing), many times surrounded by a polysaccharide-based structure known as biofilm. This structure is produced by the bacterial colony and acts as a barrier against different chemical factors and physical forces (eg, immune system response and antibiotics), providing a favorable environment for colony survival and playing an important role in its permanency and in the chronic colonization/infection process.Citation21,Citation104,Citation106,Citation107
Many of the steps in the biofilm formation process are being highly investigated as treatment targets, with many others not being completely understood yet.Citation21
Alginate is a very important virulence variable, affecting children with cystic fibrosis.Citation108,Citation109 However, cystic fibrosis patients carry mucosal strainsCitation110 which are uncommon in patients with VAP, requiring different therapeutic considerations.
Quorum sensing
Quorum sensing is an evolved adaptive strategy expressed in several gram-negative and gram-positive bacteria species, based on a highly complex cell-to-cell communication mechanism, which allows a group of bacteria to exchange information and make dynamic and coordinated changes in response to different environmental stimuli, thus playing an important role in host infection and the bacterial permanence.Citation111 This system is based on signal molecules expressed by bacteria in a density-dependent way and released to the environment; these molecules are called autoinducers and are recognized by other cells, in some cases from different species (eg, between P. aeruginosa and Burkholderia cepacia), inducing genomic changes and giving to a population of bacteria the ability to deploy coordinated responses to affront different environmental assaults.Citation111–Citation113 With three known autoinducers from the acyl-homoserine lactone (AHL) family, Las, Rhl, and the P. aeruginosa quinolone signal, P. aeruginosa has one of the most classical and understood quorum sensing models, involved in many defense mechanisms such as bio-film formation and production of antimicrobial substances and bacterial virulence factors.Citation21,Citation111,Citation112,Citation114 This communication system facilitates host infection, ensures the permanency of colonies, and makes eradication of these colonies difficult, making it a highly attractive target for novel treatments. Three targets in this communication circuit have been identified as susceptible to pharmacological intervention: the inhibition of both Las and Rhl synthesis, the autoinducers’ degradation, and the blockage of AHL receptor function,Citation21,Citation111,Citation115 with several in vitro and animal model trials demonstrating the blockade of the quorum sensing as a feasible strategy to reduce the bacterial virulence and restore some P. aeruginosa susceptibility to classical antibiotics. However, further investigations are needed to evaluate its role in the treatment of human infections due to MDR P. aeruginosa.
Monoclonal antibodies
Current research in the management of P. aeruginosa infection has been directed toward prevention of infection in high-risk patients with vaccines and modulation of virulence with monoclonal antibodies instead of focusing on bacterial clearance attainment. Its main appeal relies on multidrug therapy with one molecule targeting mechanisms of action of bacteria covering MDR strains and probably active in different infection models.
Monoclonal anti-type three secretion system antibodies
Type three secretion system, known as TTSS or T3SS, is a complex system expressed by some bacteria which allows intoxication of host cells. This system is present in many gram-negative bacteria, including P. aeruginosa, and is based in several groups of proteins (more than 20) exhibited in the bacterial wall, which acts as a syringe, making the bacteria capable of injecting modulation factors and cytotoxins into other eukaryotic organisms, including the immune host apparatus and epithelial cells, inducing cellular death and playing an important role in P. aeruginosa virulence and in the inflammatory response.Citation116–Citation119 TTSS is a marker of virulence in P. aeruginosa penumoniaCitation110 and its presence in patients with VAP is associated with worse outcomes.Citation119,Citation120 TTSS plays an important role in VAP, since worse clinical outcomes are seen when TTSS is present. An obvious implication of this is that adjunctive therapies targeting these proteins, such as antibodies, may improve outcomes of patients under MV and P. aeruginosa respiratory isolation (both colonization and infection).Citation119,Citation121 The PcrV is a needle-tip protein involved in many steps of the TTSS-mediated infection process, sensing the outside environment and helping bacteria to recognize the strange cells. It also plays a role in translocation and secretion control of some proteins involved in functional molecular syringe assembling and facilitating the union into the molecular needle and the host membrane, which makes an attractive target in TTSS-mediated virulence control, with studies showing loss of virulence capacity in bacteria with an unfunctional PcrV, both in in vitro and in vivo animal models.Citation116,Citation121 Based on this idea, antibodies have been developed for the blockage of PcrV protein function, with many studies reporting a decrease in blood bacterial colonies and a less severe inflammatory response in various animal models treated with anti-PcrV immunoglobulins.Citation117,Citation122–Citation124 One of the most successful is the KB001, a high-affinity PEGylated Fab antibody, which, in a Phase II study, has been well tolerated and showed a safety profile in mechanically ventilated patients colonized by P. aeruginosa, also showing a nonstatistically significant tendency to reduce P. aeruginosa pneumonia episodes in the intervention group ().Citation125
Table 2 Studies regarding the effect of adjunctive therapies on Pseudomonas aeruginosa infection
Monoclonal anti-alginate antibodies
Alginate is involved in many processes during P. aeruginosa infection, providing protection against a variety of host defense mechanisms and environmental factors such as antimicrobial agents; it also is highly present in mucoid bio-films and facilitates medical device colonization.Citation105,Citation107,Citation126 This exopolysaccharide, principally exhibited by mucoid strains of P. aeruginosa, is capable of reducing the host immune response by interfering with the activation of complements and polymorphonuclear chemotaxis, and also was shown to play a role in decreasing the phagocytosis of Pseudomonas spp., both those that are planktonic and those that form bio-film structure guaranteeing the P. aeruginosa survival during the first steps of primary infection, its permanency, and its chronic colonization development.Citation105,Citation107,Citation127
Different monoclonal antibodies against alginate have been developed, showing an increase in P. aeruginosa phagocytosis. In some cases, as with the monoclonal antibody F429, this improvement in immune response against P. aeruginosa infection was also reported in different models of infection such as pneumonia, sepsis, and keratitis in animal models,Citation109,Citation128 being promising as an adjunctive strategy in P. aeruginosa infection management ().
Panobacumab (AR-101)
Panobacumab is an IgM-type human monoclonal antibody that is directed against IATS 011 serotype P. aeruginosa, one of the most prevalent serotypes associated with nosocomial pneumonia.Citation16,Citation129,Citation130 A multicenter Phase II study using panobacumab in combination with different antipseudomonal antibiotics in critical patients with nosocomial pneumonia due to P. aeruginosa serotype O11, almost all with VAP, showed a good safety profile with good PKs ().Citation131,Citation132
Vaccines
P. aeruginosa’s infection mechanism and its interaction with the host immunity is highly studied and well known. With the advances in antimicrobial therapy and many sites identified as possible targets to improve the acquired immunity response and block the P. aeruginosa infection and biofilm formation, different types of vaccines are being designed to improve the immune response against many substances involved in this process. The most common targets are components of the bacterial surface, such as outer membrane proteins (Opr) and different polysaccharides (lipopolysaccharides, mucoid exopolysaccharide, and O-polysaccharides), structures involved in P. aeruginosa adhesion and movement, such as flagella, pili, and several virulence factors, such as TTSS, exotoxin A, or proteases.Citation133,Citation134 Development of an effective vaccine is difficult due to the high variability between Pseudomonas species and the complexity of its infection process and its interaction with the host immune response. In many cases during phase I, II and III studies, some molecules failed to provide an adequate coverage against different P. aeruginosa strains, or showed a low inmunogenicity capacity or an unsecure profile.Citation133–Citation135
One of the most promising targets to induce an acquired immune response are the Opr, showing an improved immune response against P. aeruginosa infection in murine models previously exposed to modified epitopes from Opr.Citation133,Citation135,Citation136 From this group, the Opr-based vaccine IC43 has been used in healthy individuals and in different groups with increased risk to develop P. aeruginosa infection, including critical patients under MV, showing a good safety profile and being well tolerated,Citation137–Citation140 and there is an ongoing Phase II/III study designed to show its effect on mortality in mechanically ventilated ICU patients.Citation141
Conclusion
P. aeruginosa VAP management requires prompt and adequate antibiotic exposure. Initial empiric therapy should be done with broad-spectrum antibiotics in combination therapy followed by de-escalation with one effective antibiotic since its effectiveness equals two antibiotics. Immunotherapy, including strategies with monoclonal antibodies, might be a new approach to treat (and perhaps prevent) P. aeruginosa infections. Future research should focus on optimizing outcomes with strategies of blocking virulence and vaccination.
Disclosure
Jordi Rello has served in the Advisory Boards and Speakers Bureau of Cubist. The authors report no other conflicts of interest in this work.
References
- ChastreJFagonJYVentilator-associated pneumoniaAm J Respir Crit Care Med2002165786790311934711
- KollefMHChastreJFagonJYGlobal prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosaCrit Care Med201442102178218725054674
- RelloJJubertPVallésJArtigasARuéMNiedermanMSEvaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosaClin Infect Dis19962359739788922788
- BerthelotPGrattardFMahulPProspective study of nosocomial colonization and infection due to Pseudomonas aeruginosa in mechanically ventilated patientsIntensive Care Med200127350351211355118
- TumbarelloMDe PascaleGTrecarichiEMClinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patientsIntensive Care Med201339468269223370828
- MelsenWGRoversMMGroenwoldRHAttributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studiesLancet Infect Dis201313866567123622939
- SafdarNDezfulianCCollardHRSaintSClinical and economic consequences of ventilator-associated pneumonia: a systematic reviewCrit Care Med200533102184219316215368
- BouadmaLSonnevilleRGarrouste-OrgeasMOUTCOMEREA Study GroupVentilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumoniaCrit Care Med20154391798180625978340
- MicekSJohnsonMTReichleyRKollefMHAn institutional perspective on the impact of recent antibiotic exposure on length of stay and hospital costs for patients with gram-negative sepsisBMC Infect Dis2012125622414209
- MicekSTWunderinkRGKollefMHAn international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistanceCrit Care20151921925944081
- NathwaniDRamanGSulhamKGavaghanMMenonVClinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysisAntimicrob Resist Infect Control2014313225371812
- VincentJLRelloJMarshallJEPIC II Group of InvestigatorsInternational study of the prevalence and outcomes of infection in intensive care unitsJAMA2009302212323232919952319
- WangCYJerngJSChenKYPandrug-resistant Pseudomonas aeruginosa among hospitalised patients: clinical features, risk-factors and outcomesClin Microbiol Infect2006121636816460548
- LeroyOd’EscrivanTDevosPDubreuilLKipnisEGeorgesHHospital-acquired pneumonia in critically ill patients: factors associated with episodes due to imipenem-resistant organismsInfection200533312913515940413
- Martin-LoechesIDejaMKoulentiDEU-VAP Study InvestigatorsPotentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factorsIntensive Care Med201339467268123358539
- LuQEggimannPLuytCEPseudomonas aeruginosa serotypes in nosocomial pneumonia: prevalence and clinical outcomesCrit Care2014181R1724428878
- SandiumengeALisboaTGomezFHernandezPCanadellLRelloJEffect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE organismsChest2011140364365121659436
- PeñaCGómez-ZorrillaSOriolIImpact of multidrug resistance on Pseudomonas aeruginosa ventilator-associated pneumonia outcome: predictors of early and crude mortalityEur J Clin Microbiol Infect Dis201332341342023344827
- RelloJBorgattaBLisboaTRisk factors for Pseudomonas aeruginosa pneumonia in the early twenty-first centuryIntensive Care Med201339122204220624146002
- VallésJMariscalDCortésPPatterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumoniaIntensive Care Med20043091768177515243686
- VeesenmeyerJLHauserARLisboaTRelloJPseudomonas aeruginosa virulence and therapy: evolving translational strategiesCrit Care Med20093751777178619325463
- RelloJAusinaVRicartMRisk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumoniaIntensive Care Med19942031931988014285
- RelloJLisboaTKoulentiDRespiratory infections in patients undergoing mechanical ventilationLancet Respir Med20142976477425151022
- RelloJBorgattaBLagunesLManagement of Pseudomonas aeruginosa pneumonia: one size does not fit allCrit Care201418213625029571
- RelloJAllegriCRodriguezARisk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence of recent antibiotic exposureAnesthesiology2006105470971417006069
- RieraJCaraltBLópezIVall d’Hebron Lung Transplant Study GroupVentilator-associated respiratory infection following lung transplantationEur Respir J201545372673725359351
- RelloJMariscalDMarchFRecurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection?Am J Respir Crit Care Med19981573 Pt 19129169517611
- VallésJMesallesEMariscalDA 7-year study of severe hospital-acquired pneumonia requiring ICU admissionIntensive Care Med200329111981198813680109
- American Thoracic Society; Infectious Diseases Society of AmericaGuidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumoniaAm J Respir Crit Care Med2005171438841615699079
- RobertsJAAbdul-AzizMHLipmanJInternational Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious DiseasesIndividualised antibiotic dosing for patients who are critically ill: challenges and potential solutionsLancet Infect Dis201414649850924768475
- TacconeFSLaterrePFDugernierTInsufficient β-lactam concentrations in the early phase of severe sepsis and septic shockCrit Care2010144R12620594297
- RobertsJAPaulSKAkovaMDALI StudyDALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients?Clin Infect Dis20145881072108324429437
- BlotSKoulentiDAkovaMDoes contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI studyCrit Care2014183R9924887569
- KettDHCanoEQuartinAAImproving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) InvestigatorsImplementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort studyLancet Infect Dis201111318118921256086
- PlanquetteBTimsitJFMissetBYOUTCOMEREA Study GroupPseudomonas aeruginosa ventilator-associated pneumonia. Predictive factors of treatment failureAm J Respir Crit Care Med20131881697623641973
- BorgattaBRelloJHow to approach and treat VAP in ICU patientsBMC Infect Dis20141421125430899
- KumarARobertsDWoodKEDuration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shockCrit Care Med20063461589159616625125
- FerrerRMartin-LoechesIPhillipsGEmpiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement programCrit Care Med20144281749175524717459
- SandiumengeADiazEBodíMRelloJTherapy of ventilator-associated pneumonia. A patient-based approach based on the ten rules of “The Tarragona Strategy”Intensive Care Med200329687688312677369
- Garnacho-MonteroJCorcia-PalomoYAmaya-VillarRMartin-VillenLHow to treat VAP due to MDR pathogens in ICU patientsBMC Infect Dis20141413525430700
- SafdarNHandelsmanJMakiDGDoes combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysisLancet Infect Dis20044851952715288826
- Garnacho-MonteroJSa-BorgesMSole-ViolanJOptimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapyCrit Care Med20073581888189517581492
- MokartDSlehoferGLambertJDe-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational studyIntensive Care Med2014401414924231857
- CapellierGMocklyHCharpentierCEarly-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatmentPLoS One201278e4129022952580
- ChastreJWolffMFagonJYPneumA Trial GroupComparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trialJAMA2003290192588259814625336
- VidaurLPlanasKSierraRVentilator-associated pneumonia: impact of organisms on clinical resolution and medical resources utilizationChest2008133362563218198250
- GoldsteinIChastreJRoubyJJNovel and innovative strategies to treat ventilator-associated pneumonia: optimizing the duration of therapy and nebulizing antimicrobial agentsSemin Respir Crit Care Med2006271829116508884
- LuytCECombesATrouilletJLChastreJValue of the serum procalcitonin level to guide antimicrobial therapy for patients with ventilator-associated pneumoniaSemin Respir Crit Care Med201132218118721506054
- QuenotJPLuytCERocheNRole of biomarkers in the management of antibiotic therapy: an expert panel review II: clinical use of biomarkers for initiation or discontinuation of antibiotic therapyAnn Intensive Care2013312123830525
- PughRGrantCCookeRPDempseyGShort-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adultsCochrane Database Syst Rev201110CD00757721975771
- Vazquez-GrandeGKumarAOptimizing antimicrobial therapy of sepsis and septic shock: focus on antibiotic combination therapySemin Respir Crit Care Med201536115416625643278
- CrandonJLBulikCCKutiJLNicolauDPClinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosaAntimicrob Agents Chemother20105431111111620038614
- RafatiMRRouiniMRMojtahedzadehMClinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patientsInt J Antimicrob Agents200628212212716815689
- ChytraIStepanMBenesJClinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trialCrit Care2012163R11322742765
- LodiseTPJrLomaestroBDrusanoGLPiperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategyClin Infect Dis200744335736317205441
- LorenteLLorenzoLMartínMMJiménezAMoraMLMeropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilliAnn Pharmacother200640221922316449546
- LorenteLJiménezAPalmeroSComparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open-label, historical chart reviewClin Ther200729112433243918158083
- DulhuntyJMRobertsJADavisJSContinuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trialClin Infect Dis201356223624423074313
- KasiakouSKSermaidesGJMichalopoulosASoteriadesESFalagasMEContinuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trialsLancet Infect Dis20055958158916122681
- RobertsJAWebbSPatersonDHoKMLipmanJA systematic review on clinical benefits of continuous administration of beta-lactam antibioticsCrit Care Med20093762071207819384201
- MohamedAFKaraiskosIPlachourasDApplication of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial killAntimicrob Agents Chemother20125684241424922615285
- PeaFBrolloLVialePPavanFFurlanutMTeicoplanin therapeutic drug monitoring in critically ill patients: a retrospective study emphasizing the importance of a loading doseJ Antimicrob Chemother200351497197512654757
- WangJTFangCTChenYCChangSCNecessity of a loading dose when using vancomycin in critically ill patientsJ Antimicrob Chemother200147224611157921
- OparaojiECSiramSShoheiberOCornwellEE3rdMezghebeHMAppropriateness of a 4 mg/kg gentamicin or tobramycin loading dose in post-operative septic shock patientsJ Clin Pharm Ther19982331851909831969
- LuQYangJLiuZGutierrezCAymardGRoubyJJNebulized Antibiotics Study GroupNebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosaAm J Respir Crit Care Med2011184110611521474643
- LuQLuoRBodinLNebulized Antibiotics Study GroupEfficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumanniiAnesthesiology201211761335134723132092
- ArnoldHMSawyerAMKollefMHUse of adjunctive aerosolized antimicrobial therapy in the treatment of Pseudomonas aeruginosa and Acinetobacter baumannii ventilator-associated pneumoniaRespir Care20125781226123322349038
- SafdarAShelburneSAEvansSEDickeyBFInhaled therapeutics for prevention and treatment of pneumoniaExpert Opin Drug Saf20098443544919538104
- WarkPMcDonaldVMNebulised hypertonic saline for cystic fibrosisCochrane Database Syst Rev20092CD00150619370568
- MichonALJumas-BilakEChironRLamyBMarchandinHAdvances toward the elucidation of hypertonic saline effects on Pseudomonas aeruginosa from cystic fibrosis patientsPLoS One201492e9016424587256
- BoeJDennisJHO’DriscollBREuropean Respiratory Society Task Force on the use of nebulizersEuropean Respiratory Society Guidelines on the use of nebulizersEur Respir J200118122824211510796
- DuijvestijnYCBrandPLSystematic review of N-acetylcysteine in cystic fibrosisActa Paediatr1999881384110090545
- BassettiMMerelliMTemperoniCAstileanANew antibiotics for bad bugs: where are we?Ann Clin Microbiol Antimicrob2013122223984642
- SaderHSCastanheiraMFlammRKFarrellDJJonesRNAntimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from US medical centers in 2012Antimicrob Agents Chemother20145831684169224379201
- BustosCDel PozoJLEmerging agents to combat complicated and resistant infections: focus on ceftobiproleInfect Drug Resist2010351421694889
- FarrellDJFlammRKSaderHSJonesRNCeftobiprole activity against over 60,000 clinical bacterial pathogens isolated in Europe, Turkey, and Israel from 2005 to 2010Antimicrob Agents Chemother20145873882388824777091
- AwadSSRodriguezAHChuangYCA phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumoniaClin Infect Dis2014591516124723282
- LahiriSDManganiSDurand-RevilleTStructural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamasesAntimicrob Agents Chemother20135762496250523439634
- Lagacé-WiensPWalktyAKarlowskyJACeftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infectionsCore Evid20149132524493994
- CurcioDMultidrug-resistant Gram-negative bacterial infections: are you ready for the challenge?Curr Clin Pharmacol201491273823489027
- HousmanSTCrandonJLNicholsWWNicolauDPEfficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection modelAntimicrob Agents Chemother20145831365137124342641
- LevasseurPGirardAMClaudonMIn vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolatesAntimicrob Agents Chemother20125631606160822214778
- CrandonJLSchuckVJBaneviciusMAComparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosaAntimicrob Agents Chemother201256126137614622985878
- VazquezJAGonzález PatzánLDStricklinDEfficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized studyCurr Med Res Opin201228121921193123145859
- LucastiCPopescuIRameshMKLipkaJSableCComparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, Phase II trialJ Antimicrob Chemother20136851183119223391714
- AstraZenecaA Study Comparing Ceftazidime-Avibactam Versus MeropenemHospitalized Adults With Nosocomial Pneumonia Available from: https://clinicaltrials.gov/ct2/show/NCT1808092. NLM identifier: NCT1808092Accessed February 26, 2015
- HongMCHsuDIBounthavongMCeftolozane/tazobactam: a novel antipseudomonal cephalosporin and β-lactamase-inhibitor combinationInfect Drug Resist2013621522324348053
- TakedaSNakaiTWakaiYIkedaFHatanoKIn vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosaAntimicrob Agents Chemother200751382683017145788
- WalktyAKarlowskyJAAdamHIn vitro activity of ceftolozane-tazobactam against Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals in the CANWARD study, 2007 to 2012Antimicrob Agents Chemother201357115707570923939895
- MoyaBZamoranoLJuanCPérezJLGeYOliverAActivity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patientsAntimicrob Agents Chemother20105431213121720086158
- ChandorkarGHuntingtonJAGotfriedMHRodvoldKAUmehOIntrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjectsJ Antimicrob Chemother201267102463246922773741
- SolomkinJHershbergerEMillerBCeftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI)Clin Infect Dis201560101462147125670823
- MatsumotoTArbekacin: another novel agent for treating infections due to methicillin-resistant Staphylococcus aureus and multidrug-resistant Gram-negative pathogensClin Pharmacol2014613914825298740
- Calixa Therapeutics, Inc.Safety and Efficacy of IV CXA-101 and IV CeftazidimePatients With Complicated Urinary Tract Infections Available from https://clinicaltrials.gov/ct2/show/study/NCT0921024. NLM identifier: NCT0921024Accessed February 28, 2015
- Cubist Pharmaceuticals Holdings LLCStudy of Intravenous Ceftolozane/Tazobactam Compared to Piperacillin/TazobactamVentilator-Associated Pneumonia Available from: https://clinicaltrials.gov/ct2/show/NCT1853982. NLM identifier: NCT1853982Accessed February 28, 2015
- AraokaHBabaMTatedaKABX Combination Therapy Study GroupIn vitro combination effects of aztreonam and aminoglycoside against multidrug-resistant Pseudomonas aeruginosa in JapanJpn J Infect Dis2012651848722274165
- FunatsuYHasegawaNFujiwaraHPharmacokinetics of arbekacin in bronchial epithelial lining fluid of healthy volunteersJ Infect Chemother2014201060761124973909
- SrinivasNJetterPUeberbacherBJPeptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosaScience201032759681010101320167788
- Antibiotic POL7080 [webpage on the Internet]AllschwilPolyphor Ltd [updated March 25, 2015]. Available from: http://www.polyphor.com/products/pol7080Accessed March 29, 2015
- Polyphor LtdPharmacokinetics, Safety and Efficacy of POL7080Patients With Ventilator Associated Pseudomonas Aeruginosa Pneumonia Available from: https://clinicaltrials.gov/ct2/show/NCT2096328. NLM identifier: NCT2096328Accessed March 1, 2015
- HustonWMPotterAJJenningsMPRelloJHauserARMcEwanAGSurvey of ferroxidase expression and siderophore production in clinical isolates of Pseudomonas aeruginosaJ Clin Microbiol20044262806280915184477
- BattleSEMeyerFRelloJKungVLHauserARHybrid pathogenicity island PAGI-5 contributes to the highly virulent phenotype of a Pseudomonas aeruginosa isolate in mammalsJ Bacteriol2008190217130714018757543
- BattleSERelloJHauserARGenomic islands of Pseudomonas aeruginosaFEMS Microbiol Lett20092901707819025565
- LavertyGGormanSPGilmoreBFBiomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formationPathogens20143359663225438014
- SharmaGRaoSBansalADangSGuptaSGabraniRPseudomonas aeruginosa biofilm: potential therapeutic targetsBiologicals20144211724309094
- LeidJGWillsonCJShirtliffMEHassettDJParsekMRJeffersAKThe exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killingJ Immunol2005175117512751816301659
- GellatlySLHancockREPseudomonas aeruginosa: new insights into pathogenesis and host defensesPathog Dis201367315917323620179
- MaiGTMcCormackJGSeowWKPierGBJacksonLAThongYHInhibition of adherence of mucoid Pseudomonas aeruginosa by alginase, specific monoclonal antibodies, and antibioticsInfect Immun19936110433843438406822
- PierGBBoyerDPrestonMHuman monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strainsJ Immunol200417395671567815494518
- SordéRPahissaARelloJManagement of refractory Pseudomonas aeruginosa infection in cystic fibrosisInfect Drug Resist20114314121694907
- JuhasMEberlLTümmlerBQuorum sensing: the power of cooperation in the world of PseudomonasEnviron Microbiol20057445947115816912
- de KievitTRQuorum sensing in Pseudomonas aeruginosa biofilmsEnviron Microbiol200911227928819196266
- WhiteleyMLeeKMGreenbergEPIdentification of genes controlled by quorum sensing in Pseudomonas aeruginosaProc Natl Acad Sci U S A19999624139041390910570171
- LespritPFaurissonFJoin-LambertORole of the quorum-sensing system in experimental pneumonia due to Pseudomonas aeruginosa in ratsAm J Respir Crit Care Med2003167111478148212569080
- HraiechSHiblotJLafleurJInhaled lactonase reduces Pseudomonas aeruginosa quorum sensing and mortality in rat pneumoniaPLoS One2014910e10712525350373
- SatoHFrankDWMulti-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other gram-negative bacteriaFront Microbiol2011214221772833
- SawaTItoENguyenVHHaightMAnti-PcrV antibody strategies against virulent Pseudomonas aeruginosaHum Vaccin Immunother201410102843285225483637
- LynchSVFlanaganJLSawaTPolymorphisms in the Pseudomonas aeruginosa type III secretion protein, PcrV – implications for anti-PcrV immunotherapyMicrob Pathog201048619720420211240
- HauserARCobbEBodiMType III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosaCrit Care Med200230352152811990909
- SchulertGSFeltmanHRabinSDSecretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumoniaJ Infect Dis2003188111695170614639541
- GoureJPastorAFaudryEChabertJDessenAAttreeIThe V antigen of Pseudomonas aeruginosa Is required for assembly of the functional PopB/PopD translocation pore in host cell membranesInfect Immun20047284741475015271936
- WangQLiHZhouJPcrV antibody protects multi-drug resistant Pseudomonas aeruginosa induced acute lung injuryRespir Physiol Neurobiol2014193212824418353
- WarrenerPVarkeyRBonnellJCA novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection modelsAntimicrob Agents Chemother20145884384439124841258
- ShimeNSawaTFujimotoJTherapeutic administration of anti-PcrV F(ab’)(2) in sepsis associated with Pseudomonas aeruginosaJ Immunol2001167105880588611698464
- FrançoisBLuytCEDugardASafety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trialCrit Care Med20124082320232622622405
- NakagawaAHosoyamaTChubachiKTakahashiSOhkuboTIyobeSA search for Pseudomonas alginate biosynthesis inhibitors from microbial metabolitesJ Antibiot (Tokyo)1997503286288
- OliverAMWeirDMThe effect of Pseudomonas alginate on rat alveolar macrophage phagocytosis and bacterial opsonizationClin Exp Immunol19855911901963918817
- ZaidiTPierGBProphylactic and therapeutic efficacy of a fully human immunoglobulin G1 monoclonal antibody to Pseudomonas aeruginosa alginate in murine keratitis infectionInfect Immun200876104720472518644881
- de KievitTRLamJSMonoclonal antibodies that distinguish inner core, outer core, and lipid A regions of Pseudomonas aeruginosa lipopolysaccharideJ Bacteriol199417623712971397525538
- LazarHHornMPZuercherAWPharmacokinetics and safety profile of the human anti-Pseudomonas aeruginosa serotype O11 immunoglobulin M monoclonal antibody KBPA-101 in healthy volunteersAntimicrob Agents Chemother20095383442344619451304
- LuQRoubyJJLaterrePFPharmacokinetics and safety of panobacumab: specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumoniaJ Antimicrob Chemother20116651110111621398296
- Summary of AR-101 clinical data [webpage on the Internet]San JoseAridis Pharmaceuticals Available from: http://www.aridispharma.com/ar101clinicaldata.htmlAccessed June 7, 2015
- SharmaAKrauseAWorgallSRecent developments for Pseudomonas vaccinesHum Vaccin2011710999101121941090
- PierGApplication of vaccine technology to prevention of Pseudomonas aeruginosa infectionsExpert Rev Vaccines20054564565616221066
- BaumannUMansouriEvon SpechtBURecombinant OprF-OprI as a vaccine against Pseudomonas aeruginosa infectionsVaccine200422784084715040936
- von SpechtBUKnappBMuthGProtection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteinsInfect Immun1995635185518627729895
- SorichterSBaumannUBaumgartAWalterspacherSvon SpechtBUImmune responses in the airways by nasal vaccination with systemic boosting against Pseudomonas aeruginosa in chronic lung diseaseVaccine200927212755275919366571
- MansouriEBlome-EberweinSGabelsbergerJGermannGvon SpechtBUClinical study to assess the immunogenicity and safety of a recombinant Pseudomonas aeruginosa OprF-OprI vaccine in burn patientsFEMS Immunol Med Microbiol2003372–316116612832120
- MansouriEGabelsbergerJKnappBSafety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteersInfect Immun19996731461147010024596
- Valneva Austria GmbHStudy Assessing Immunogenicity and Safety of IC43Intensive Care Patients Available from: https://clinicaltrials.gov/ct2/show/NCT0876252. NLM identifier: NCT0876252Accessed April 6, 2015
- IntercellA confirmatory phase II/III study assesing efficacy, immunogenecity and safety of IC43 recombinant Pseudomonas vaccie in intensive care patients Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-004771-36/ES. EudraCT identifier: 2011-004771-36Accessed April 6, 2015
