Abstract
Influenza virus infection is a major cause of morbidity and mortality in children and adults globally. Seasonal epidemics are common due to the rapid virus evolution, whereas the frequent emergence of antigenic variants can result in pandemics and sporadic/endemic avian influenza virus infections. Although annual vaccination is the mainstay for influenza prevention and control, the use of antiviral agents must be considered for treatment and prophylaxis against influenza. Currently available antiviral drugs include neuraminidase inhibitors (NAIs), adamantanes, and a novel polymerase inhibitor (favipiravir). Peramivir is a recently US Food and Drug Administration-approved NAI for the treatment of acute uncomplicated influenza in adults. The chemical structure of peramivir allows it to bind to the influenza neuraminidase with much higher affinity than oseltamivir. Peramivir is effective against a variety of influenza A and B subtypes and has a lower half-maximal inhibitory concentration compared to other NAIs in in vitro studies. Peramivir can be administered intravenously, a route that is favorable for hospitalized, critically ill patients with influenza. The long half-life of peramivir allows for once-daily dosing. The drug is eliminated primarily by the kidneys, warranting dose adjustments in patients with renal dysfunction. Studies have assessed the clinical efficacy of peramivir for treatment of pandemic influenza A (H1N1). Although anecdotal evidence supports the use of peramivir in pediatric patients, pregnant women, and hospitalized patients with severe influenza receiving continuous renal replacement therapy and extracorporeal membrane oxygenation, well-designed, controlled clinical trials should be conducted in order to assess its clinical efficacy in these patient populations.
Introduction
The influenza virus causes a highly infectious, acute respiratory illness that causes significant morbidity and mortality in children and adults both in the US as well as globally.Citation1 Seasonal influenza affects between 5% and 20% of the population in the US annually, resulting in 25–50 million cases each year.Citation1 This significant number of influenza cases leads to approximately 225,000 hospitalizations and is responsible for 36,000 deaths each year in the US alone.Citation2 Globally, the WHO (World Health Organization) estimates that up to 20% of the population is infected with influenza each year, causing up to one billion infections, three-to-five-million cases of severe disease, and up to 300,000–500,000 deaths.Citation3 Although endemics and pandemics of influenza have been surfacing for centuries, the pandemic influenza A (H1N1) that arose in the spring of 2009 was particularly devastating. The 2009 H1N1 virus infected individuals in almost all countries globally and was responsible for 60.8 million cases, 273,304 hospitalizations, and 12,469 deaths, many of which were documented in pregnant women, indigenous populations, and in patients who were morbidly obese or had serious comorbidities.Citation4,Citation5
Despite the fact that the H1N1 pandemic revealed the need for better pandemic planning, it also illustrated the need for more effective antiviral agents for the treatment of severe influenza.Citation6,Citation7 In 2009, available therapies for acute influenza treatment included the adamantanes or M2 channel inhibitors and neuraminidase inhibitors (NAIs). M2 channel inhibitors include amantadine and rimantidine and have activity only against influenza A; however, the circulating H1N1 viruses were resistant to adamantanes and not recommended for treatment of influenza in the US.Citation6,Citation8 NAIs included oseltamivir (Tamiflu®; Genentech USA, Inc., South San Francisco, CA, USA) and zanamivir (Relenza®; GlaxoSmithKline, Brentford, UK), which have activity against both influenza A and B virus.Citation6,Citation8 Due to the fact that oseltamivir is administered orally and zanamivir is administered via the inhalation route, an unmet need for an intravenous (IV) antiviral agent existed for patients with severe influenza who were mechanically ventilated or critically ill.Citation8 Peramivir (Rapivab™; BioCryst Pharmaceuticals, Inc., Durham, NC, USA), an investigational NAI that was in advanced clinical development during the pandemic of 2009, is an IV NAI that was a promising therapy for patients with contraindications or poor response to available antivirals.Citation8,Citation9 Peramivir binds tightly to the neuraminidase (NA) enzyme compared to other NAIs and inhibits the growth of influenza A and B virus in vitro.Citation10
Due to the favorable route of administration and promising Phase II trials, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for this drug on October 23, 2009.Citation8,Citation9 Hospitalized patients were eligible for peramivir treatment if they were unresponsive to or were unable to tolerate available antivirals, or if oral and inhaled drug delivery routes were deemed unreliable.Citation9 Under the EUA, health care providers were required to monitor and report medication errors, adverse events (AEs), and deaths to the FDA Adverse Event Reporting System.Citation9
From the date of the EUA issuance through June 23, 2010, 1,371 requests for peramivir were submitted to the Centers for Disease Control and Prevention (CDC), and 2,129 5-day adult treatment courses were delivered to 563 hospitals.Citation8 Following a survey of health care providers, 1,274 patients had received at least one dose of peramivir.Citation8 The median age of peramivir recipients was 43 years (range, 0–92 years); 49% of patients were male.Citation8 The Adverse Event Reporting System reports were completed for 344 patients and included 28 children and 3 pregnant womenCitation9; 41% of patients for whom reports were received were critically ill and on mechanical ventilation, and 19% were on renal replacement therapies.Citation9
Serious AEs reported included death (15%), H1N1 influenza (8%), respiratory failure (8%), acute renal failure (7%), and acute respiratory distress syndrome (7%); additionally, six medication errors were reported.Citation9 Most patients who died during or following peramivir therapy were obese, immunosuppressed, >65 years, or had also received oseltamivir.Citation9 The only treatment-emergent AE that was reported and found to be attributable to peramivir was development of rash.Citation9 Other reports of AEs with peramivir use were confounded by severity of influenza disease, comorbidities, and concomitant medications.Citation9 Following the evaluation of reported AEs during the EUA, it was concluded that many of the patients who suffered AEs were critically ill and therefore at risk of developing complications that may or may not be attributable to the drug use itself.Citation9 Peramivir has been the subject of several excellent reviews.Citation10–Citation12
Peramivir has been approved for influenza treatment in both Japan and South Korea since 2010. In December 2014, peramivir was approved by the FDA for the treatment of acute, uncomplicated influenza in adults who have been symptomatic for ≤2 days.Citation13 This review will outline the new literature that has been published in recent years so as to provide a better understanding of peramivir’s mechanism of action, pharmacology, clinical efficacy, safety, and tolerability, as well as its current place in influenza therapy.
Microbiology overview and pharmacology of peramivir
Structure and mechanism of action
Peramivir, like other NAIs, binds to the active site of the influenza virus NA enzyme in order to prevent spread of infectious virions. Two glycoproteins are important to facilitate the influenza virus to infect host cells, the aforementioned NA, as well as hemaglutinin. The hemaglutinin protein allows viral entry, while NA promotes release of the virus from infected host cells so as to continue to spread the infection.Citation14 When peramivir binds to the active NA site on the virus, it is able to mimic the sialic acid residues present in the viral membrane to compete with neuraminic acid binding, which in turn prevents virion release.Citation7,Citation15–Citation18
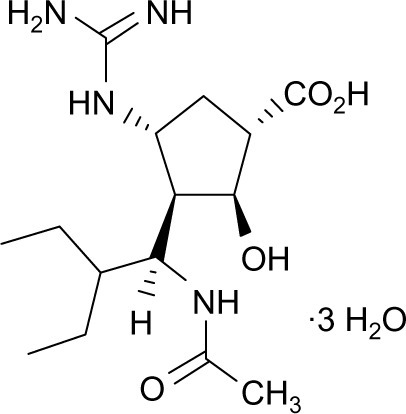
The peramivir molecule () contains a carboxylate group, a guanidino group, and lipophilic side chains off the cyclopentane backbone.Citation19 Its empirical formula is C15H34N4O4·3H2O, with a molecular weight of 328.45 g/mol. The chemical structure of peramivir allows it to bind to the influenza NA with much higher affinity than oseltamivir carboxylate, despite the similar structure in the cyclopentane backbone of the two drugs.Citation20,Citation21 The interaction between the NA arginine residue at position 292 and the carboxylic acid groups on zanimivir and oseltamivir allow binding of the drugs to the influenza NA. Additionally, the glutamic acid residue at position 119 in the NA enzyme interacts with the basic structures of zanimivir (guanidinium) and oseltamivir (amine) and allows for the difference in drug resistance profiles.Citation19,Citation20,Citation22,Citation23 Peramivir’s negatively charged carboxylate group allows for hydrogen bonding, while the acetamido group binds to the NA’s hydrophobic pocket. Strong hydrogen bonds and electrostatic interactions are also formed between the peramivir’s positively charged guanidinium group and the carboxylates of the NA.Citation19,Citation20,Citation22,Citation23 Due to these structural characteristics, the peramivir molecule has the ability to bind to the N9 site of the NA for a prolonged period of time (about 24 hours) with a slow dissociation rate.Citation20,Citation23
Pharmacokinetics
The pharmacokinetic (PK) parameters of peramivir are outlined in .Citation24 Oral administration of peramivir was assessed in a Phase III clinical trial, but was not found to achieve statistical significance in the treatment group.Citation25 Subsequently, it was found to have very low bioavailability (<3%), which led to the development and assessment of parenteral formulations.Citation23 Several Phase I clinical trialsCitation24 have been conducted to assess the PK parameters of both intamuscular (IM) and IV administration of peramivir in healthy adults, which have shown a linear relationship between the dose and maximum serum concentration (Cmax) as well as area under the curve (AUC).Citation24 Additionally, single-dose injections of IV and IM peramivir have been found to be bioequivalent. A Phase I, two-period crossover study was conducted in healthy volunteers, which found that peramivir administered via the IV and IM route to the same subjects at doses of 75 to 600 mg led to bioequivalence of the two routes when AUC was compared. Researchers also determined that the absolute bioavailability of IM peramivir ranged from 92% to 100% and that overall drug administration was safe and well tolerated.Citation26
Table 1 PK parameters after a single dose of peramivir
Matsuo et alCitation27 conducted a recent study of population PK in both healthy volunteers and influenza patients to assess factors that influence the PK parameters of peramivir. The study utilized 3,199 plasma concentration samples from 332 subjects that were analyzed from six trials conducted in Japan and the US. Subjects included healthy patients, patients with influenza, and patients with renal impairment. Researchers found that the plasma concentrations of peramivir were well described by a three-compartment model. Additionally, they determined that the most important factor influencing peramivir PK was creatinine clearance (CLCR), owing to the fact that peramivir is primarily renally excreted. Age was also found to be related to peramivir clearance, while body weight was a factor in the volume of distribution of the drug, primarily when it came to the central compartment of the model. There were no differences between the Japanese or US subjects, and sex was also found to have no effects on peramivir PK. The study also utilized Monte Carlo simulations to determine that patients with a CLCR >50 mL/min required no dose adjustment, while patients with a CLCR of 30–50 mL/min should have a 1/3-fold dose adjustment, and those with CLCR 10–30 mL/min should have a 1/6-fold dose adjustment in order to achieve AUCs comparable to patients with normal renal function.Citation27 Studies have also been conducted to assess PK parameters of peramivir following single doses of the drug in both healthy and infected patients.
Zhang et alCitation28 explored the PK profile of peramivir in healthy Chinese subjects through an open-label, randomized, single-dose, crossover study. They utilized doses of 300 and 600 mg, both given intravenously, and collected blood and urine samples at 17 designated time points and seven designated time intervals for up to 36 hours postdose. They found that the drug displays linear PKs at the tested doses and noted increases in Cmax and AUC in proportion to the dose given.Citation28 Sato et alCitation29 assessed 28 pediatric patients who were treated with 10 mg/kg of IV peramivir to determine the predictive peramivir concentration–time curve against the viruses with decreased susceptibility to NAIs. They found that peramivir concentration decreased to <0.1% of the Cmax at 24 hours postadministration. They concluded that peramivir readministration should be considered in the event of lack of clinical improvement in patients with normal susceptibility to influenza A and B and that better viral inhibition and lower frequency of adverse effects may be expected with divided administration.Citation29
To date, no clinical trials have been specifically conducted to assess the PK parameters of peramivir in patients undergoing continuous renal replacement therapy (CRRT) or extracorporeal membrane oxygenation (ECMO). Case reports of peramivir use in patients on CRRT have shown that peramivir has high clearance by various forms of CRRT, including continuous venovenous hemofiltration. A recent report by Bentley et alCitation30 assessed the PK parameters of a 29-year-old female receiving peramivir during continuous venovenous hemodiafiltration, which were consistent with those of previous reports. The dialysate flow rate was 16.7 mL/min, producing a total ultrafiltrate of 14.2 mL/min. They obtained both pre- and postfilter peramivir samples from blood and effluent at 4.5 and 8.5 hours following the third dose of 480 mg of peramivir, as well as plasma concentrations at several time points for measurement of AUC. Researchers found the Cmax at 30 minutes to be 19,477 ng/mL, Cmin to be 2,750 ng/mL, and AUC0–24 h to be 196,166 ng·h/mL. The plasma half-life was estimated to be 8.2 hours, and researchers noted a log–linear decrease over the 24-hour period. Thus, peramivir was found to have significant clearance with continuous venovenous hemodiafiltration with a calculated saturation coefficient of 0.98, which was very close to the estimated saturation coefficient of 1.Citation30 Additionally, Tang et alCitation31 describe the successful use of peramivir in a patient with acute respiratory distress syndrome caused by the influenza A virus H7N9 who underwent continuous venovenous hemodiafiltration in addition to venovenous ECMO. Though they do not report PK parameters in their patient, they do describe that they were able to remove her from venovenous ECMO support after 13 days. Dedicated studies of peramivir use in advanced forms of life support as well as in CRRT will provide valuable information for clinicians who desire to use peramivir in their clinical practice with critically ill patients.
Spectrum of antivirus activity
Peramivir has activity against both influenza A and influenza B viruses as demonstrated by both in vivo and in vitro studies.Citation20,Citation23 The potent antiviral effects of the drug confer activity against pandemic and highly pathogenic strains of influenza subtypes H5N1 and H9N2. Additionally, peramivir has the ability to inhibit the replication of nine different types of avian influenza viruses.Citation32 In an experimental mouse model of H7N9 avian influenza virus infection, repeated doses of peramivir at 30 mg/kg body weight demonstrated antiviral activity resulting in the resolution of clinical signs, improved survival, and prevention of recurrence of neurological symptoms.Citation33
Recent studies have been conducted on isolates of previous influenza seasons to determine the susceptibility of the viruses to NAIs. outlines the susceptibility profiles of these viruses as evaluated by two studies conducted by Dapat et alCitation34 and Leang et al.Citation35 Dapat et alCitation34 assessed the susceptibility of influenza virus isolates during the 2009–2010 and 2010–2011 seasons in Japan and showed that type A influenza isolates were more susceptible to the NAIs in comparison to type B isolates. They noted that the half-maximal inhibitory concentration (IC50) values for peramivir and laninamivir were significantly lower than those for oseltamivir and zanamivir, signifying a higher in vitro susceptibility of the viruses to peramivir and laninamivir. Researchers also examined eight A(H1N1)pdm09 viruses and one type B virus from patients following treatment with NAIs. The authors determined that there was an increase in prevalence of the H274Y mutation in the influenza A(H1N1)pdm09 from the 2009–2010 season to the 2010–2011 season and that patients with this mutation were resistant to treatment with oseltamivir and peramivir but retained susceptibility to zanamivir and laninamivir. Despite the discovery of other mutations in the isolated viruses and the widespread use of NAIs, researchers concluded that the prevalence of NAI-resistant influenza viruses in Japan remains low.Citation34
Table 2 In vitro neuraminidase inhibitor activity against influenza A and B viruses
Leang et alCitation35 collected influenza viruses from 19 countries in Asia, Africa, and Oceania between 2009 and 2012 to evaluate their susceptibility to peramivir and laninamivir. In addition to the IC50 values recorded in for viruses with normal inhibition, researchers determined that 19 of the total 599 A(H1N1)pdm09 viruses had highly reduced peramivir inihibition due to H275Y NA substitution upon genetic evaluation. Despite this increase in H275Y variants, the authors concluded that there were no marked changes in the frequency of peramivir-resistant variants despite the widespread use of NAIs.Citation35 Both these studies highlight the necessity for continuous susceptibility monitoring to determine if the viruses are developing resistance to these new NAIs.
Development of antiviral resistance
Emerging resistance of influenza viruses to antiviral agents, including the NAIs, is becoming an important public health concern for clinicians. Treatment options are affected by circulating resistant strains of the influenza virus and put patients at risk for treatment failure. Due to structural differences involving the chemical moieties involved in NA binding among the available NAIs, influenza resistance has been shown to be agent-specific.Citation36 Despite this, research by the WHO shows that influenza viruses in the Western Pacific, the Americas, and Europe have developed highly reduced inhibition to at least one of the four NAIs.Citation37
One of the most well-studied mutations that confers resistance among influenza viruses is the H275Y substitution in the NA protein. Mutations of this substitution have been found to confer a 400-fold decrease in susceptibility to oseltamivir and 140-fold decrease in susceptibility to peramivir in comparison to wild-type viruses.Citation38 Two case reports by Memoli et alCitation39 demonstrate the ability of wild-type influenza viruses to develop mutations during therapy, specifically in immunocompromised hosts. One patient with a history of myelodysplastic syndrome and stem cell transplantation was treated with oseltamivir for influenza A infection for an extended period of time. Upon analysis of viral isolates throughout her treatment course, it was found that the initial wild-type virus had acquired an H275Y mutation by day 9 of oseltamivir therapy. Similarly, in a second patient, an H275Y mutation that resulted in significant reduction in viral susceptibility was recovered after 14 days of treatment with oseltamivir.Citation39 While development of NA substitutions has been specifically noted in patients who have received treatment with NAIs, Takashita et alCitation40 have also identified patients with H275Y mutations that have never received prior treatment with NAIs. Six viruses in Japan were isolated in November and December of 2013, and though no epidemiological link was identified between the viruses, they were found to be closely genetically related. This finding suggests spread of a single resistant virus.Citation40
A CDC analysis of 87 specimens from 58 patients utilized pyrosequencing to determine that H275Y mutations were present in 38% of isolates from the 2009 influenza pandemic. In isolates that exhibited the H275Y variant at ≥50%, resistance to oseltamivir and peramivir was detected, though full susceptibility to zanamivir was retained. Additional substitutions were recovered from two patients, I223K or I22KR substitutions, and were found to have 38–52-fold enhancement in susceptibility to oseltamivir and 33–97-fold enhancement to peramivir.Citation41
Takashita et alCitation38 aimed to monitor the emergence of NAI-resistant viruses in four influenza seasons from 2008 to 2012. Using allelic discrimination, gene sequencing, and susceptibility profiles, researchers determined that the detection rate of resistant viruses had increased from 1% in the 2009 pandemic season to 2% in the postpandemic period. They also found that 1.3% of over 12,000 A(H1N1)pdm09 isolates had acquired an H275Y substitution and that, in general, patients who were between 0 and 9 years had the highest detection rate for resistant viruses.Citation38 A report of community transmission of oseltamivir-resistant A(H1N1) pdm09 in Australia advocates for rapid analysis of potentially transmissible virus strains. Twenty-nine viruses analyzed contained H275Y substitutions, and upon hemagglutinin and NA sequence analysis, it was determined that the strains were closely genetically related and likely accounted for the spread of a single variant.Citation42
Other mutations are less common but have been associated with reduced susceptibility to NAIs. A report of an A(H3N2) virus, A/Ohio/88/2012, was found to contain two rare substitutions, S245N and S247P. Researchers found that there was a 31-fold reduction in oseltamivir susceptibility and 66-fold reduction in peramivir susceptibility, which was mostly due to the SN247P mutation.Citation43 Additionally, highly reduced inhibition was demonstrated in an A(H3N2) virus with an NA E119V mutation, a B/Yamagata-lineage with an H273Y mutation, as well as a B/Victoria-lineage strain with an NA E117G mutation.Citation37 Overall, the concern for development of viral resistance to the currently available NAIs is justified and suggests that continued research be done to determine ways to decrease the spread of resistant influenza strains.Citation44
Clinical efficacy and comparative trials of peramivir
A compilation of randomized controlled trials of IV and IM peramivir in adult patients is provided in .Citation45–Citation52 Additional clinical trials, including open-label randomized trials as well as retrospective observational studies, are provided in .Citation9,Citation53–Citation55,Citation60
Table 3 Clinical efficacy of peramivir: randomized controlled trials of IV or IM peramivir in adults
Table 4 Other clinical studies of peramivir in influenza infection in adult and pediatric patients
Clinical trials of ambulatory patients with influenza
Two multicenter, phase II, randomized, double-blind, placebo-controlled trials were conducted by BioCryst Pharmaceuticals, Inc. in adult patients with positive rapid antigen tests (RATs) for influenza.Citation45,Citation46 The first study assessed 344 subjects who were randomized to receive a single dose of peramivir 150 mg IM, peramivir 300 mg IM, or placebo within 48 hours of symptom onset. The study found no statistically significant differences between treatment groups for the primary end point, time to alleviation of symptoms.Citation45 The second study evaluated peramivir 600 mg IM as a single dose versus placebo, also in adult patients with symptom duration of <48 hours. From the 334 patients who completed the efficacy analysis, researchers concluded that there were no statistically significant differences between treatment groups for time to alleviation of symptoms.Citation46 In general, these studies found that peramivir was safe and well tolerated.Citation45,Citation46
In 2010, Kohno et alCitation47 conducted an additional multicenter, phase II, randomized, double-blind, placebo-controlled trial in adult patients aged 20–64 years with influenza diagnosed by RATs. Subjects received peramivir 300 mg or 600 mg as a single IV dose or placebo within 48 hours of symptom onset. The primary end point was time to symptom alleviation, and 296 patients were included in the efficacy analysis. Statistically significant differences were found for both doses of peramivir compared to placebo, with a hazard ratio of 0.681 (P=0.0092) for the peramivir 300 mg group and 0.666 (P=0.0092) for the peramivir 600 mg group. This corresponded to a median time to symptom alleviation of 59.1 and 59.9 hours in the 300 and 600 mg peramivir groups, respectively, compared to 82 hours in the placebo group.Citation47 To assess the time to symptom alleviation in ambulatory patients with high-risk influenza symptoms, Kohno et alCitation48 then conducted a phase III, randomized, double-blind, uncontrolled study in which patients received peramivir 300 mg IV or peramivir 600 mg IV for 1–5 days. Thirty-seven patients were included, and the study determined that the median time to symptom alleviation was 68.6 hours in all patients. Researchers found that the 600 mg peramivir group had a significantly shorter duration of illness compared to the 300 mg group. Additionally, they determined that multiple doses of peramivir conferred more rapid symptom alleviation than single doses of peramivir.Citation48
Kohno et alCitation49 conducted a multicenter, phase III, randomized, double-blind, double-dummy trial in adult patients with acute uncomplicated influenza A or B diagnosed by RATs. Patients were randomized to receive peramivir IV at doses of 300 or 600 mg as a single dose or oral osteltamivir at a dose of 75 mg twice daily for 5 days. The study found that the 1,091 patients included in the efficacy analyses had similar time to alleviation of symptoms in all three treatment groups: 78 hours for the 300 mg peramivir group, 81 hours for the 600 mg peramivir group, and 81.8 hours for the oseltamivir group. Based on the hazard ratios of 0.946 (95% CI, 0.793–1.129) for the 300 mg peramivir group and 0.970 (95% CI, 0.814–1.157) for the 600 mg peramivir group, researchers concluded that both peramivir regimens were noninferior to the oseltamivir group.Citation49
Clinical trials of hospitalized patients with influenza
Ison et alCitation50 conducted a multicenter, phase II trial in hospitalized patients with acute or potentially life-threatening influenza in which patients were randomized to receive peramivir 200 mg, peramivir 400 mg, or placebo intravenously for 5 days in combination with oseltamivir or oral placebo. Researchers found no significant differences in the time to clinical stability or time to resumption of normal activities between groups and similarly found no differences between changes in viral titers at 48 hours. Thus, they concluded that peramivir was noninferior to oseltamivir in this patient population and had no significant differences in dose response between peramivir groups.Citation50
A phase III, randomized, open-label study was conducted by the National Institutes of Health in hospitalized patients aged 14–92 years with confirmed or suspected H1N1 influenza.Citation51 Patients received peramivir 300 mg IV twice daily or peramivir 600 mg IV once daily and were found to have similar reduction in influenza virus titers over the first 24 hours. An analysis of the intention-to-treat population revealed a median time to fever resolution of 25.3 hours, with time to clinical resolution of 92 hours, time to symptom alleviation of 145 hours, and time to resumption of usual activities of 26.8 days.Citation51 An additional randomized, double-blind, controlled, phase III study was conducted by the National Institutes of Health to assess peramivir use in hospitalized adults and adolescents with influenza confirmed through RAT. Time to clinical resolution at day 5 was the primary outcome and was determined to be 42.5 hours for peramivir versus 49.5 hours for placebo (P=0.97). Due to these results, this study was terminated following a preplanned interim analysis.Citation52
Additional studies have demonstrated the clinical effects of peramivir in adult patients.Citation53,Citation54 An open-label, randomized trial of hospitalized patients of ≥6 years of age was conducted by Ison et alCitation54 to determine viral titer decline between two peramivir regimens. A total of 234 patients were randomized to receive either peramivir 300 mg IV twice daily (or 5 mg/kg twice daily for patients <18 years) or peramivir 600 mg IV once daily (or 10 mg/kg once daily for patients <18 years). The study found that viral titers declined similarly between the two regimens and that there were no significant differences in virologic end points between the arms. In general, peramivir was found to be both safe and well tolerated in both regimens.Citation54
Observational studies of pediatric patients with influenza
While retrospective and uncontrolled trials have been emerging to assess the use of peramivir in pediatric patients in recent years, randomized controlled trials are yet to have been conducted in this patient population. A multicenter, open-label trial was conducted by Sugaya et alCitation55 that evaluated patients ≥28 days old but <16 years old who were hospitalized during the 2009 H1N1 epidemic. Patients received peramivir 10 mg/kg IV at a maximum of 600 mg once daily. The median time to alleviation of symptoms was 29.1 hours, and the drug was found to be both clinically and virologically effective as well as safe in pediatric patients.Citation55 An observational trial of pediatric outpatients was conducted to assess time to fever alleviation with use of various NAIs.Citation56 Out of a total of 263 patients, four received peramivir as a single dose of 10 mg/kg (maximum of 600 mg). The peramivir group was found to have a median time to fever alleviation of 17 hours, which was the fastest of all the NAI groups and was found to be a statistically significant time difference when compared to the oseltamivir group (P=0.044).Citation56
Hikita et alCitation57 also conducted a retrospective chart review of pediatric outpatients and found that in patients aged 5–18 years with influenza A, the duration of fever with peramivir was shorter in comparison to patients treated with zanamivir. In patients with influenza B within the same age group, fever duration was found to be significantly shorter in patients treated with peramivir versus those treated with laninamivir (P=0.0097). Although a number of studies of peramivir use in children and adolescents has been reported, it is difficult to draw conclusions given small sample sizes or lack of information regarding number of patients treated with peramivir.Citation58,Citation59 Further studies should assess both the safety and efficacy of peramivir in children.
Retrospective studies of critically ill adults and children
In one retrospective comparative analysis, researchers evaluated hospitalized, critically ill patients aged 5 months to 81 years, of whom 57 received peramivir. They found that 51% of patients who received peramivir died, but that fatal peramivir cases were more likely to have developed acute renal failure, had a shorter length of hospital stay, and received a shorter course of peramivir as compared to patients who did not receive the drug. While patients who received peramivir were found to be more likely to die than those who did not receive it (P<0.0001), researchers hypothesized that this was likely due to the preexisting severity of the patient cases prior to receiving peramivir treatment itself, but also that more studies should be conducted to assess the safety of peramivir in the critically ill patient population.Citation60
Antiviral combination therapy for severe influenza
The role of antiviral combinations and antiviral–immuno-modulator combination therapy for severe influenza has been reviewed recently.Citation61 Preclinical studies demonstrate a potential for combination therapy including NAIs (such as oseltamivir or IV zanamivir or IV peramivir as a foundation drug) in conjunction with antiviral agents with different mechanisms of action. No key PK interactions have been observed in healthy volunteers receiving IV peramivir and oral oseltamivir.Citation62 Well-designed controlled clinical trials must be conducted to investigate the efficacy of novel combination therapies for severe influenza infections, especially those caused by novel viruses such as 2009 H1N1, avian influenza A H5N1, or the avian influenza A H7N9.Citation61
Safety and tolerability in clinical use of peramivir
The most commonly reported AEs as well as the warnings and precautions as outlined in the package insert by BioCryst Pharmaceuticals are outlined in .Citation63
Table 5 Safety summary for peramivir
Two studies assessing adverse events of peramivir were conducted by BioCryst Pharmaceuticals in hospitalized patients with influenza.Citation64 BCX1812-301 was a randomized, double-blind, placebo-controlled trial that assessed the incidence of adverse effects in patients receiving peramivir 600 mg IV once daily for 5–10 days in addition to standard of care, which could include an additional NAI.Citation64 A total of 398 patients were included in the study, 88 of whom received peramivir alone and 176 of whom received peramivir in addition to another NAI. Researchers determined that the incidence of adverse effects did not increase with the use of peramivir in addition to oseltamivir and that the incidence of AEs in patients receiving peramivir was similar to that of patients receiving standard of care alone. In this trial, the majority of adverse effects involved the gastrointestinal tract, laboratory parameters, or were infectious or respiratory in nature.Citation64 The second trial, BCX1812-303, prohibited the use of concomitant NAIs and assessed the differences in adverse effects between a peramivir regimen of 600 mg IV daily versus peramivir 300 mg IV twice daily. The trial concluded that the incidence of adverse effects was similar between groups and that gastrointestinal side effects were seen most commonly.Citation64
Komeda et alCitation65,Citation66 conducted postmarketing drug use investigations of peramivir in both adult and pediatric populations. Safety was assessed in 1,174 adult patients from October 2010 to February 2012, and it was determined that the incidence rate of adverse drug reactions (ADRs) was 4.34% (51/1,174). Diarrhea, vomiting, and nausea were the most frequently reported, with incidence rates of 1.87%, 0.85%, and 0.68%, respectively. No ADRs were reported as serious in this patient population, and 91% of ADRs arose within 3 days of peramivir administration, with 96.2% of them being resolved or improved within 7 days of onset. Safety was also assessed in 1,199 pediatric patients aged <15 years. Two hundred forty-five AEs were observed, corresponding to an incidence rate of 14.01% (168/1,199). ADRs accounted for 115 of these events, and commonly reported symptoms were diarrhea and abnormal behavior. Fourteen serious ADRs were observed among 12 patients and included five cases of abnormal behavior as well as five cases of decreased neutrophil count. The majority of events (87%) occurred within 3 days of peramivir initiation, and 87.8% had resolved or improved within 7 days of onset. Overall, these two studies concluded that peramivir is safe in both adult and pediatric populations.Citation65,Citation66
While peramivir has been found to be generally safe and effective in most patient populations, case reports have been published which demonstrate that rare adverse effects have occurred. Hayashi et alCitation67 reported a case of a 73-year-old woman with myasthenia gravis who experienced acute respiratory failure and an exacerbation of myasthenia gravis following IV peramivir treatment for influenza A. Investigators reported a decrease in oxygen saturation and altered consciousness within 20 minutes of peramivir administration in this patient.Citation68 Additionally, Harada-Shirado et alCitation68 reported a case of severe immune thrombocytopenia in a 44-year-old male patient being treated with peramivir for influenza A infection. Bone marrow findings and peripheral blood examination were consistent with immune thrombocytopenia, and further testing revealed a drug-induced lymphocyte-stimulating test with positivity to peramivir.
Patient-focused perspectives
In general, peramivir has been found to be well tolerated by patients in clinical trials. Peramivir can be administered intravenously, a route that is favorable for hospitalized, critically ill patients with influenza. Since peramivir is a parenteral agent that requires IV or IM administration, it is most appropriate to administer in a clinic or in inpatient setting. Many patients who will be treated with full a course of peramivir are likely to be critically ill; therefore, adherence to the once-daily medication regimen will be overseen by the inpatient health care team. Widespread seasonal immunization and optimal use of antiviral agents including peramivir are key tools in our armamentarium against the influenza virus.
Data regarding drug interactions with peramivir are lacking. The manufacturer labeling recommends to avoid use of live attenuated influenza vaccine within 2 weeks before or 48 hours after peramivir administration due to the NAI’s ability to inhibit viral replication and therefore reduce vaccine efficacy. Since peramivir is not hepatically metabolized, the concern for cytochrome P450-mediated drug interactions is very low. In clinical trials, peramivir was not shown to have interactions with oral rimantidine, oseltamivir, oral contraceptives, or probenacid.Citation63
Conclusion
Peramivir is a recently FDA-approved NAI for the treatment of influenza A and B. As a parenteral agent, it can be given via IV administration, a route that may be favorable for the critically ill population, although studies have shown that enteral administration of standard doses of oseltamivir has adequate absorption with therapeutic blood levels in critically ill adult patients.Citation69,Citation70 The long half-life of peramivir allows for once-daily dosing; however, due to elimination primarily by the kidneys, dose adjustments are required in patients with renal dysfunction. Peramivir has been shown to be efficacious against a variety of influenza A and B subtypes and has been found to have a lower IC50 compared to other NAIs in in vitro studies. Several studies have been conducted to assess its clinical efficacy for treatment of A(H1N1)pdm09, and trials continue to be conducted to assess its utility in postpandemic influenza. Studies of peramivir demonstrating an effect on hospitalizations or mortality are lacking. The efficacy of peramivir in hospitalized patients with severe influenza needs further investigation. Though case reports and retrospective studies support the use of peramivir in pediatric patients, pregnant women, and patients undergoing CRRT and ECMO, well-designed, controlled clinical trials should be conducted in order to assess its clinical efficacy in these patient populations.
Disclosure
The authors report no conflicts of interest in this work.
References
- RuuskanenOLahtiEJenningsLCMurdochDRViral pneumoniasLancet201137797731264127521435708
- FioreAEUyekiTMBroderKPrevention and control of influenza with vaccinesMMWR Morb Mortal Wkly Rep201059RR–816220075837
- World Health Organization (WHO)Influenza (seasonal). Fact Sheet; March 2014Geneva, SwitzerlandWorld Health Organization2014 Available from: http://www.who.int/mediacentre/factsheets/fs211/en/Accessed June 23, 2014
- GirardMPCherianTPervikovYKienyMPA review of vaccine research and development: human acute respiratory infectionsVaccine200523505708572416154667
- ShresthaSSSwerdlowDLBorseRHEstimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–2010)Clin Infect Dis201152Suppl 1S75S8221342903
- LouieJKAcostaMWinterKthe California Pandemic (H1N1) Working GroupFactors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in CaliforniaJAMA2009302171896190219887665
- HaydenFDeveloping new antiviral agents for influenza treatment: what does the future hold?Clin Infect Dis200948Suppl 1S3S1319067613
- YuYGargSPatriciaAPeramivir use for treatment of hospitalized patients with influenza A (H1N1) pdm09 under emergency use authorization, October 2009–June 2010Clin Infect Dis201255181522491506
- SorbelloAJonesSCCarterWEmergency use authorization for intravenous peramivir: evaluation of safety in the treatment of hospitalized patients infected with 2009 H1N1 influenza A virusClin Infect Dis20125511722491501
- HataAAkashi-UedaRTakamatsuKMatsumuraTSafety and efficacy of peramivir for influenza treatmentDrug Des Devel Ther2014820172038
- IsonMGOptimizing antiviral therapy for influenza: understanding the evidenceExpert Rev Anti Infect Ther201513441742525695406
- McLaughlinMMSkoglundEWIsonMGPeramivir: an intravenous neuraminidase inhibitorExpert Opin Pharmacother201516121889190026153242
- US Food Drug AdministrationFDA approves Rapivab to treat flu infectionSilver Spring, MDUS Food and Drug Administration Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427755.htmAccessed June 23, 2015
- MosconaANeuraminidase inhibitors for influenzaN Engl J Med2005353131363137316192481
- LiWEscarpePAEisenbergEJIdentification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071Antimicrob Agents Chemother19984236476539517946
- HaydenFGTreanorJJBettsRFSafety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenzaJAMA199627542952998544269
- NguyenHTSheuTGMishinVPKlimovAIGubarevaLVAssessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibitors in three enzyme activity inhibition assaysAntimicrob Agents Chemother20105493671367720585136
- MendelDBTaiCYEscarpePAOral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infectionAntimicrob Agents Chemother19984236406469517945
- BabuYSChandPBantiaSBCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug designJ Med Chem200043193482348611000002
- BantiaSArnoldCSParkerCDAnti-influenza virus activity of peramivir in mice with single intramuscular injectionAntiviral Res2006691394516325932
- BantiaSUpshawRBabuYSCharacterization of the binding affinities of peramivir and oseltamivir carboxylate to the neuraminidase enzymeAntiviral Res201191328829121722670
- MinenoTMillerMJStereoselective total synthesis of racemic BCX-1812 (RWJ-270201) for the development of neuraminidase inhibitors as anti-influenza agentsJ Org Chem200368176591659612919021
- CastilloRHollandLEBoltzDAPeramivir and its use in H1N1 influenzaDrugs Today (Barc)201046639940820571608
- BeigelJHarmonLACollisPJPharmacokinetics and safety evaluations of escalating doses of peramivir administered intravenously in healthy volunteersPresented at: 47th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC)September 17–20, 2007Chicago, IL Abstract A-1408
- BarrosoLTreanorJGubarevaLEfficacy and tolerability of the oral neuraminidase inhibitor in experimental human influenza: randomized, controlled trials for prophylaxis and treatmentAntivir Ther200510890191016430195
- AtieeGCollisPMcCulloughADoboSElderJSheridanBSingle dose injections of iv and im peramivir are bioequivalent and well toleratedPoster presented at: Interscience Conference on Antimicrobial Agents ChemotherapySeptember 5–9, 2014Washington, DC
- MatsuoYIshibashiTHollisterASWajimaTPopulation pharmacokinetics of peramivir in healthy volunteers and influenza patientsAntimicrob Agents Chemother201559116755676226282420
- ZhangDDuAZhangLPharmacokinetics of peramivir after single intravenous doses in healthy Chinese subjectsXenobiotica201445323924325231091
- SatoMItoMSuzukiSInfluenza viral load and peramivir kinetics after single administration and proposal of regimens for peramivir administration against resistant variantsAntimicrob Agents Chemother20155931643164925547357
- BentleyMLHollisteraASHansenbACSmithJACainJSPeramivir pharmacokinetics in a patient receiving continuous veno-venous hemodiafiltration during the 2009 H1N1 influenza A pandemicInt J Clin Pharm Ther2014521211051111
- TangXHeHSunBARDS associated with pneumonia caused by avian influenza A H7N9 virus treated with extracorporeal membrane oxygenationClin Respir J20159338038424725670
- GovorkovaEALenevaIAGoloubevaOGComparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza virusesAntimicrob Agents Chemother200145102723273211557461
- FarooquiAHuangLWuSAssessment of antiviral properties of peramivir agains H7N9 avian influenza virus in an experimental mouse modelAntimicrob Agents Chemother201559127255726426369969
- DapatCKondoHDapatICNeuraminidase inhibitor susceptibility profile of pandemic and seasonal influenza viruses during the 2009–2010 and 2010–2011 influenza seasons in JapanAntiviral Res201399326126923791870
- LeangSKKwokSSullivanSGPeramivir and laninamivir susceptibility of circulating influenza A and B virusesInfluenza Other Respir Viruses20138213513924734292
- BazMAbedYBoivinGCharacterization of drug-resistant recombinant influenza A/H1N1 viruses selected in vitro with peramivir and zanamivirAntiviral Res200774215916217137644
- TakashitaEMeijerALackenbyAGlobal update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2013–2014Antiviral Res2015117273825721488
- TakashitaEFujisakiSKishidaNCharacterization of neuraminidase inhibitor-resistant influenza A (H1N1) pdm09 viruses isolated in four seasons during pandemic and post-pandemic periods in JapanInfluenza Other Respir Viruses2013761390139923745712
- MemoliMJHrabalRJHassantoufighiAEichelbergerMCTaubenbergerJKRapid selection of oseltamivirand peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hostsClin Infect Dis20105091252125520345239
- TakashitaEEjimaMItohRA community cluster of influenza A (H1N1) pdm09 virus exhibiting cross-resistance to oseltamivir and peramivir in Japan, November to December 2013Euro Surveill201419116
- NguyenHTTrujilloAASheuTGAnalysis of influenza viruses from patients clinically suspected of infection with an oseltamivir resistant virus during the 2009 pandemic in the United StatesAntiviral Res201293338138622330888
- HurtACHardieKWilsonNJCommunity transmission of oseltamivir-resistant A (H1N1) pdm09 influenzaN Engl J Med2011365262541254222204735
- SleemanKMishinVPGuoZAntiviral susceptibility of variant influenza A (H3N2) viruses isolated in the United States from 2011 to 2013Antimicrob Agents Chemother20145842045205124449767
- FarrukeeRMosseJHurtACReview of the clinical effectiveness of the neuraminidase inhibitors against influenza B virusesExpert Rev Anti Infect Ther201311111135114524093683
- BioCryst PharmaceuticalsEvaluation of the efficacy and safety of peramivir in subjects with uncomplicated acute influenza Available from: https://clinicaltrials.gov/ct2/show/NCT0419263. NLM identifier: NCT0419263Accessed November 30, 2015
- AtieeGLaughlinATellierGVirologic analysis of influenza viruses after therapy with a single intramuscular dose of the neuraminidase inhibitor peramivir versus placebo in patients with influenza in the outpatient settingPresented at: 49th Annual Meeting of the Infectious Diseases Society of AmericaOctober 20–23, 2011Boston, MA
- KohnoSKidaHMizuguchiMShimadaJEfficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infectionAntimicrob Agents Chemother201054114568457420713668
- KohnoSKidaHMizuguchiMIntravenous peramivir for treatment of influenza A and B virus infection in high-risk patientsAntimicrob Agents Chemother20115562803281221464252
- KohnoSYenMYCheongHJComparison of single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection: a phase III randomized, double-blind studyAntimicrob Agents Chemother201155115267527621825298
- IsonMGHuiDSClezyKA clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adultsAntivir Ther201318565166123111657
- HernandezJEAdigaRArmstrongRClinical experience in adults and children treated with intravenous peramivir for 2009 influenza A (H1N1) under an emergency IND program in the United StatesClin Infect Dis2011526696706
- de JongMDIsonMGMontoASEvaluation of intravenous peramivir for treatment of influenza in hospitalized patientsClin Infect Dis20145912e172e18525115871
- YoshinoYSeoKKogaIKitazawaTOtaYClinical efficacy of peramivir in adult patients with seasonal influenza during the winter of 2012 in JapanClin Respir J20149222823224612842
- IsonMGFraizJHellerBIntravenous peramivir for treatment of influenza in hospitalized patientsAntivir Ther201419434936123985625
- SugayaNKohnoSIshibashiTWajimaTTakahashiTEfficacy, safety, and pharmacokinetics of intravenous peramivir in children with 2009 pandemic A (H1N1) influenza virus infectionAntimicrob Agents Chemother201256136937722024821
- ShobugawaYSaitoRSatoIClinical effectiveness of neuraminidase inhibitors –oseltamivir, zanamivir, laninamivir, and peramivir – for treatment of influenza A (H3N2) and A (H1N1) pdm09 infection: an observational study in the 2010–2011 influenza season in JapanJ Infect Chemother201218685886422644080
- HikitaTHikitaHHikitaFHikitaNHikitaSClinical effectiveness of peramivir in comparison with other neuraminidase inhibitors in pediatric influenza patientsInt J Pediatr2012201214
- SugayaNShinjohMMitamuraKTakahashiTVery low pandemic influenza A (H1N1) 2009 mortality associated with early neuraminidase inhibitor treatment in Japan: analysis of 1,000 hospitalized childrenJ Infect201163428829421722665
- LouieJKYangSSamuelMCUyekiTMSchechterRNeuraminidase inhibitors for critically ill children with influenzaPediatrics20131326e1539e154524276847
- LouieJKYangSYenCAcostaMSchechterRUyekiTMUse of intravenous peramivir for treatment of severe influenza A (H1N1) pdm09PLoS One201276e4026122768265
- DunningJBaillieJKCaoBAntiviral combinations for severe influenzaLancet Infect Dis201414121259127025213733
- AtieeGLasseterKBaughmanSAbsence of pharmacokinetic interaction between intravenous peramivir and oral oseltamivir or rimantadine in humansJ Clin Pharmacol20125291410141921960669
- Peramivir® (Rapivab) [package insert]Durham, NCBioCryst Pharmaceuticals, Inc2014
- DoboSMElderJCollisPSafety of peramivir in hospitalized influenzaPoster #1169 presented at: 53rd Annual Meeting of the Infectious Diseases Society of AmericaOctober 8–11Philadelphia, PA
- KomedaTIshiiSItohYPost-marketing safety and effectiveness evaluation of the intravenous anti-influenza neuraminidase inhibitor peramivir (I): a drug use investigationJ Infect Chemother2014201168969525131292
- KomedaTIshiiSItohYPost-marketing safety and effectiveness evaluation of the intravenous anti-influenza neuraminidase inhibitor peramivir (II): a pediatric drug use investigationJ Infect Chemother201421319420125523716
- HayashiKIwasaKMorinagaAOnoKYamadaMExacerbation of myasthenia gravis by intravenous peramivirMuscle Nerve201551693593625656959
- Harada-ShiradoKIkedaKFurukawaMSevere immune thrombocytopenia possibly elicited by the anti-influenza viral agent peramivirIntern Med201453202369237125318805
- ArianoRESitarDSZelenitskySAEnteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenzaCMAJ2010182435736320159892
- TaylorWRThinhBNAnhGTOseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenzaPLoS One2008310e3410.1618923671