Abstract
Raltegravir is the first licensed compound in 2007 of the new integrase inhibitor drug class. At the dose of 400 mg twice daily, raltegravir showed a potent antiviral action in antiretroviral-naïve patients when associated with tenofovir and emtricitabine. Raltegravir was also found to be highly active in antiretroviral-experienced patients with virological failure and displaying multiresistant virus, as shown with the BENCHMRK and ANRS 139 TRIO trials. Finally, the use of raltegravir was assessed in the context of a switch strategy in antiretroviral-experienced patients with virological success [human immunodeficiency virus type 1 (HIV-1) RNA below detection limit], highlighting the following mandatory criteria in this strategy: the nucleoside reverse transcriptase inhibitors associated with raltegravir have to be fully active. In the different studies, raltegravir had a favorable safety and tolerability profile. In the clinical situation a switch in virologically suppressed patients receiving a protease inhibitor, an improvement of the lipid profile was observed. Overall, when analyzing the Phase II and III trials together, only a few patients on raltegravir discontinued for adverse events. The development of resistance to raltegravir mainly involved three resistance mutations in integrase gene: Q148H/K/R, N155H, and Y143C/H/R. In conclusion, raltegravir improved the clinical management of HIV-1 infection both in antiretroviral-naïve and in antiretroviral-experienced patients.
Rationale for the development of new antiretroviral drugs in HIV infection management
Since 1996, highly active antiretroviral therapy is still the standard of care for patients infected with human immunodeficiency virus (HIV) and displaying advanced immunodeficiency.Citation1 Combination regimens have resulted in improved survival and decreased morbidity for patients with a CD4 cell count below 350 cells/mm3.Citation2 However, viral suppression cannot always be achieved or sustained with standard antiretroviral-based regimens because of the development of viral resistance, toxic effects of drugs, drug intolerance, or problems of adherence.Citation3 The majority of HIV-infected patients in whom highly active antiretroviral therapy fails displayed plasma-resistant virus.Citation4 When only two viral enzymes (ie, reverse transcriptase and protease) were targeted by antiretroviral drugs, cross-resistance rapidly limits therapeutic options. Thus, antiretroviral drugs directed at new HIV targets were urgently needed for patients exhibiting detectable viremia despite treatment.
In this context, HIV type 1 (HIV-1) integrase represented one of the possible new therapeutic target.Citation5,Citation6 Consequently, HIV-1 integrase inhibitors would be expected to retain activity against HIV-1 that is resistant to other classes of antiretroviral drugs. In October 2007, raltegravir (MK-0518; Isentress, Merck, Whitehouse Station, NJ) was the first approved HIV-1 integrase inhibitor; it targets the strand transfer step of HIV-1 integration. It is currently approved by the US Food and Drug Administration in a large indication: “in combination with other antiretroviral agents for the treatment of HIV-1 infection in adult patients” without any specific criteria, both in antiretroviral-naïve and in antiretroviral-experienced patients.
Twenty years after the discovery of the therapeutic activity of zidovudine, the first antiretroviral drug used in the clinic, more than 25 antiretroviral drugs are now available, representing six different drug classes.
Overview of pharmacology and mechanism of action of raltegravir
Mechanism of action
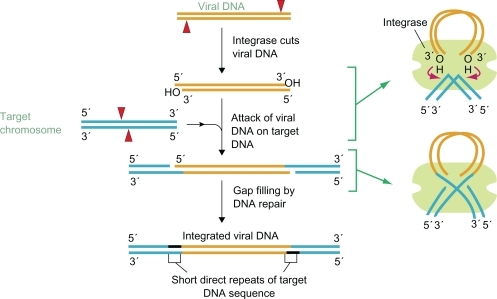
The complete viral life cycle requires integration of HIV-1 viral DNA into the host cell genome. It occurs in three steps ().Citation5,Citation7,Citation8 The double-stranded DNA copy generated by reverse transcription of the HIV-1 viral RNA genome is a part of the “preintegration complex”, containing both cellular and viral proteins, including integrase. In a first step, termed “3′ processing”, two nucleotides are removed from the 3′ ends of the viral DNA. In a second step, termed “strand transfer”, the proviral DNA is inserted into the host DNA and joined with it. Then, gaps in DNA are repaired by cellular enzymes by removing the two unpaired nucleotides at the 5′ end of the proviral DNA. The HIV integrase catalyses all steps of integration process except the last one, ie, DNA repair and ligation. A large series of selective inhibitors of strand transfer, β-diketo acid derivates, were tried to be developed as orally bioavailable agents.Citation6,Citation9–Citation12 Mechanistic studies showed that L-731,988, a β-diketo acid derivate, recognizes and selectively binds the integrase donor substrate complex exclusively in the context of catalytically active protein assembled on the viral DNA end. Thus, L-731,988 binds within the integraseactive site and inhibits strand transfer by competing with the target DNA substrate.Citation13
Figure 1 Steps of viral integration. Copyright © 2002. Reproduced with permission from NCBI DNA Replication, Repair, and Recombination. In: “Basic Genetic Mechanisms”, Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, editors. Molecular Biology of the Cell. New York, NY: Garland Science; 2002.
Chemical optimization of these compounds led to naphthyridine derivatives and L-900612 or MK-0518 (raltegravir) as a promising candidate compound. A recent study based on the diffracting crystals of the full-length integrase from the prototype foamy virus in complex with its cognate viral DNA enabled to determine more precisely the contacts between raltegravir and integrase structure.Citation14 The latter particularly included the metal cofactors of the active site, the bases of the invariant CA dinucleotide, and integrase residues 212, 214, and 215. Thus, the mechanism of action of the integrase strand-transfer inhibitors was more precisely described. Thus, the drug binding to integrase leads to the displacement of the reactive viral DNA end from the active site, disarming the viral nucleoprotein complex.Citation14 This could explain why the integrase strand-transfer inhibitors preferentially interact with and inhibit the DNA-bound form of HIV-1 integrase.Citation14
Raltegravir has potent in vitro activity against HIV-1, with a 95% inhibitory concentration (IC95; ±SD) in human T lymphoid cell cultures of 31 ± 20 nmol/L. It is active against a wide range of wild-type and drug-resistant HIV-1 isolates harboring resistance mutations in reverse transcriptase and protease coding regions, including both virus using CCR5 coreceptor and CXCR4 coreceptor.Citation9
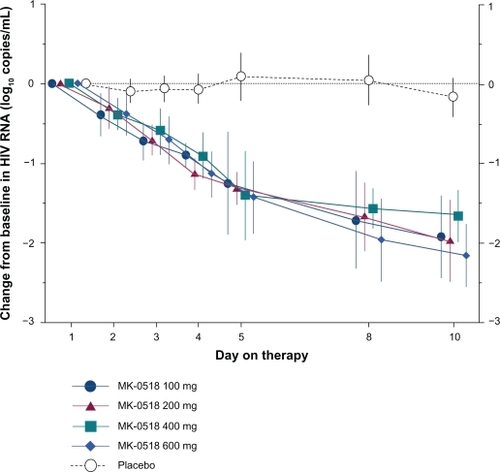
The in vivo activity of raltegravir was first described in the Phase II protocol 004, in which increasing doses of 100, 200, 400, and 600 mg of raltegravir administrated twice daily were compared with the placebo.Citation15 Thirty-five subjects received a 10-day course of raltegravir monotherapy at the doses described earlier or placebo. After 10 days, the mean HIV-1 RNA decrease was 1.66–2.16 log10 copies/mL in raltegravir recipients as compared with 0.17 log10 copies/mL in placebo-treated patients. No significant difference in HIV-1 RNA decrease was evidenced in the different raltegravir arms ().
Figure 2 Change from baseline at day 10 in HIV-1 RNA log10 copies/mL (with 95% CI). Error bars indicate 95% CI. Data for a total of 7 patients (from 4 of the 5 treatment groups) were not available on day 5. Copyright © 2007. Adapted with permission from Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naïve patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46(2):125–133.
Thus, in vitro and in vivo activities of raltegravir against HIV-1 were demonstrated. Furthermore, additive or synergistic activity was observed when cell culture samples, infected with HIV-1, were incubated with raltegravir and a panel of available nucleoside analog reverse transcriptase inhibitors (NRTI), nonnucleoside reverse transcriptase inhibitors (NNRTI), and protease inhibitors (PI). Taken together, these findings argue for the use of raltegravir with other active antiretroviral drugs.
Pharmacology of raltegravir
Oral absorption of raltegravir is rapid with a median time to maximum concentration of 0.5–1.3 hours, and oral bioavailability is approximately 30%.Citation16 Plasma protein binding reaches 83%. There was a biphasic decrease in drug concentrations, with an initial-phase half-life of ∼1 hour and a terminal-phase half-life of 7–12 hours (Merck). These characteristics were not affected by food with moderate-to-high fat content. Raltegravir is mainly metabolized by the glucuronidation enzyme UGT1A1, not by the CYP450 enzyme system. Thus, the potential for drug–drug interactions is decreased and administration of raltegravir may be of interest in combination with antituberculosis drugs or immunosuppressive agents, for example, with the exception of rifampicin. Thus, rifampicin is the inducer of UGT1A1, resulting in lower plasma raltegravir concentrations and needing to double the raltegravir dose to 800 mg.Citation17 Finally, excretion in feces and in urine accounts for most of the elimination.
Raltegravir is administered twice daily. No dose adjustments are recommended for severe renal impairment or mild-to-moderate hepatic impairment. The independence of the compound from “boosting” of drug levels with ritonavir is an attractive feature for many patients suffering from ritonavir-associated side effects. Interestingly, atazanavir inhibits UGT1A1, thus atazanavir is a likely combination partner for raltegravir, and pharmacokinetic studies with atazanavir, with or without ritonavir, showed a 30%–70% increase in raltegravir area under the plasma concentration time curve (AUC).Citation18 However, further studies are needed to assess the clinical relevance of this mechanism.
Efficacy studies
Raltegravir-containing regimens were demonstrated to have potent antiretroviral activity and to be well tolerated in HIV-1-infected individuals. Clinical trials were performed in different groups of HIV-1-infected patients, ie, treatment-naïve patients and antiretroviral-experienced patients with virological failure and exhibiting multiresistant plasma virus. Finally, the use of raltegravir was also assessed in switch strategy in antiretroviral-experienced patients with virological success.
Treatment-naïve patients
Patients included in the 10-day course of raltegravir monotherapy protocol (protocol 004) described earlier (n = 30) and 171 additional patients were randomized to receive either raltegravir (100, 200, 400, or 600 mg twice daily) or efavirenz in combination with tenofovir and lamivudine for 48 weeks.Citation15 At baseline, mean viral load was 4.7 and 4.8 log10 copies/mL and mean CD4 cell count was 280 and 305 cells/mm3 in the raltegravir and efavirenz arms, respectively. At week 24, the HIV-1 RNA level was <50 copies/mL in 85%–98% of raltegravir recipients and in 92% of efavirenz recipients. The mean change in CD4 cell count ranged from 144 to 221 cells/mm3 across all raltegravir groups at week 48. After week 48, all patients in the raltegravir arms were given 400 mg twice daily. The virological response was sustained up to 96 weeks.Citation19
This latter study confirmed the efficacy of raltegravir at the dose of 400 mg twice daily in antiretroviral-naïve patients, allowing to initiate Phase III trials using raltegravir-based regimen in first-line.
The STARTMRK study was an international, double-blind, Phase III randomized, noninferiority trial.Citation20 The study compared the safety and efficacy of raltegravir vs efavirenz in combination with tenofovir and emtricitabine in treatment-naïve patients. Inclusion criteria are as follows: HIV-1 infected patients naïve of antiretroviral treatment with HIV-1 RNA > 5000 copies/mL. Most patients were at an advanced stage of disease. Thus, 267 (47%) patients had a CD4 cell count of ≤200 cells/mm3 at baseline, of whom 58 (22%) had ≤50 cells/mm3. More than half [n = 297 (53%)] of participants had HIV-1 RNA level of >5 log10 copies/mL.
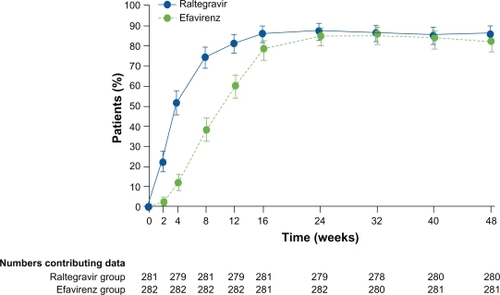
In the main analysis that recorded as failures all patients who did not complete the study, 86.1% (n = 241) of the raltegravir group achieved the primary endpoint of <50 copies/mL at week 48, compared with 81.9% (n = 230) of the efavirenz group [difference 4.2%, 95% confidence interval (CI): −1.9 to 10.3], indicating that raltegravir was noninferior to efavirenz (P < 0.0001 for noninferiority). The mean change in CD4 cell count at week 48 was 189 cells/mm3. The noninferiority of the raltegravir arm was also demonstrated in patients exhibiting HIV-1 RNA > 5 log10 copies/mL. Interestingly, the time to achieve such viral suppression was shorter for patients on raltegravir than those on efavirenz (log-rank test P < 0.0001) (); however, the clinical significance of a more rapid HIV-1 RNA decline has not yet been established but might be of interest to limit the selection of drug-resistant variants during the phase of viral load decay.Citation21
Figure 3 Proportion of patients with HIV-1 RNA < 50 copies/mL. Patients who did not complete the study were recorded as failures. Error bars = 95% CI.
Copyright © 2009. Adapted with permission from Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-na naïve ve patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806.
The results of the STARTMRK study at week 96 have recently been published and showed that the non-inferiority of raltegravir relative to efavirenz was sustained (difference 2%, 95% CI: −4 to 9).Citation22 Thus, raltegravir combined with tenofovir and emtricitabine is a durably efficacious regimen for treatment-naïve patients.
The results of the STARTMRK study led to the approval of raltegravir in antiretroviral-naïve patients.
Treatment-experienced patients with virological failure
Protocol 005 was a multicenter, randomized, double-blind, placebo-controlled dose-ranging trial in treatment-experienced patients.Citation23 Inclusion criteria were as follows: HIV-1 infected patients with HIV-1 RNA > 5000 copies/mL and CD4 < 50 cells/mm3, on stable antiretroviral therapy for more than 3 months and infected with HIV-1 with documented genotypic or phenotypic resistance to at least one NNRTI, one NRTI, and one PI. One hundred seventy-eight patients were included in this protocol. Investigators selected an optimal background regimen (OBR) according to all resistance tests available during therapeutic history. Randomization was performed in a 1:1:1:1 fashion to raltegravir doses of 200, 400, and 600 mg twice daily, or placebo, stratified by PI resistance at baseline and prior enfuvirtide use. Primary end-points were response and toxicity on week 24. Virological response was significantly better for all raltegravir arms as compared to placebo, with no significant difference between the raltegravir arms. This difference in favor of raltegravir was observed with any extent of resistance to the compounds of the background regimen, as assessed by genotypic or phenotypic sensitivity scores corresponding to the number of drugs received by the patient predicted to be active at baseline. Use of additional enfuvirtide was also associated with a better virological outcome. The mean change in CD4 cell count from baseline to week 24 was 113 cells/mm3 (95% CI: 73–150), with raltegravir 400 mg vs 5 cells/mm3 in the placebo group. There was no obvious difference of toxicity profile for any raltegravir dose in comparison with placebo.
The results of the protocol 005 confirmed the efficacy of raltegravir at the dose of 400 mg twice daily in antiretroviral-experienced patients, allowing to initiate Phase III clinical trials using raltegravir as part of a salvage regimen in patients with prior virological failures and exhibiting multidrug resistant virus.
Clinical trials BENCHMRK-1 (performed in Europe, Asia, the Pacific, and Peru) and BENCHMRK-2 (performed in the United States) were two parallel, double-blind, placebo-controlled studies.Citation24–Citation26 In both trials, an investigator-selected, resistance analysis-based OBR was combined with either raltegravir or placebo. Randomization was performed in a 2:1 manner. Four hundred sixty-two patients displaying triple-class resistant virus at baseline and exhibiting plasma HIV-1 RNA levels above 1000 copies/mL were included. The primary end-point was viral suppression to HIV-1 RNA < 400 copies/mL at week 16, with virus suppression to HIV-1 RNA < 50 copies/mL and change from baseline viral load and CD4 cell count evaluated as secondary end-points. The raltegravir arms in both trials were superior over placebo with regard to all end-points. The difference was maintained irrespective of the extent of baseline resistance (as assessed by genotypic and phenotypic sensitivity scores), baseline HIV-1 RNA levels, and baseline CD4 cell count. A superior response of raltegravir was sustained until week 24 in a combined analysis of both trials and confirmed after 48 weeks of follow-up. The difference in favor of raltegravir persisted regardless of the background regimen. The mean change in CD4 cell count between baseline and week 48 was 109 cells/mm3 (95% CI: 98–121) in the raltegravir recipients as compared with 45 cells/mm3 in the placebo recipients. The response rate (HIV-1 RNA < 50 copies/mL) in the raltegravir group at week 48 was 89% in the subgroup of patients receiving raltegravir in association with enfuvirtide and darunavir, when both drugs were used for the first time.Citation24 This approaches response rates in previously untreated patients and therefore represents a marked improvement for clinical management of patients with resistant virus. Results from combined BENCHMRK-1 and BENCHMRK-2 studies after 96 weeks of follow-up have been recently published, confirming the long-term efficacy of this strategy.Citation23 Thus, 57% and 61% of the patients in the raltegravir arm, compared with 26% and 28% of the patients in the placebo group, sustained an HIV-1 RNA level < 50 copies/mL and <400 copies/mL at week 96, respectively, (P < 0.001 for both comparisons), demonstrating a superior and durable virological efficacy of the raltegravir-based regimen.
Another trial assessing raltegravir in HIV-experienced patients in virological failure was the Agence Nationale de Recherches sur le SIDA (ANRS) 139 TRIO trial.Citation24 The objective of the ANRS 139 TRIO trial was to assess the virological efficacy and safety of an antiretroviral regimen containing raltegravir and two new drugs: the second generation NNRTI etravirine and darunavir boosted with ritonavir in HIV-1-infected patients who experienced virological failure of a combination antiretroviral therapy and displaying plasma multidrug-resistant virus. Patients enrolled in this noncomparative, open multicenter trial were naïve to the three investigational drugs and had plasma HIV-1 RNA levels >1000 copies/mL, a history of virological failure while receiving NNRTI, baseline plasma virus exhibiting ≥3 major PI-resistance mutations and NRTI resistance-associated mutations, and ≥3 darunavir and NNRTI resistance-associated mutations. The primary end point was the proportion of patients with plasma HIV-1 RNA levels <50 copies/mL at week 24. A total of 103 patients were included, and 87% of them received an OBR that included NRTI (86 patients) or enfuvirtide (12 patients). At week 24, 90% of patients (95% CI: 85–96) had an HIV-1 RNA level of <50 copies/mL. At week 48, 86% (95% CI: 80–93) had an HIV-1 RNA level of <50 copies/mL. The median CD4 cell count increase from baseline to week 48 was 108 cells/mm3 [interquartile range (IQR): 58–169]. Thus, highly antiretroviral-experienced patients harboring multidrug-resistant virus and who have few remaining treatment options may benefit from an antiretroviral therapy regimen containing three new drugs, raltegravir, etravirine, and darunavir/ritonavir, and may achieve high levels of virological suppression comparable to that of treatment-naïve patients.
Switch to raltegravir in treatment-experienced patients with virological success
The aim of these studies was to evaluate a switch from enfuvirtide (ANRS 138 EASIER trial) or a boosted PI as lopinavir (SWITCHMRK trial) to raltegravir in virologically suppressed patients.
The SWITCHMRK 1 and 2 studies were identically desig ned, double-blind, randomized, Phase III, active-controlled, non-inferiority clinical trials.Citation25 Patients virologically suppressed, receiving a lopinavir-based regimen for at least 3 months were included from 81 centers in five continents. The aims of the study were to assess the relative effects of a switch from lopinavir to raltegravir vs continuation of lopinavir on different parameters: i) serum lipid concentrations, ii) viral suppression, and iii) adverse events. In the combined analysis, viral suppression to <50 copies/mL was achieved by 293 (84.4%) patients in the raltegravir group compared with 319 (90.6%) patients in the lopinavir group at week 24, failing to establish non-inferiority of raltegravir to lopinavir. The studies were terminated at week 24 because of lower than expected virological efficacy in the raltegravir group compared with the lopinavir group. Overall, 49 patients met the protocol definition of confirmed virological failure, 32 in the raltegravir arm and 17 in the lopinavir arm. It is important to note that 18 (56%) and 4 (23%) patients had a history of virological failure on previous regimens, with the selection of NRTI resistance mutations, in the raltegravir and lopinavir arms, respectively. These results can be explained by the heterogeneous population enrolled in the SWITCHMRK studies. Of note, the inclusion criteria did not take account of genotypic resistance test criteria, or analysis of the patients’ antiretroviral history, including the occurrence of prior virological failure. Thus, it was not possible to ensure that the antiretroviral drugs present in the OBR were fully active. This study emphasizes that raltegravir had to be associated with fully active antiretroviral drugs to exclude a functional monotherapy context, which will strongly favor the development of resistance.
The SPIRAL study is a multicenter, noninferiority study assessing the efficacy of a switch from ritonavir-boosted PI to raltegravir in selected patients.Citation27 To be included, the patients had to i) receive a ritonavir-boosted PI added to at least two antiretroviral drugs and ii) show plasma HIV-1 RNA below 50 copies/mL for at least the previous 6 months. At week 48, 124 raltegravir recipients (89.2%) and 116 ritonavir-boosted PI recipients (86.6%) were still on virological success (difference 1.8%, 95% CI: −5.2 to 10.6), reaching noninferior efficacy. The differences of efficacy results observed between the SWITCHMRK and SPIRAL switch studies strengthen the need to reliably select the eligible patients for such strategies.
The ANRS 138 EASIER trial is a prospective, randomized, open-label, noninferiority trial to compare the antiviral efficacy and safety of a switch to raltegravir with the efficacy and safety of continuing enfuvirtide.Citation28,Citation29 Inclusion criteria were as follows: HIV-1-infected patients with multidrug-resistant virus and exhibiting plasma HIV-1 RNA levels of <400 copies/mL for at least 3 months with an enfuvirtidebased regimen. A total of 170 patients were randomized 1:1 to maintain enfuvirtide or to switch to raltegravir. The switch to raltegravir was noninferior to the maintenance of enfuvirtide, with only one patient experiencing virological failure in each arm, leading to a difference of 0.01% between treatments (95% CI: −6.7–6.8). At week 24, 88% of patients in both arms had plasma HIV-1 RNA levels of <50 copies/mL. Thus, a switch from enfuvirtide to raltegravir within a virologically suppressive regimen was successful in maintaining inhibition of plasma HIV-1 replication at least over 24 weeks. A long-term follow up is needed to accurately confirm the sustained efficacy of this strategy.
HIV genetic diversity and antiretroviral activity of raltegravir
HIV is characterized by high level of genetic diversity, within HIV-1 and HIV type 2 (HIV-2). HIV-1 is subdivided into four groups: M (major), O (outlier), N (non-M/non-O), and P.
Despite 40% of heterogeneity between the HIV-1 and HIV-2 integrase genes, the in vitro phenotypic susceptibility of HIV-2 clinical isolates to raltegravir was found in similar range to that of HIV-1.Citation30 In addition, previous studies reported a potent in vivo clinical and virological efficacy of raltegravir in HIV-2 infected patients.Citation31,Citation32
HIV-1 group O (HIV-O) is endemic in Western Central Africa, including Cameroon, where the prevalence of this group is estimated at about 1% of all HIV infections. HIV-O displays strong genetic divergence from HIV-1 group M and a high degree of intragroup diversity. A study assessing integrase polymorphism of 117 HIV-O clinical samples suggests that HIV-O presents natural mutations associated with in vitro or in vivo resistance to integrase inhibitors in HIV-1 group M genetic context.Citation33 However, preliminary data reported on in vivo virological response in a few number of HIV-O-infected patients receiving raltegravir-based regimen.Citation34,Citation35
Safety and tolerability
Overall, tolerability of raltegravir in clinical trials was excellent, and the toxicity profile of this drug is nonoverlapping with other agents, with no clear neuropsychiatric, gastrointestinal, or metabolic toxicity.
In the protocol 004, adverse events were infrequent, of mild to moderate intensity, with no obvious difference between the treatment arms.Citation15,Citation19
In the STARTMRK studies, significantly more patients on efavirenz than on raltegravir had clinical adverse events that were judged to be drug-related. Post-hoc analysis showed that 16% and 32% of patients on raltegravir and efavirenz, respectively, had drug-related clinical adverse events of moderate-to-severe intensity (P < 0.0001).Citation20,Citation22 Specific drug-related clinical adverse events of any severity occurring in more than 10% of participants on either raltegravir or efavirenz were dizziness, headache, and abnormal dreams. Immune reconstitution syndrome was reported as an adverse event in 17 (6%) raltegravir recipients and 11 (4%) efavirenz recipients. Analysis at week 8 showed that at least one central nervous system (CNS)-related adverse event had occurred in 10% of patients on raltegravir vs 18% of those on efavirenz (P = 0.0149). Serious adverse events occurred at a similar frequency in both treatment groups. Fewer laboratory-associated adverse events were recorded in patients on raltegravir than on efavirenz, but the difference was not significant. At week 48, the mean changes from baseline in total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglyceride concentrations were smaller for raltegravir than for efavirenz recipients and the change in the total cholesterol/HDL-cholesterol ratio between treatment groups was not significant. At week 96 of the STARTMRK studies, fewer drug-related clinical adverse events (47% vs 78%; P < 0.001) occurred in raltegravir than in efavirenz recipients. Both regimens had modest effects on serum lipids and glucose levels and on body fat composition. Consistent with the week 48 findings, mean changes from baseline in total cholesterol, LDL-cholesterol, HDL cholesterol, and triglyceride levels were smaller in raltegravir than in efavirenz recipients at week 96 (P < 0.001 for each comparison).Citation22 Changes in fat content at each time point were comparable in both treatment groups. Relatively few patients who had not developed drug-related clinical adverse events after 48 weeks of the study later developed such side effects. Serious adverse events and discontinuations due to adverse events were comparably infrequent in both treatment groups.
The protocol 005 and the BENCHMRK studies were not the most appropriate trials to assess the safety profile of raltegravir because all participants included in these studies previously received complex regimens and were highly pretreated.Citation23–Citation25 However, side effects and laboratory abnormalities were balanced between the groups in both trials, with no significant difference between the study arms. Of note, no dose-related toxicities were reported in the Phase II protocol 005 dose-ranging trial.Citation23 In the BENCHMRK studies, a disproportionate diagnosis of several cancers was observed in the raltegravir groups (1.3%) as compared with the placebo groups (0.3%) at the time of the 16-week analyses.Citation24,Citation25 Following these preliminary results, a global analysis including all Phase II and Phase III raltegravir studies, as well as data from the expanded access program, was performed, showing an adjusted risk of malignancy per 100 patient-years not significantly different (2.5% in the raltegravir group vs 1.9% in the group of control subjects; Merck).
The ANRS 139 TRIO trial was an open trial without control arm, so it is difficult to identify which adverse events were related to the investigational drugs: raltegravir, etravirine, and darunavir boosted with ritonavir.Citation36 In this study, grade 3 or 4 clinical adverse events were reported in 15 patients (14.6%) and only one patient discontinued the regimen because of an adverse event. This combination was well tolerated, most drug-related adverse events proved to be mild or moderate in severity.
The SWITCHMRK studies, assessing the switch from lopinavir to raltegravir in virologically suppressed patients, are of interest because two of the three primary endpoints of the study carried out the safety profile: i) the mean percentage change in serum lipid concentrations from baseline to week 12 and ii) the frequency of adverse events up to 24 weeks.Citation30 At week 12, percentage changes in lipid concentrations (total cholesterol, non-HDL cholesterol, and triglycerides) from baseline were significantly greater (P < 0.0001) in raltegravir recipients than in lopinavir recipients. In the combined analysis, the changes in lipid concentrations for the raltegravir group compared with the lopinavir group were −12.6% vs 1.0% for total cholesterol, −15.0% vs 2.6% for non-HDL cholesterol, and −42.2% vs 6.2% for triglycerides. Changes in LDL cholesterol and HDL cholesterol were similar in the raltegravir and lopinavir groups. Clinical and laboratory adverse events occurred at similar frequencies in both treatment groups. There were no serious drug-related adverse events or deaths. Overall, virologically suppressed patients switching to raltegravir had significantly greater reductions in some lipid parameters such as total cholesterol and triglycerides. Similarly, the SPIRAL study demonstrated that switching to raltegravir was associated with significant decreases in plasma lipids (triglycerides, total, LDL, and HDL cholesterols; P < 0.0001 for each comparison) and total-to-HDL cholesterol ratio (P < 0.05) relative to continuing ritonavir-boosted PI. In term of adverse events, their overall incidence was similar in the raltegravir group than in the PI group.
In the ANRS 138 EASIER trial, grade 3–4 adverse events and laboratory abnormalities were uncommon, not different between the treatment arms, and there was no difference in glucose levels between treatment arms.Citation28
Thus, in the different studies, raltegravir had a favorable safety and tolerability profile compared with efavirenz in antiretroviral-naïve patients. In the clinical situation of a switch in virologically suppressed patients receiving a boosted PI-based regimen, an improvement of the lipid profile was observed. Overall, when analyzing the Phase II and III trials together, only a few patients on raltegravir discontinued the drug for adverse events.
HIV resistance to raltegravir
The development of resistance to raltegravir is associated with the selection of mutations in its viral target: integrase gene. Three major raltegravir resistance-associated mutations are characterized and frequently detected in vivo in case of virological failure on a raltegravir-containing regimen: Q148H/K/R, N155H, and Y143C/H.Citation24,Citation37 All these positions are located near the catalytic site of the enzyme. A recent crystal structure study enabled to describe the interactions between HIV-1 integrase residue Tyr 143 and the methyloxadiazole group of raltegravir, which could explain the role of the Y143C/H/R mutations in the development of resistance to raltegravir.Citation14 The presence of any of these key resistance mutations is sufficient to reach high level of phenotypic resistance to raltegravir (at least fold change >15).Citation38 Furthermore, in vitro selection experiments under drug pressure showed the rapid appearance of virus with a high level of phenotypic resistance to raltegravir.Citation39,Citation40 Thus, raltegravir is considered as a molecule with a low genetic barrier to resistance. In most cases, the development of key raltegravir resistance-associated mutations is followed by the selection of secondary mutations specific to the resistance genetic pathway. The secondary mutations G140A/S, E92Q, and T97A are preferentially linked to the Q148, N155, and Y143 genetic pathways, respectively.Citation40,Citation41 Several studies reported that these primary resistance mutations Y143C/R, Q148H/K/R, and N155H represent mutually exclusive and nonoverlapping genotypic resistance pathways.Citation41–Citation43
In the BENCHMRK studies, a total of 105 of the 462 patients receiving raltegravir (23%) had virological failure by week 48.Citation24 Among these samples, integrase genotyping performed both at baseline and after virological failure showed that 68% had genotypic evidence of viral resistance to raltegravir. Virological failure was generally associated with mutations at one of the three residues: Y143, Q148, or N155, usually in combination with at least one other mutation.Citation24
In the STARTMRK studies, in case of virological failure, genotypic resistance tests were performed only in samples with plasma HIV-1 RNA levels of >400 copies/mL. Among the eight eligible samples for this analysis in the raltegravir arm, four displayed resistant virus with key raltegravir resistance-associated mutations at positions 143, 148, and 155.Citation20,Citation22 Between weeks 48 and 96, 12 additional patients met the protocol definition of virological failure in the raltegravir group, and 4 had plasma HIV-1 RNA level of >400 copies/mL. None of the viruses from the four evaluable raltegravir recipients had detectable resistance to any of the drugs in their regimen.Citation22 Of note, in both BENCHMRK and STARTMRK studies, integrase genotyping was performed only in patients exhibiting HIV-1 RNA level >400 copies/mL for technical reasons; however, the selection of raltegravir resistance mutations has been previously described in the context of low-level viremia, between 100 and 400 copies/mL.Citation43–Citation45
In the ANRS 139 TRIO trial, among the 103 patients included in the study, 14 had a virological failure, defined as a plasma HIV-1 RNA level >50 copies/mL at week 24 or between week 24 and week 48 on two consecutive specimens for those undetectable at week 24.Citation36 HIV-1 RNA level at time of virological failure was low with a median of 90 copies/mL. No raltegravir resistance-associated mutations were detected at time of virological failure either by bulk sequencing or by clonal analyses, performed in three subject, despite adequate plasma drug concentrations in almost all patients.Citation46
In the ANRS 138 EASIER trial, no raltegravir resistance mutations was detected by direct sequencing in the only patient with virological failure according to the trial criteria.Citation28 However, 39 (29%) of the patients in the raltegravir arm of the EASIER study displayed at least one episode of low-level viremia on treatment and significant integrase resistance-associated mutations were detected in three subject (7.7%), including N155H in two subjects and P145S in one subject.Citation45
In the SWITCHMRK studies, genotypic resistance testing was done in 14 of 16 patients with confirmed virological failure and HIV-1 RNA >400 copies/mL.Citation30 Of the 11 assessable patients who displayed a viral rebound on raltegravir-based therapy, virus with mutations known to confer raltegravir resistance was found in eight patients: N155H (n = 6); Q148H/K/R ± G140S (n = 2); Y143C (n = 1). In five of these eight patients, resistance mutations were also found in the reverse transcriptase. Of note, the studies were terminated at week 24 because of lower than expected virological efficacy in the raltegravir group compared with the lopinavir group.Citation30
Interestingly, in the drug class of integrase inhibitors, several studies reported on the replacement of one resistant pathway by another one in patients continuing on a failing raltegravir-containing regimen.Citation41,Citation42,Citation47 Most of these shifts in raltegravir-resistance profiles were characterized by the loss of variants containing N155H and the emergence of variants containing Q148R/H or, in a few cases, Y143C/R.Citation41,Citation42,Citation47 Phenotypic studies assessing viral replicative capacity and phenotypic resistance levels of raltegravir-resistant viruses showed that among single mutants, the N155H had the highest selective-advantage profile.Citation48 Among raltegravir-resistant double mutants, the highest selective-advantage profile was seen with G140S + Q148H.Citation48 This finding likely explains why N155H can be selected early in the course of raltegravir resistance evolution in vivo but is later replaced by genotypes of the Q148 pathway. The same mechanism was described for the Y143 resistance pathway. Indeed, the level of phenotypic resistance to raltegravir associated with N155H is always much lower than that associated with Q148 or Y143 mutation (>100 times higher).Citation49 Moreover, the characterization of the phenotypic evolution showed that a switch from N155H to Y143C/R was linked to an increase in resistance to raltegravir.Citation50 Taken together, these findings showed that these changes in phenotype may help to explain the shifts in integrase genotype under raltegravir treatment failure observed in different studies.Citation41,Citation42,Citation47,Citation49
Little is known about the role of integrase-mutated minority variants in the development of resistance to raltegravir. Previous studies assessed the presence of such quasispecies at the baseline of raltegravir-based regimen, using different sensitive techniques (ultra-deep sequencing, allele-specific PCR assay). Thus, integrase-mutated minority variants were detected in some cases in minority proportions (<1%) at baseline; however, no impact of their presence on the virological outcome was evidenced.Citation51–Citation53
A question remains unclear; several studies actually described the absence of selection of raltegravir resistance-associated mutations in patients exhibiting low-level viremia on raltegravir-based therapy.Citation46,Citation54,Citation55 In all Phase II and Phase III clinical trials, only the current characterized raltegravir resistance-associated mutations are reported, but we can hypothesize that the presence of other determinants, either in the integrase region or located in other parts of the pol gene, could impact on resistance.
Conclusions, place of raltegravir in therapy
The international guidelines recommend the use of raltegravir in different stages of HIV infection, corresponding to distinct profiles of clinical situations, as we described in this paragraph.
Guidelines from the European AIDS Clinical Society (EACS) include raltegravir (400 mg twice daily) as an alternative in the first-line regimen in antiretroviral-naïve patients (http://www.europeanaidsclinicalsociety.org/guidelinespdf). Regarding the use of raltegravir, the recently updated French 2010 recommendations (see http://www.sante-sports.gouv.fr/IMG/pdf/Prise_en_charge_medicale_des_personnes_infectees_par_le_VIH.pdf) were similar to the EACS ones. According to the recommendations of the Department of Health and Human Services, United States guidelines (see http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf), raltegravir (400 mg twice daily) associated with tenofovir and emtricitabine is indicated as the preferred regimen for the first-line ART.
About the use of raltegravir in a switch strategy in patients virologically suppressed, the EACS switch strategy indicates that switching from a ritonavir-boosted protease inhibitor to raltegravir in a antiretroviral-based regimen is only possible if (1) there is no history of prior virological failure with plasma virus exhibiting NRTI resistance-associated mutations and (2) NRTI backbone is fully active according to the different genotypic resistance tests available during the whole therapeutic history of the patient. A switch from enfuvirtide to raltegravir for simplification and adherence facilitation can also be considered in patients with HIV-1 RNA < 50 copies/mL, following similar criteria to that described earlier for a ritonavir-boosted PI. Of note, the association of only one NRTI with raltegravir is not recommended by the EACS.
In summary, raltegravir is a potent and well-tolerated antiretroviral drug, active in vivo against a large variety of HIV strains (HIV-1 group M non-B subtypes, HIV-1 group O, HIV-2) and with many possible clinical uses:
in antiretroviral-naïve patients (associated with tenofovir and emtricitabine)
in antiretroviral-experienced patients in virological failure, associated with at least two fully active drugs
in antiretroviral-experienced patients in virological success (HIV-1 RNA < 50 copies/mL), associated with at least two fully active drugs and with no prior history of virological failure
In conclusion, the use of raltegravir had improved the clinical management of HIV-1 infection both in antiretroviral-naïve and in antiretroviral-experienced patients. Ongoing clinical trials currently assess new therapeutic strategies such as antiretroviral bitherapy of raltegravir combined with a PI and a new therapeutic scheme with a daily-dosing strategy. Results from ongoing trials will also help to delineate the place of raltegravir in other clinical contexts, such as the particular case of post-exposure prophylaxis, the treatment of children or adolescents infected with HIV, pregnant women, and adults co-infected with HIV and tuberculosis.
Disclosure
The authors report no conflicts of interest in this work.
References
- HammerSMSaagMSSchechterMTreatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panelJAMA2006296782784316905788
- PalellaFJJrDelaneyKMMoormanACDeclining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study InvestigatorsN Engl J Med1998338138538609516219
- TozziVZaccarelliMBonfigliSDrug-class-wide resistance to antiretrovirals in HIV-infected patients failing therapy: prevalence, risk factors, and virological outcomeAntivir Ther200611555356016964822
- CostagliolaDDescampsDAssoumouLPrevalence of HIV-1 drug resistance in treated patients: a French nationwide studyJ Acquir Immune Defic Syndr2007461121817514016
- HazudaDJFelockPWitmerMInhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cellsScience2000287545364665010649997
- EspesethASFelockPWolfeAHIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integraseProc Natl Acad Sci U S A20009721112441124911016953
- CraigieRHIV integrase, a brief overview from chemistry to therapeuticsJ Biol Chem200127626232132321611346660
- LaFeminaRLSchneiderCLRobbinsHLRequirement of active human immunodeficiency virus type 1 integrase enzyme for productive infection of human T-lymphoid cellsJ Virol19926612741474191433523
- HazudaDBlauCUFelockPIsolation and characterization of novel human immunodeficiency virus integrase inhibitors from fungal metabolitesAntivir Chem Chemother1999102637010335400
- HazudaDJAnthonyNJGomezRPA naphthyridine carboxamide provides evidence for discordant resistance between mechanistically identical inhibitors of HIV-1 integraseProc Natl Acad Sci U S A200410131112331123815277684
- PaisGCZhangXMarchandCStructure activity of 3-aryl-1,3-diketo-containing compounds as HIV-1 integrase inhibitorsJ Med Chem200245153184319412109903
- EmbreyMWWaiJSFunkTWA series of 5-(5,6)-dihydrouracil substituted 8-hydroxy-[1,6]naphthyridine-7-carboxylic acid 4-fluorobenzylamide inhibitors of HIV-1 integrase and viral replication in cellsBioorg Med Chem Lett200515204550455416102965
- EspesethASFelockPWolfeAHIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integraseProc Natl Acad Sci U S A20009721112441124911016953
- HareSGuptaSSValkovEEngelmanACherepanovPRetroviral intasome assembly and inhibition of DNA strand transferNature2010464728623223620118915
- MarkowitzMNguyenBYGotuzzoERapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled studyJ Acquir Immune Defic Syndr200746212513317721395
- IwamotoMWenningLAPetryASSafety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjectsClin Pharmacol Ther200883229329917713476
- WenningLAHanleyWDBrainardDMEffect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of ralteg ravirAntimicrob Agents Chemother20095372852285619433563
- IwamotoMWenningLAMistryGCAtazanavir modestly increases plasma levels of raltegravir in healthy subjectsClin Infect Dis200847113714018513146
- MarkowitzMNguyenBYGotuzzoESustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naive patients with HIV-1 infectionJ Acquir Immune Defic Syndr200952335035619648823
- LennoxJLDeJesusELazzarinASafety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trialLancet2009374969279680619647866
- MetznerKJAllersKRauchPHarrerTRapid selection of drug-resistant HIV-1 during the first months of suppressive ART in treatment-naive patientsAIDS200721670371117413691
- LennoxJLDejesusEBergerDSRaltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analysesJ Acquir Immune Defic Syndr2010551394820404738
- GrinsztejnBNguyenBYKatlamaCSafety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trialLancet200736995691261126917434401
- CooperDASteigbigelRTGatellJMSubgroup and resistance analyses of raltegravir for resistant HIV-1 infectionN Engl J Med2008359435536518650513
- SteigbigelRTCooperDAKumarPNRaltegravir with optimized background therapy for resistant HIV-1 infectionN Engl J Med2008359433935418650512
- SteigbigelRTCooperDATepplerHLong-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trialsClin Infect Dis201050460561220085491
- MartínezELarrousseMLlibreJMSubstitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL studyAIDS201024111697170720467288
- de CastroNBraunJCharreauISwitch from enfuvirtide to raltegravir in virologically suppressed multidrug-resistant HIV-1-infected patients: a randomized open-label trialClin Infect Dis20094981259126719757993
- RoquebertBDamondFCollinGHIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvitegravir in vitroJ Antimicrob Chemother200862591492018718922
- EronJJYoungBCooperDASwitch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trialsLancet2010375971239640720074791
- DamondFLarivenSRoquebertBVirological and immunological response to HAART regimen containing integrase inhibitors in HIV-2-infected patientsAIDS200822566566618317013
- GarrettNXuLSmitEFernsBEl-GadiSAndersonJRaltegravir treatment response in an HIV-2 infected patient: a case reportAIDS20082291091109218520356
- LeozMDepatureauxAVessièreAIntegrase polymorphism and HIV-1 group O diversityAIDS200822101239124318525277
- BrizVGarridoCPovedaERaltegravir and etravirine are active against HIV type 1 group OAIDS Res Hum Retroviruses200925222522719239363
- CharpentierCUnalGDepatureauxAIn vivo Virological Response in HIV-1 Group O-infected Patients to Different Antiretroviral Line RegimensInternational HIV and Hepatitis Virus Drug Resistance Workshop and Curative StrategiesDubrovnik, Croatia201068–12 Abstract 111
- YazdanpanahYFagardCDescampsDHigh rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trialClin Infect Dis20094991441144919814627
- MouscadetJFDelelisOMarcelinAGTchertanovLResistance to HIV-1 integrase inhibitors: a structural perspectiveDrug Resist Updat Epub201064
- FransenSGuptaSDanovichRLoss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathwaysJ Virol20098322114401144619759152
- SekiTKobayashiMWakasa-MorimotoCS/GSK1349572 is a potent next generation HIV integrase inhibitor and demonstrates a superior resistance profile substantiated with 60 integrase mutant molecular clones. [Abstract 555]17th Conference on Retroviruses and Opportunistic InfectionsSan Francisco, CA, USA2010 Feb.
- Bar-MagenTSloanRDDonahueDAIdentification of novel mutations responsible for resistance to MK-2048, a second-generation HIV-1 integrase inhibitorJ Virol201084189210921620610719
- FransenSKarmochkineMHuangWWeissLPetropoulosCJCharpentierCLongitudinal analysis of raltegravir susceptibility and integrase replication capacity of human immunodeficiency virus type 1 during virologic failureAntimicrob Agents Chemother200953104522452419667293
- MaletIDelelisOSoulieCQuasispecies variant dynamics during emergence of resistance to raltegravir in HIV-1-infected patientsJ Antimicrob Chemother200963479580419221102
- CharpentierCKarmochkineMLaureillardDDrug resistance profiles for the HIV integrase gene in patients failing raltegravir salvage therapyHIV Med20089976577018651855
- GhosnJMazetAAAvettand-FenoelVRapid selection and archiving of mutation E157Q in HIV-1 DNA during short-term low-level replication on a raltegravir-containing regimenJ Antimicrob Chemother200964243343419457931
- GallienSDelaugerreCHuZIntegrase inhibitor resistance mutations in treatment-experienced HIV-1-infected patients with low-level viremia receiving raltegravir-containing antiretroviral therapy: an ANRS 138-EASIER trial substudyInternational HIV and Hepatitis Virus Drug Resistance Workshop and Curative StrategiesDubrovnik, Croatia201068–12; Abstract 49.
- CharpentierCRoquebertBColinCResistance analyses in highly-experienced patients failing raltegravir, etravirine and darunavir/ritonavir regimen (ANRS 139 TRIO trial)AIDS Epub2010826
- MillerMDDanovichRMKeYLongitudinal analysis of resistance to the HIV-1 integrase inhibitor raltegravir: results from P005, a phase II study in treatment-experienced patientsAntivir Ther200813Suppl 3A8
- QuerciaRDamEPerez-BercoffDClavelFSelective-advantage profile of human immunodeficiency virus type 1 integrase mutants explains in vivo evolution of raltegravir resistance genotypesJ Virol20098319102451024919605484
- HatanoHLampirisHFransenSEvolution of integrase resistance during failure of integrase inhibitor-based antiretroviral therapyJ Acquir Immune Defic Syndr201054438939320300008
- ReigadasSAniesGMasquelierBThe HIV-1 integrase mutations Y143C/R are an alternative pathway for resistance to raltegravir and impact the enzyme functionsPLoS One201054e1031120436677
- CharpentierCLaureillardDPikettyCHigh frequency of integrase Q148R minority variants in HIV-infected patients naïve of integrase inhibitorsAIDS201024686787320160635
- Ceccherini-SilbersteinFvan BaelenKArmeniaDSecondary integrase resistance mutations found in HIV-1 minority quasispecies in integrase therapy-naive patients have little or no effect on susceptibility to integrase inhibitorsAntimicrob Agents Chemother20105493938394820479206
- LiuJMillerMDanovichRDetection of low-frequency mutations associated with drug resistance to raltegravir before ART [Abstract 685]16th Conference on Retroviruses and Opportunistic InfectionsMontreal; Canada2009 Feb
- CabyFValinNMarcelinAGRaltegravir as functional monotherapy leads to virological failure and drug resistance in highly treatment-experienced HIV-infected patientsScand J Infect Dis2010426/752753220222846
- AllavenaCMounouryORodallecARaltegravir in HIV-1 ARV-experienced patients: high efficacy and absence of emergence of resistance mutations in low-grade virologic failures. [Abstract PE7.9/15]12th European AIDS Conference/EACSKoln, GermanyNov 2009