Abstract
Infections caused by Gram-positive pathogens remain a major public health burden and are associated with high morbidity and mortality. Increasing rates of infection with Gram-positive bacteria and the emergence of resistance to commonly used antibiotics have led to the need for novel antibiotics. Daptomycin, a cyclic lipopeptide with rapid bactericidal activity against a wide range of Gram-positive bacteria including methicillin-resistant Staphylococcus aureus, has been shown to be effective and has a good safety profile for the approved indications of complicated skin and soft tissue infections (4 mg/kg/day), right-sided infective endocarditis caused by S. aureus, and bacteremia associated with complicated skin and soft tissue infections or right-sided infective endocarditis (6 mg/kg/day). Based on its pharmacokinetic profile and concentration-dependent bactericidal activity, high-dose (>6 mg/kg/day) daptomycin is considered an important treatment option in the management of various difficult-to-treat Gram-positive infections. Although daptomycin resistance has been documented, it remains uncommon despite the increasing use of daptomycin. To enhance activity and to minimize resistance, daptomycin in combination with other antibiotics has also been explored and found to be beneficial in certain severe infections. The availability of daptomycin via a 2-minute intravenous bolus facilitates its outpatient administration, providing an opportunity to reduce risk of health care-associated infections, improve patient satisfaction, and minimize health care costs. Daptomycin, not currently approved for use in the pediatric population, has been shown to be widely used for treating Gram-positive infections in children.
Introduction
Infections caused by Gram-positive pathogens remain common, and resistance to traditional, established antibiotics is increasingly recognized. Daptomycin, a cyclic lipopeptide parenteral antibiotic, exhibits rapid concentration-dependent bactericidal activity against Gram-positive pathogens such as methicillin-resistant Staphylococcus aureus (MRSA).Citation1 The in vitro bactericidal activity of daptomycin showed that the minimum inhibitory concentrations (MICs) at which 90% of clinically relevant Gram-positive bacteria tested were inhibited were ≤1 µg/mL, except for enterococci, for which the MICs were 2–4 µg/mL.Citation2 Daptomycin has a distinct mechanism of action involving the calcium-dependent insertion of the compound into the bacterial cytoplasmic membrane.Citation3,Citation4 The interactions with the Gram-positive bacterial surface lead to rapid disruption of the membrane without penetrating into the cytoplasm or causing lysis.Citation5 Daptomycin also inhibits the synthesis of protein, DNA, RNA, and lipoteichoic acid, and is effective at all growth phases including the stationary phase. This property may be particularly useful in the treatment of indolent and deep-seated infections, such as endocarditis and osteomyelitis, where bacteria may exist within biofilm.Citation5
Daptomycin is approved for the treatment of complicated skin and soft tissue infections (cSSTIs) (4 mg/kg/day), right-sided infective endocarditis (RIE) caused by S. aureus, and bacteremia associated with cSSTIs or RIE (6 mg/kg/day).Citation6 Daptomycin is not indicated for the treatment of pneumonia.Citation7
Published data on pharmacokinetics, clinical safety and efficacy/effectiveness (including high dose and combination therapy), utility in outpatient parenteral antimicrobial therapy (OPAT), and drug resistance of daptomycin are summarized.
Pharmacokinetics of daptomycin
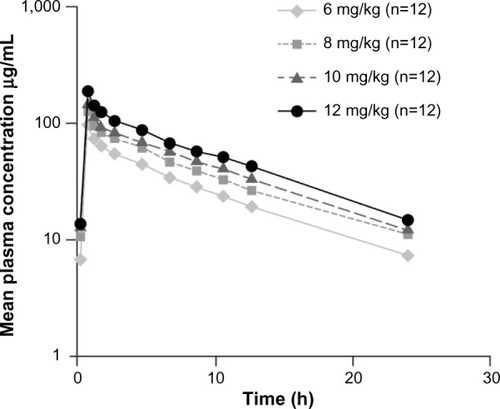
Daptomycin’s pharmacokinetics has been examined in healthy subjects and in patients in both single- and multiple-dose studies. Pharmacokinetics is generally linear and time independent at doses up to at least 12 mg/kg/day administered for 14 days ().Citation8 Following repeated once-daily doses, steady-state concentrations are achieved by the third dose. The serum half-life of the drug is 8–9 hours.Citation9 Daptomycin is renally excreted, and its systemic clearance in healthy adults is 0.011 L/kg/h.Citation10 Dosing must be adjusted for adult subjects who have impaired creatinine clearance.Citation11,Citation12 In patients with chronic kidney disease stage 4 (creatinine clearance <30 mL/min) and in subjects undergoing hemodialysis or peritoneal dialysis, a dosage adjustment of daptomycin is recommended.Citation6,Citation9 Daptomycin has a very small volume of distribution of ∼0.1 L/kg. It is distributed primarily to extracellular fluid, does not cross cell membranes, and is ∼92% reversibly bound to serum proteins.Citation9,Citation13
Figure 1 Plasma daptomycin concentration–time curves at steady state (day 4).
Notes: Antimicrob Agents Chemother, 2006;50(10):3245–3249. DOI: 10.1128/AAC.00247-06,Citation8 reproduced with permission from American Society for Microbiology.
Pharmacokinetic studies of daptomycin have been reported in pediatric patients suggesting that higher doses of daptomycin may be required due to increased plasma clearance and hence lower area under the plasma concentration–time curve as compared with adults.Citation14 In a recently completed study (NCT0711802) of daptomycin in patients aged 1–17 years with cSSTIs, age-adjusted daptomycin doses were administered once daily to achieve exposures demonstrated to be successful in adult studies of cSSTIs: 12–17 years, 5 mg/kg/day; 7–11 years, 7 mg/kg/day; 2–6 years, 9 mg/kg/day; and 1 to <2 years, 10 mg/kg/day.Citation15 As described in the 2011 Infectious Diseases Society of America (IDSA) treatment guidelines, high-dose (6–10 mg/kg/day) daptomycin may be used in pediatric patients as an alternative drug for the management of MRSA bacteremia, infective endocarditis, acute hematogenous osteomyelitis, and septic arthritis.Citation16
As shown in , the pharmacokinetics of daptomycin has been assessed in specific populations, such as critically ill patients and patients with morbid obesity, moderate hepatic failure and renal disease.Citation11,Citation17–Citation21
Table 1 Pharmacokinetics of daptomycin in special populations
Safety studies of daptomycin
Daptomycin has generally been shown to have a good safety profile in several randomized clinical trials ().Citation22–Citation28 In two randomized, controlled, Phase III clinical trials in 1,092 patients with cSSTIs, daptomycin (4 mg/kg/day) therapy was compared with conventional antibiotics such as penicillinase-resistant penicillins.Citation28 Daptomycin was well tolerated. The discontinuation rates were low and similar to standard therapy (2.8% in both groups). The most commonly reported adverse events (AEs) were constipation, nausea, and headache. In an open-label, randomized trial (246 patients) with daptomycin (6 mg/kg/day) use in bacteremia and endocarditis, similar overall incidence of AEs was observed in daptomycin and standard therapy groups.Citation27 Clinically significant renal dysfunction was lower in patients who received daptomycin as compared to those who received standard therapy (11.0% and 26.3%, respectively, P=0.004).
Table 2 Characteristics of the main efficacy and safety studies for daptomycin
Post-marketing real-world studies (mainly from large US [CORE] and European [EU-CORE] registries) support the safety of daptomycin and have shown similar findings as the clinical trials.Citation29–Citation35 A recent systematic review and meta-analysisCitation36 showed that the overall incidence of treatment-related AEs was similar between daptomycin and comparator therapy groups. A significantly lower incidence of renal impairment, nausea, and headache was observed in the daptomycin therapy group. Elevated creatine phosphokinase (CPK) levels were reported, but these resolved rapidly after the discontinuation of daptomycin therapy. CPK elevation and associated skeletal muscle toxicity were frequently reported in early clinical studies that used multiple daily injections of daptomycin. The use of once-daily injection of daptomycin reduced the risk of this toxicity.Citation2 The results from the two clinical Phase III trials showed that elevation in CPK was low (2.1% with daptomycin and 1.4% with standard treatment).Citation28 Only two patients discontinued daptomycin; one had CPK elevation, and the second had symptoms of muscle toxicity. In another study, patients treated with daptomycin (6 mg/kg/day) for endocarditis and bacteremia experienced significantly more CPK elevation compared to standard treatment (6.7% vs 0.9%, P=0.04).Citation27 However, only three patients discontinued daptomycin. The results from real-world clinical experience have shown that a small proportion of patients experienced serum CPK elevation (<2%) and severe skeletal muscle toxicity (0.1%).Citation29
As shown in a few studies, high-dose daptomycin may elevate CPK level at an incidence of 2.5%–8.3%.Citation26,Citation37,Citation38 However, CPK elevation during daptomycin therapy is not always associated with muscle toxicity.Citation39–Citation42 In healthy volunteers, doses up to 12 mg/kg/day were not associated with muscle toxicity.Citation8 In a study on patients treated with high-dose (>6 mg/kg/day) daptomycin, CPK elevations were low (<3.0%).Citation43 Concomitant use of daptomycin and statins may increase CPK levels. There was a twofold risk of CPK elevation with concurrent daptomycin and statin therapy as compared to daptomycin alone.Citation44 The safety analysis of high-dose daptomycin showed similar rates of CPK elevation in those who received concomitant statin therapy (8%) as compared to those who did not (10%).Citation45 CPK levels should be monitored weekly, or more often, in patients with myalgia or concomitant renal failure, or when drugs associated with elevated CPK levels and myopathy are coadministered.Citation46
Daptomycin-induced acute eosinophilic pneumonia is a very rare, unpredictable, and potentially serious AE. It should be suspected in the context of fever, hypoxia, and pulmonary infiltrates.Citation47,Citation48 These symptoms resolve following the discontinuation of daptomycin; however, supportive therapy, including corticosteroids, may be required.Citation49
Although not considered as an AE, apparent prolongation of the prothrombin time may be observed in patients receiving daptomycin due to an interaction with some test reagents, potentially leading to difficulties in therapeutic monitoring for anticoagulation therapy.Citation50
Efficacy studies of daptomycin
Efficacy of daptomycin in patients with cSSTIs, bacteremia, and infective endocarditis caused by S. aureus was demonstrated in several randomized, controlled clinical trials (). Daptomycin (4 mg/kg/day) was compared in two randomized double-blind trials with vancomycin or penicillinase-resistant penicillin for the treatment of cSSTIs.Citation28 Among 902 evaluated patients, the clinical efficacy of daptomycin was 83.4% as compared with 84.2% in the comparator group, and daptomycin required a shorter duration of administration in patients who were successfully treated with intravenous therapy alone. In another study designed to evaluate the efficacy of daptomycin at 6 mg/kg/day in patients with bacteremia or infective endocarditis caused by S. aureus, daptomycin was non-inferior to standard treatment (either vancomycin or an anti-staphylococcal penicillin).Citation27 At the request of the Committee for Medicinal Products for Human Use, a comparative study of daptomycin in the elderly population was conducted, which showed that daptomycin had similar efficacy to semisynthetic penicillins and vancomycin in the treatment of cSSTIs.Citation23 A recent meta-analysis including 13 randomized controlled trials compared the efficacy of daptomycin with that of comparator therapy.Citation36 The results showed that daptomycin was as efficacious as standard therapy in the eradication of pathogens (methicillin-susceptible S. aureus, MRSA, Streptococcus pyogenes, Enterococcus faecalis, and Streptococcus pneumoniae), and significantly reduced the intravenous treatment duration.
Clinical trials with rigorous inclusion and exclusion criteria may not reflect true clinical experience. The EU-CORE study provided insight into the real-world clinical experience and supported findings from these clinical trials.Citation29 Analyses from the EU-CORE study in patients with various Gram-positive infections (cSSTIs, bacteremia, right- and left-sided infective endocarditis, enterococcal infections, foreign body/prosthetic infections, and osteomyelitis) showed high and consistent rates of effectiveness.Citation29–Citation32,Citation34,Citation35
High-dose daptomycin
In difficult-to-treat infections, on the basis of its pharmacokinetic profile and concentration-dependent bactericidal activity, high-dose (>6 mg/kg/day) daptomycin may be considered due to the potential for more rapid bacterial clearance and reduced risk of emergence of resistance.Citation51,Citation52 The IDSA MRSA guidelines recommend consideration of high-dose (10 mg/kg/day) daptomycin in patients with persistent MRSA bacteremia associated with vancomycin failure.Citation16 Several other national and international treatment guidelines include high-dose (8–10 mg/kg/day) daptomycin as a therapeutic option for difficult-to-treat infections including endocarditis, bacteremia, and bone/joint infection.Citation53–Citation55 High-dose daptomycin may also be advantageous in patients with sepsis and high volumes of distribution, or when there is a difficulty in achieving adequate local antibiotic concentration at the infection site.Citation56,Citation57
Several studies have suggested that higher doses (>6 mg/kg/day) of daptomycin are safe and effective in patients with bacteremia, osteomyelitis, foreign body/prosthetic infection (mainly orthopedic, intracardiac, and intravascular devices), and endocarditis ().Citation26,Citation38,Citation43,Citation58–Citation60
Table 3 Clinical outcome and safety in high-dose daptomycin studies
Clinicians should be cautious when using non-licensed doses of daptomycin in obese patients as they may achieve higher exposure from reduced volume of distribution when compared to nonobese patients.Citation18,Citation61
Daptomycin within combination antimicrobial therapy
In clinical practice, daptomycin is recommended at doses that are higher than those currently approved (4–6 mg/kg/day) for the treatment of certain infections (osteomyelitis, foreign body/prosthetic infections, and enterococcal infections).Citation16 However, emerging reports of the development of daptomycin resistanceCitation62 during therapy are cause of concern, and it may be appropriate to consider combination antibiotic therapy in patients at higher risk of developing daptomycin resistance.Citation63 Interactions between daptomycin and other antibiotics have been studied in vitro, and it has been shown that activity of gentamicin and rifampicin administered concomitantly with daptomycin was not affected.Citation64–Citation67 Other in vitro models demonstrated that the combination of daptomycin and linezolid was synergistic and bactericidal for MRSA and for enterococci.Citation68–Citation70 In an in vitro simulated endocarditis pharmacokinetic/pharmacodynamic model, with daptomycin-nonsusceptible MRSA isolates (SA-684 and R6003), daptomycin plus trimethoprim–sulfamethoxazole was bactericidal (8 hours) and superior to daptomycin alone between 8 and 72 hours (P<0.001).Citation71 In a clinical study, the overall clinical outcome was slightly enhanced with the addition of a β-lactam; this trend was better for bacteremia associated with endocarditis or bone/joint infection.Citation72
Despite appropriate antimicrobial therapy, bacteremia due to MRSA remains a challenge.Citation73 To increase activity and to prevent resistance, high-dose daptomycin used concomitantly with other antimicrobial agents has been considered in treating severe infections and is recommended within IDSA guidelines.Citation16 Daptomycin combined with β-lactams prevents the emergence of resistance to daptomycin in clinical MRSA isolates and in enterococci.Citation64,Citation74–Citation76 Mechanistically, β-lactams increase the negative charge of the bacterial cell surface leading to a better daptomycin binding and therefore improving its bactericidal activity.Citation77 The synergy between daptomycin and β-lactams leading to bacterial killing and growth inhibition could also be explained by the penicillin-binding protein 1 inactivation following exposure to β-lactams.Citation78,Citation79 Daptomycin has also been used in combination with rifampin, trimethoprim–sulfamethoxazole, fosfomycin, tigecycline, and linezolid in order to achieve the resolution of MRSA infections.Citation80–Citation83 A combination of high-dose daptomycin and fosfomycin may be effective in the treatment of both native- and prosthetic-valve endocarditis caused by methicillin-susceptible S. aureus or MRSA.Citation84
Daptomycin use in pediatric patients
Daptomycin is currently not approved for use in the pediatric population, and appropriate dosages for pediatric patients of different ages are yet to be clearly defined.Citation85 A pharmacokinetic study in 25 children (2–17 years of age) receiving 4 mg/kg/day of daptomycin showed a more rapid clearance in those younger than 6 years.Citation86 Another study conducted later showed that use of high-dose (8–10 mg/kg/day) daptomycin in children aged 2–6 years provided systemic exposure comparable to that seen in adults treated with the approved daptomycin doses of 4–6 mg/kg/day.Citation87 Daptomycin’s role in pediatric Gram-positive infections has been evaluated in several studies, and a good safety profile has been observed.Citation9,Citation10,Citation86–Citation89 The IDSA MRSA treatment guidelines recommend the use of daptomycin (6–10 mg/kg/day) for managing MRSA bacteremia, infective endocarditis, acute hematogenous osteomyelitis, and septic arthritis in pediatric patients.Citation16
In a recent multicenter, randomized, Phase III trial that included in total 396 children with cSSTIs, daptomycin given at age-appropriate doses was shown to be efficacious, safe, and generally well tolerated compared with the standard of care.Citation15 Results from the EU-CORE registry showed that children and adolescent patients with a variety of Gram-positive infections treated with daptomycin had a high clinical success rate when daptomycin was used as a first- or second-line therapy.Citation90
To further investigate the safety and efficacy of daptomycin in pediatric patients aged 1–17 years, a study (NCT1728376) comparing daptomycin to the standard of care for the treatment of S. aureus bacteremia was recently completed, and another study (NCT1922011) comparing daptomycin to vancomycin or nafcillin for the treatment of acute hematogenous osteomyelitis is currently ongoing.
Daptomycin use in outpatient practice
OPAT has been used in many countries to facilitate the cost-effective, safe administration of antibiotics as an alternative to an extensive and expensive hospital stay,Citation91,Citation92 and to reduce health care-associated infection risk and improve patient satisfaction.Citation93,Citation94 The most frequently treated infections in OPAT programs are skin and soft tissue infections (cellulitis, erysipelas, wound infection, and bursitis) and bone and joint infections (discitis, septic arthritis, diabetic foot osteomyelitis, and prosthetic joint and other metalwork-related infections).Citation95 Careful attention to risk assessment and management can minimize potential risks in the OPAT setting,Citation96 and several national guidelines have been developed to guide service development and patient management.Citation93,Citation97–Citation99 Many studies have demonstrated that OPAT is indeed an effective and safe service.Citation100–Citation105 The clinical efficacy of OPAT has also been demonstrated for endocarditis or S. aureus bacteremia when delivered through a formal service model,Citation106,Citation107 and OPAT is now included in European, UK, and US guidelines on the management of endocarditis.Citation108
The pharmacokinetic properties of daptomycin allow convenient once-daily 2-minute intravenous injection,Citation6 which facilitates outpatient or home administration.Citation95,Citation109 This avoids multiple dosing or continuous infusion as required with other available antibiotics. Daptomycin in the OPAT setting is also associated with significantly fewer AEs and antimicrobial interventions compared to vancomycin.Citation110 In EU-CORE, 12% of patients received daptomycin within an OPAT setting with an overall clinical success in 89% of these patients; the highest clinical success rates were in patients with bacteremia or endocarditis.Citation95 Because of its ease of administration and an overall good safety profile, daptomycin has been considered as a first-line agent for use within OPAT.Citation111–Citation113
Daptomycin resistance
Emergence of antibiotic resistance in Gram-positive pathogens has become a major clinical and public health problem worldwide.Citation78,Citation114–Citation116 Considering the limited number of new antimicrobial agents, the use of antibiotics in hospitals or elsewhere demands an acute awareness of the increasing problems with resistant organisms.Citation5,Citation62 According to the antimicrobial resistance global report on surveillance, antimicrobial resistance has been a growing threat to the effective treatment of an ever-increasing range of bacterial infections.Citation117 To assess and monitor the magnitude and trends of the antibiotic resistance problem, major surveillance systems have been implemented.Citation118
Daptomycin resistance has been previously documented for S. aureus and enterococci.Citation119–Citation123 Also, few clinical reports showing emergence of daptomycin resistance have been published until now. The prevalence of de novo resistance to daptomycin without prior exposure has been reported to be extremely rare, showing that only 0.04% among 10,000 S. aureus isolates tested had an MIC of 2 µg/mL.Citation124 The Worldwide Surveillance Programme reported resistance data on daptomycin and numerous comparator agents in 164,457 Gram-positive clinical isolates (S. aureus, coagulase-negative staphylococci, enterococci, hemolytic streptococci) across five continents between 2005 and 2012. The results indicated that daptomycin remained potent against these indicated pathogens.Citation125
The mechanisms of daptomycin resistance are multifactorial.Citation74,Citation116,Citation126–Citation128 Perturbation of the bacterial cell membrane and overexpression of dltA seem to be considered as the most common factors of resistance.Citation124 This dysregulation of dltA transcription may result in a change of the bacterial cell membrane fluidity and therefore lead to a reduced affinity of daptomycin to its target site.Citation129 The resistance pathways may vary among Gram-positive organisms.Citation130 Mutations in the genes involved in phospholipid synthesis seem to be associated with the development of daptomycin resistance in S. aureus and E. faecalis.Citation130,Citation131
Future directions
Further clinical trials are warranted to better understand how to use high-dose daptomycin, that is, in which patient groups and for which pathogens, and to investigate the utility of short-course regimen. Such trials could provide data on how to minimize the risk of development of resistance and to optimize clinical outcomes. Also, understanding the optimal dosing of daptomycin remains a clinical priority, as well as increasing awareness of the relative benefits of combination therapy. Further research is needed in the form of well-designed, adequately powered trials comparing the efficacy and safety of daptomycin with other new anti-Gram-positive agents for treating different infections and eradicating pathogens.
Prosthetic joint infection is a devastating and costly complication of arthroplasty. Antibiotic-impregnated cement spacers are a useful tool for facilitating the penetration of the drug into the local infected area.Citation132 The first clinical use of daptomycin-impregnated cement was described by Cortes et al who demonstrated a successful treatment in one patient with multiple allergies treated for chronic MRSA hip prosthetic infection.Citation133 However, clinical data are lacking, and studies are needed to further evaluate daptomycin’s utility in this setting. More generally, implant-associated infection is serious and costly and associated with high morbidity.Citation134 The presence of biofilm allows persistence of bacteria in a difficult-to-eradicate physiological state and increases the risk of resistance development.Citation135 Further investigation of daptomycin’s role within biofilm-related infection is warranted.Citation136
Conclusion
Daptomycin exhibits linear pharmacokinetics at doses up to 12 mg/kg/day and has been shown to be safe and efficacious for the treatment of cSSTIs, bacteremia, and RIE caused by susceptible Gram-positive bacteria in adults. In addition, according to post-marketing studies, daptomycin is a valuable treatment option in the management of other Gram-positive and difficult-to-treat infections, including left-sided endocarditis, osteomyelitis, prosthetic infections, and enterococcal infections.
On the basis of its pharmacokinetic profile and dose-dependent bactericidal activity, higher doses of daptomycin may be beneficial in treating severe infections. High-dose (>6 mg/kg/day) daptomycin used in the post-marketing observational studies exhibited a good safety and tolerability profile.
Of particular interest, daptomycin given in combination with other antibiotics may lead to resolution of various complex and resistant Gram-positive infections and may have a role (along with high-dose therapy) in minimizing the development of resistance.
Daptomycin appears promising as a safe and efficacious drug for the treatment of pediatric diseases caused by Gram-positive pathogens.
Daptomycin is recognized as an important agent in OPAT practice due to its spectrum of activity and ease of administration.
Although daptomycin resistance has been documented, it remains uncommon.
Acknowledgments
Medical writing support was provided by Farid Khalfi (Novartis Ireland Ltd, Dublin, Ireland).
Disclosure
AGR received fees from Novartis, Pfizer, Cubist, and Gilead for staff training, and is a member of advisory boards and speaker panels. RAS received consultancy fees and honoraria for speaking at Novartis-sponsored symposia. KH is an employee of Novartis Pharmaceuticals Corporation. The authors report no other conflicts of interest in this work.
References
- van HalSJPatersonDLNew Gram-positive antibiotics: better than vancomycin?Curr Opin Infect Dis201124651552021844804
- DvorchikBHBrazierDDeBruinMFArbeitRDDaptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjectsAntimicrob Agents Chemother20034741318132312654665
- NakhatePHYadavVKPathakANA review on daptomycin; the first US-FDA approved lipopeptide antibioticsJ Sci Innov Res201325970980
- LaPlanteKLRybakMJDaptomycin – a novel antibiotic against Gram-positive pathogensExpert Opin Pharmacother20045112321233115500379
- HancockREMechanisms of action of newer antibiotics for Gram-positive pathogensLancet Infect Dis20055420921815792738
- Novartis Europharm LtdCubicin® (daptomycin) summary of product characteristics2014
- PotashmanMHFormellaDNHamedKMohrJFComment on: efficacy and safety of daptomycin for the treatment of infectious disease: a meta-analysis based on randomized controlled trialsJ Antimicrob Chemother20157041274127525583745
- BenvenutoMBenzigerDPYankelevSViglianiGPharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteersAntimicrob Agents Chemother200650103245324917005801
- BaiettoLCorcioneSPaciniGPerriGDD’AvolioADe RosaFGA 30-years review on pharmacokinetics of antibiotics: is the right time for pharmacogenetics?Curr Drug Metab201415658159824909419
- GostelowMGonzalezDSmithPBCohen-WolkowiezMPharmacokinetics and safety of recently approved drugs used to treat methicillin-resistant Staphylococcus aureus infections in infants, children and adultsExpert Rev Clin Pharmacol20147332734024716805
- DvorchikBModerate liver impairment has no influence on daptomycin pharmacokineticsJ Clin Pharmacol200444771572215199076
- SchrieverCAFernandezCRodvoldKADanzigerLHDaptomycin: a novel cyclic lipopeptide antimicrobialAm J Health Syst Pharm200562111145115815914875
- EstesKSDerendorfHComparison of the pharmacokinetic properties of vancomycin, linezolid, tigecyclin, and daptomycinEur J Med Res2010151253354321163728
- DurandCBruecknerASampadianCWillettKCBelliveauPDaptomycin use in pediatric patientsAm J Health Syst Pharm201471141177118224973375
- GlasserCAgarwalRBradleyJYoonMBokeschPSafety and efficacy of daptomycin in children with complicated skin and skin structure infections (cSSSI) caused by Gram-positive pathogensAbstract presented at: 33rd Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID)May 14; 2015Leipzig
- LiuCBayerACosgroveSEClinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summaryClin Infect Dis201152328529221217178
- Di PaoloATasciniCPolilloMPopulation pharmacokinetics of daptomycin in patients affected by severe Gram-positive infectionsInt J Antimicrob Agents201342325025523891432
- DvorchikBHDamphousseDThe pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjectsJ Clin Pharmacol2005451485615601805
- CortiNRudigerAChiesaAPharmacokinetics of daily daptomycin in critically ill patients undergoing continuous renal replacement therapyChemotherapy201359214315124051895
- KhadzhynovDSlowinskiTLiekerIPlasma pharmacokinetics of daptomycin in critically ill patients with renal failure and undergoing CVVHDInt J Clin Pharmacol Ther2011491165666522011690
- KullarRMcClellanIGeriakMSakoulasGEfficacy and safety of daptomycin in patients with renal impairment: a multicenter retrospective analysisPharmacotherapy201434658258924658897
- AikawaNKusachiSMikamoHEfficacy and safety of intravenous daptomycin in Japanese patients with skin and soft tissue infectionsJ Infect Chemother201319344745523085743
- KonychevAHeepMMoritzRKSafety and efficacy of daptomycin as first-line treatment for complicated skin and soft tissue infections in elderly patients: an open-label, multicentre, randomized phase IIIb trialDrugs Aging2013301082983623990341
- QuistSRFierlbeckGSeatonRALoefflerJChavesRLComparative randomised clinical trial against glycopeptides supports the use of daptomycin as first-line treatment of complicated skin and soft-tissue infectionsInt J Antimicrob Agents2012391909121982144
- PertelPEEisensteinBILinkASThe efficacy and safety of daptomycin vs. vancomycin for the treatment of cellulitis and erysipelasInt J Clin Pract200963336837519222623
- KatzDELindfieldKCSteenbergenJNA pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by gram-positive bacteriaInt J Clin Pract20086291455146418662172
- FowlerVGJrBoucherHWCoreyGRDaptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureusN Engl J Med2006355765366516914701
- ArbeitRDMakiDTallyFPThe safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infectionsClin Infect Dis200438121673168115227611
- Gonzalez-RuizAGargalianos-KakolyrisPTimermanADaptomycin in the clinical setting: 8-year experience with Gram-positive bacterial infections from the EU-CORE(SM) registryAdv Ther201532649650926108157
- CogoAGonzalez-RuizAPathanRHamedKReal-world treatment of complicated skin and soft tissue infections with daptomycin: results from a large European registry (EU-CORE)Infect Dis Ther20154327328226168987
- LubbertCRodloffACHamedKReal-world treatment of enterococcal infections with daptomycin: insights from a large European registry (EU-CORE)Infect Dis Ther20154325927126168986
- KeilFDaikosGLSkoutelisADominguezJIPathanRHamedKDaptomycin for Gram-positive infections in patients with neutropenia: clinical experience from a European outcomes registryAdv Ther201532871572626239201
- MohrJFFriedrichLVYankelevSLampKCDaptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE)Int J Antimicrob Agents200933654354819201165
- MalizosKSarmaJSeatonRADaptomycin for the treatment of osteomyelitis and orthopaedic device infections: real-world clinical experience from a European registryEur J Clin Microbiol Infect Dis201635111111826563898
- GuleriAUtiliRDohmenPDaptomycin for the treatment of infective endocarditis: results from European Cubicin® Outcomes Registry and Experience (EU-CORE)Infect Dis Ther20154328329626168988
- HeWZhangYChenHZhaoCWangHEfficacy and safety of daptomycin for the treatment of infectious disease: a meta-analysis based on randomized controlled trialsJ Antimicrob Chemother201469123181318925063779
- FigueroaDAManginiEAmodio-GrotonMSafety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical programClin Infect Dis200949217718019500039
- MoisePAHershbergerEAmodio-GrotonMILampKCSafety and clinical outcomes when utilizing high-dose (> or =8 mg/kg) daptomycin therapyAnn Pharmacother20094371211121919584384
- Durante-MangoniECasilloRBernardoMHigh-dose daptomycin for cardiac implantable electronic device-related infective endocarditisClin Infect Dis201254334735422100575
- De RosaFGMollarettiOComettoCPaganiNMontrucchioCDi PerriGEarly experience with high-dosage daptomycin for prosthetic infectionsClin Infect Dis200949111772177319891570
- KingEAMcCoyDDesaiSNyirendaTBickingKVancomycin-resistant enterococcal bacteraemia and daptomycin: are higher doses necessary?J Antimicrob Chemother20116692112211821697178
- KullarRDavisSLLevineDPHigh-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective studyPharmacotherapy201131652753621923436
- SeatonRAMenichettiFDalekosGEvaluation of effectiveness and safety of high-dose daptomycin: results from patients included in the European Cubicin® Outcomes Registry and ExperienceAdv Ther201532121192120526610384
- BergMLEstesLLDierkhisingRACurranBEnzlerMJEvaluation of impact of statin use on development of CPK elevation during daptomycin therapyAnn Pharmacother201448332032724321853
- Parra-RuizJDuenas-GutierrezCTomas-JimenezCLinares-PalominoJPGarrido-GomezJHernandez-QueroJSafety analysis of high dose (>6 mg/kg/day) daptomycin in patients with concomitant statin therapyEur J Clin Microbiol Infect Dis20123181771177422160888
- HairPIKeamSJDaptomycin: a review of its use in the management of complicated skin and soft-tissue infections and Staphylococcus aureus bacteraemiaDrugs200767101483151217600394
- KimPWSorbelloAFWasselRTPhamTMTonningJMNambiarSEosinophilic pneumonia in patients treated with daptomycin: review of the literature and US FDA adverse event reporting system reportsDrug Saf201235644745722612850
- MillerBAGrayALeblancTWSextonDJMartinARSlamaTGAcute eosinophilic pneumonia secondary to daptomycin: a report of three casesClin Infect Dis20105011e63e6820420515
- PatelJJAntonyAHerreraMLipchikRJDaptomycin-induced acute eosinophilic pneumoniaWMJ2014113519920125739164
- WhiteBSeatonRAComplicated skin and soft tissue infections: literature review of evidence for and experience with daptomycinInfect Drug Resist2011411512721753891
- WuGAbrahamTRappJVasteyFSaadNBalmirEDaptomycin: evaluation of a high-dose treatment strategyInt J Antimicrob Agents201138319219621549573
- BassettiMNiccoEGinocchioFAnsaldiFde FlorentiisDViscoliCHigh-dose daptomycin in documented Staphylococcus aureus infectionsInt J Antimicrob Agents201036545946120846832
- GarauJBouzaEChastreJGudiolFHarbarthSManagement of methicillin-resistant Staphylococcus aureus infectionsClin Microbiol Infect200915212513619291144
- GudiolFAguadoJMPascualAConsensus document for the treatment of bacteremia and endocarditis caused by methicillin-resistant Staphylococcus aureus. Sociedad Española de Enfermedades Infecciosas y Microbiología ClínicaEnferm Infecc Microbiol Clin2009272105115 Spanish19254641
- MensaJBarberanJLlinaresPGuía de tratamiento de la infección producida por Staphylococcus aureus resistente a meticilina [Guidelines for the treatment on infections caused by methicillin-resistant Staphylococcus aureus]Rev Esp Quimioter2008214234258 Spanish19031124
- FalconeMRussoAVendittiMNovelliAPaiMPConsiderations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremiaClin Infect Dis201357111568157624046298
- GouldIMMiroJMRybakMJDaptomycin: the role of high-dose and combination therapy for Gram-positive infectionsInt J Antimicrob Agents201342320221023845504
- ByrenIRegeSCampanaroERandomized controlled trial of the safety and efficacy of Daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplastyAntimicrob Agents Chemother201256115626563222908174
- CarugatiMBayerASMiroJMHigh-dose daptomycin therapy for left-sided infective endocarditis: a prospective study from the international collaboration on endocarditisAntimicrob Agents Chemother201357126213622224080644
- CasapaoAMKullarRDavisSLMulticenter study of high-dose daptomycin for treatment of enterococcal infectionsAntimicrob Agents Chemother20135794190419623774437
- PaiMPNorenbergJPAndersonTInfluence of morbid obesity on the single-dose pharmacokinetics of daptomycinAntimicrob Agents Chemother20075182741274717548489
- KaurDCChateSSStudy of antibiotic resistance pattern in methicillin resistant Staphylococcus aureus with special reference to newer antibioticJ Glob Infect Dis201572788426069428
- EntenzaJMGiddeyMVouillamozJMoreillonPIn vitro prevention of the emergence of daptomycin resistance in Staphylococcus aureus and enterococci following combination with amoxicillin/clavulanic acid or ampicillinInt J Antimicrob Agents201035545145620185277
- NadrahKStrleFAntibiotic combinations with daptomycin for treatment of Staphylococcus aureus infectionsChemother Res Pract2011201161932122312555
- SteenbergenJNMohrJFThorneGMEffects of daptomycin in combination with other antimicrobial agents: a review of in vitro and animal model studiesJ Antimicrob Chemother20096461130113819825818
- YangSJXiongYQBoyle-VavraSDaumRJonesTBayerASDaptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”)Antimicrob Agents Chemother20105483161316920547804
- BarberKEWerthBJIrelandCEPotent synergy of ceftobiprole plus daptomycin against multiple strains of Staphylococcus aureus with various resistance phenotypesJ Antimicrob Chemother201469113006301024990867
- Parra-RuizJBravo-MolinaAPena-MonjeAHernandez-QueroJActivity of linezolid and high-dose daptomycin, alone or in combination, in an in vitro model of Staphylococcus aureus biofilmJ Antimicrob Chemother201267112682268522796888
- EntenzaJMGiddeyMVouillamozJMoreillonPManciniSAssessment of the in vitro synergy of daptomycin plus linezolid against multidrug-resistant enterococciJ Global Antimicrob Resistance20142306308
- LeJBookstaverPBRudisillCNTreatment of meningitis caused by vancomycin-resistant Enterococcus faecium: high-dose and combination daptomycin therapyAnn Pharmacother201044122001200621119097
- SteedMEVidaillacCRybakMJNovel daptomycin combinations against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro model of simulated endocardial vegetationsAntimicrob Agents Chemother201054125187519220921318
- MoisePAAmodio-GrotonMRashidMMulticenter evaluation of the clinical outcomes of daptomycin with and without concomitant beta-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairmentAntimicrob Agents Chemother20135731192120023254428
- SakoulasGMoisePACasapaoAMAntimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftarolineClin Ther201436101317133325017183
- BertiADWerginJEGirdaukasGGHetzelSJSakoulasGRoseWEAltering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibioticsAntimicrob Agents Chemother201256105046505322802248
- MehtaSSinghCPlataKBbeta-Lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivativesAntimicrob Agents Chemother201256126192620022985884
- RoseWESchulzLTAndesDAddition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activityAntimicrob Agents Chemother201256105296530222869564
- OrtwineJKWerthBJSakoulasGRybakMJReduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: taking advantage of the back door left open?Drug Resist Updat2013163–5737924268586
- BertiADSakoulasGNizetVTewheyRRoseWEbeta-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureusAntimicrob Agents Chemother201357105005501223896478
- BertiADTheisenESauerJDPenicillin binding protein 1 is important in the compensatory response of Staphylococcus aureus to daptomycin-induced membrane damage and is a potential target for beta-lactam-daptomycin synergyAntimicrob Agents Chemother201560145145826525797
- ClaeysKCSmithJRCasapaoAMImpact of the combination of daptomycin and trimethoprim-sulfamethoxazole on clinical outcomes in methicillin-resistant Staphylococcus aureus infectionsAntimicrob Agents Chemother20155941969197625605354
- DhandASakoulasGDaptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremiaClin Ther201436101303131625444563
- KelesidisTHumphriesRWardKLewinskiMAYangOOCombination therapy with daptomycin, linezolid, and rifampin as treatment option for MRSA meningitis and bacteremiaDiagn Microbiol Infect Dis201171328629021855248
- AhmadNMRojtmanADSuccessful treatment of daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus bacteremia with the addition of rifampin to daptomycinAnn Pharmacother201044591892120354160
- MiroJMEntenzaJMDel RioAHigh-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditisAntimicrob Agents Chemother20125684511451522644033
- PrincipiNCaironiMVenturiniFPaniLEspositoSDaptomycin in paediatrics: current knowledge and the need for future researchJ Antimicrob Chemother201570364364825406298
- Abdel-RahmanSMBenzigerDPJacobsRFJafriHSHongEFKearnsGLSingle-dose pharmacokinetics of daptomycin in children with suspected or proved gram-positive infectionsPediatr Infect Dis J200827433033418316988
- Abdel-RahmanSMChandorkarGAkinsRLSingle-dose pharmacokinetics and tolerability of daptomycin 8 to 10 mg/kg in children aged 2 to 6 years with suspected or proved Gram-positive infectionsPediatr Infect Dis J201130871271421317681
- AntachopoulosCIosifidisESarafidisKSerum levels of daptomycin in pediatric patientsInfection201240436737122271402
- ArduraMIMejiasAKatzKSRevellPMcCrackenGHJrSanchezPJDaptomycin therapy for invasive Gram-positive bacterial infections in childrenPediatr Infect Dis J200726121128113218043450
- SyriopoulouVDailianaZDmitriyNUtiliRPathanRHamedKClinical experience with daptomycin for the treatment of Gram-positive infections in children and adolescentsPediatr Infect Dis J Epub201622
- PaladinoJAPoretzDOutpatient parenteral antimicrobial therapy todayClin Infect Dis201051Suppl 2S198S20820731577
- WilliamsDNBakerCAKindACSannesMRThe history and evolution of outpatient parenteral antibiotic therapy (OPAT)Int J Antimicrob Agents201546330731226233483
- ChapmanALSeatonRACooperMAGood practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statementJ Antimicrob Chemother20126751053106222298347
- SeatonRABarrDAOutpatient parenteral antibiotic therapy: principles and practiceEur J Intern Med201324761762323602223
- SeatonRAGonzalez-RamalloVJPriscoVDaptomycin for outpatient parenteral antibiotic therapy: a European registry experienceInt J Antimicrob Agents201341546847223473943
- HalilovicJChristensenCLNguyenHHManaging an outpatient parenteral antibiotic therapy team: challenges and solutionsTher Clin Risk Manag20141045946524971015
- HowdenBPGraysonML5: Hospital-in-the-home treatment of infectious diseasesMed J Aust2002176944044512056999
- NathwaniDZambrowskiJJAdvisory group on Home-based and Outpatient Care (AdHOC): an international consensus statement on non-inpatient parenteral therapyClin Microbiol Infect20006946447611168180
- TiceADRehmSJDalovisioJRPractice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelinesClin Infect Dis200438121651167215227610
- EspositoSOutpatient parenteral treatment of bacterial infections: the Italian model as an international trend?J Antimicrob Chemother200045672472710837423
- HitchcockJJepsonAPMainJWickensHJEstablishment of an outpatient and home parenteral antimicrobial therapy service at a London teaching hospital: a case seriesJ Antimicrob Chemother200964363063419549671
- ChapmanALDixonSAndrewsDLilliePJBazazRPatchettJDClinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspectiveJ Antimicrob Chemother20096461316132419767623
- CorwinPToopLMcGeochGRandomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospitalBMJ2005330748312915604157
- PartridgeDGO’BrienEChapmanALOutpatient parenteral antibiotic therapy for infective endocarditis: a review of 4 years’ experience at a UK centrePostgrad Med J201288104137738122366395
- MatthewsPCConlonCPBerendtAROutpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 yearsJ Antimicrob Chemother200760235636217566002
- GouldFKDenningDWElliottTSGuidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial ChemotherapyJ Antimicrob Chemother201267226928922086858
- BarrDASempleLSeatonRAOutpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 yearsInt J Antimicrob Agents201239540741322445493
- HabibGHoenBTornosPGuidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and CancerEur Heart J200930192369241319713420
- ChakrabortyARoySLoefflerJChavesRLComparison of the pharmacokinetics, safety and tolerability of daptomycin in healthy adult volunteers following intravenous administration by 30 min infusion or 2 min injectionJ Antimicrob Chemother200964115115819389714
- ShresthaNKMasonPGordonSMAdverse events, healthcare interventions and healthcare utilization during home infusion therapy with daptomycin and vancomycin: a propensity score-matched cohort studyJ Antimicrob Chemother20146951407141524398341
- CerveraCMestresCADaptomycin in outpatient antimicrobial parenteral therapyEnferm Infecc Microbiol Clin201230Suppl 15963 Spanish22541978
- MartoneWJLindfieldKCKatzDEOutpatient parenteral antibiotic therapy with daptomycin: insights from a patient registryInt J Clin Pract20086281183118718705821
- NathwaniDDevelopments in outpatient parenteral antimicrobial therapy (OPAT) for Gram-positive infections in Europe, and the potential impact of daptomycinJ Antimicrob Chemother200964344745319584105
- PatelHShahAMistryMChandaSIn vitro antimicrobial susceptibility study in clinical isolates of streptococci and enterococciAfr J Microbiol Res2011513741378
- VentolaCLThe antibiotic resistance crisis: part 1: causes and threatsP T201540427728325859123
- Garcia-de-la-MariaCPericasJMDel RioAEarly in vitro and in vivo development of high-level daptomycin resistance is common in mitis group Streptococci after exposure to daptomycinAntimicrob Agents Chemother20135752319232523478959
- WHOAntimicrobial resistance: global report on surveillance2014 Available from: http://www.who.int/drugresistance/documents/surveillancereport/en/Accessed April 2016
- BrandtCMakarewiczOFischerTThe bigger picture: the history of antibiotics and antimicrobial resistance displayed by scientometric dataInt J Antimicrob Agents201444542443025216545
- TraczewskiMMKatzBDSteenbergenJNBrownSDInhibitory and bactericidal activities of daptomycin, vancomycin, and teicoplanin against methicillin-resistant Staphylococcus aureus isolates collected from 1985 to 2007Antimicrob Agents Chemother20095351735173819223623
- DiederenBMvan DuijnIWillemsePKluytmansJAIn vitro activity of daptomycin against methicillin-resistant Staphylococcus aureus, including heterogeneously glycopeptide-resistant strainsAntimicrob Agents Chemother20065093189319116940127
- KrauseKMRenelliMDifuntorumSWuTXDebabovDVBentonBMIn vitro activity of telavancin against resistant gram-positive bacteriaAntimicrob Agents Chemother20085272647265218443122
- LewisJS2ndOwensACadenaJSabolKPattersonJEJorgensenJHEmergence of daptomycin resistance in Enterococcus faecium during daptomycin therapyAntimicrob Agents Chemother20054941664166515793168
- Munoz-PriceLSLolansKQuinnJPEmergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infectionClin Infect Dis200541456556616028170
- StefaniSCampanileFSantagatiMMezzatestaMLCafisoVPaciniGInsights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: a review of the available evidenceInt J Antimicrob Agents201546327828926143590
- SaderHSFarrellDJFlammRKJonesRNDaptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005–2012)Int J Antimicrob Agents201443546546924636430
- KelesidisTTewheyRHumphriesRMEvolution of high-level daptomycin resistance in Enterococcus faecium during daptomycin therapy is associated with limited mutations in the bacterial genomeJ Antimicrob Chemother20136881926192823580562
- MishraNNBayerASCorrelation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureusAntimicrob Agents Chemother20135721082108523254419
- NanniniEMurrayBEAriasCAResistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureusCurr Opin Pharmacol201010551652120598637
- MishraNNBayerASWeidenmaierCPhenotypic and genotypic characterization of daptomycin-resistant methicillin-resistant Staphylococcus aureus strains: relative roles of mprF and dlt operonsPLoS One201499e10742625226591
- TranTTMunitaJMAriasCAMechanisms of drug resistance: daptomycin resistanceAnn N Y Acad Sci20151354325326495887
- HumphriesRMPollettSSakoulasGA current perspective on daptomycin for the clinical microbiologistClin Microbiol Rev201326475978024092854
- LegoutLSennevilleEPeriprosthetic joint infections: clinical and bench researchScientificWorldJournal2013201354909124288493
- CortesNJLloydJMKoziolLO’HaraLSuccessful clinical use of daptomycin-impregnated bone cement in two-stage revision hip surgery for prosthetic joint infectionAnn Pharmacother2013471e223324502
- TsarasGOsmonDRMabryTIncidence, secular trends, and outcomes of prosthetic joint infection: a population-based study, olmsted county, Minnesota, 1969–2007Infect Control Hosp Epidemiol201233121207121223143357
- ArciolaCRCampocciaDSpezialePMontanaroLCostertonJWBiofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materialsBiomaterials201233265967598222695065
- GbejuadeHOLoveringAMWebbJCThe role of microbial biofilms in prosthetic joint infectionsActa Orthop201586214715825238433
