Abstract
Background
Pulmonary hypertension (PH), a disease which carries substantial morbidity and mortality, has been reported to occur in 25%–45% of dialysis patients. No prospective evaluation of the prevalence or clinical significance of PH in chronic dialysis patients in the United States (US) has been undertaken.
Methods
Echocardiograms were performed prospectively in chronic hemodialysis patients prior to dialysis at a single dialysis center. PH was defined as a tricuspid regurgitant jet ≥2.5 m/s and “more severe PH” as ≥3.0 m/s. Clinical outcomes recovered were all-cause hospitalizations and death at 12 months.
Results
In a cohort of 90 patients, 42 patients (47%) met the definition of PH. Of those, 18 patients (20%) met the definition of more severe PH. At 12 months, mortality was significantly higher in patients with PH (26%) compared with patients without PH (6%). All-cause hospitalizations were similar in patients with PH and without PH. Echocardiographic findings suggesting impaired left ventricular function and elevated pulmonary capillary wedge pressure were significantly associated with PH.
Conclusion
This prospective cross-sectional study of a single dialysis unit suggests that PH may be present in nearly half of US dialysis patients and when present is associated with increased mortality. Echocardiographic findings demonstrate an association between elevated filling pressures, elevated pulmonary artery pressures, and higher mortality, suggesting that the PH may be secondary to diastolic dysfunction and compounded by volume overload.
Background
Pulmonary hypertension (PH) regardless of etiology has been associated with increased morbidity and mortality. In patients with end-stage renal disease (ESRD) requiring chronic hemodialysis (HD) retrospective record reviews of largely non-US populations suggest that PH occurs in about 25%–45% of patients.Citation1–Citation3 Various mechanisms have been postulated for its development, including disturbances in endothelial and vascular smooth muscle function,Citation4,Citation5 high output stateCitation3,Citation6 related to grafts and fistulas, microbubble pulmonary emboliCitation7 and pre-existing or acquired left-sided cardiac dysfunction. Citation3,Citation8 It is estimated that there are about 350,000 people receiving chronic HD in the US.Citation9 The US dialysis patient demographic differs substantially from other reported populations with theoretically different prevalence and outcome of PH. Despite the potential burden of pulmonary hypertension in this large population, prospective data regarding the prevalence of PH, clinical determinants, and prognostic significance is limited. Thus, the purpose of this study was to prospectively determine the prevalence of PH by echocardiography in dialysis patients in a single dialysis center in the US and identify the clinical significance of PH on all-cause mortality and morbidity.
Methods
We prospectively performed predialysis echocardiograms on all patients routinely attending a single dialysis center. Echocardiograms were performed predialysis as opposed to post dialysis as it was felt that the predialysis status of the patient more closely reflects the chronic volume status of the patient than the immediate post dialysis “dry weight” state. Baseline demographics were collected through review of the medical records. It was attempted to consent, enroll, and recover data on all the patients in this dialysis unit. All aspects of this study were approved by the Baylor institutional review board, Fresenius (the dialysis unit operator), and the medical director(s) of the dialysis unit.
Echocardiograms and estimation of pulmonary hypertension
Complete echocardiograms (2-dimensional, M-Mode, and Doppler studies) were performed (using the Biosound system), digitally stored and analyzed. Standard 2D echocardiographic parameters of cardiac structure and function, Doppler assessment of pulmonary veins, mitral inflow, tricuspid inflow, left ventricular outflow tract, as well as tissue Doppler imaging at the mitral and tricuspid annulus were acquired with the patient in the left lateral position according to recommendations from the American Society of Echocardiography.Citation10–Citation13 All echocardiograms were analyzed by a single reader, who was aware that all patients were dialysis-dependent patients but was blinded to additional clinical and outcomes data.
Previous studies calculated pulmonary artery systolic pressure (PAP) echocardiographically using the Bernoulli equation: PAP = 4 × (peak tricuspid regurgitant (TR) jet velocity)Citation2 + 10 mmHg (estimated right atrial pressure (RAP)). As the RAP estimate is yet another estimate, to maximize the integrity of the data, PH was defined by the TR jet only. Thus, in the present study, PH was defined as a TR jet ≥2.5 m/s (this would be approximately equivalent to PAP ≥35 mmHg assuming a RAP of 10 mmHg); ‘more severe PH’ as TR jet ≥3.0 m/s. This methodology would underestimate PAP compared with prior studies but would more strictly define patients with PH. Patients with insufficient TR jet who had echo-Doppler characteristics similar to those with normal TR jet velocity and no indirect echo-Doppler evidence of PH (37 patients), were grouped with patients without PH (11 patients). Patients were divided into 3 groups based on peak TR jet velocity: group 1, no TR and TR <2.5 m/s; group 2, TR 2.5–3.0 m/s; group 3, TR >3.0 m/s.
Clinical outcomes
Data on all-cause hospitalizations and death at 4, 8, and 12 months post echocardiogram was collected. For hospitalization data, complete follow up was available in 76 patients. For the survival analysis, all 90 patients were accounted for either through chart review or the United States Renal Data System database. The research nurse recording outcomes data was blinded to the results of the echo data.
Statistical analysis
Differences across the 3 groups were examined using ANOVA for continuous variables and the Chi-square test for categorical variables. Variables that were not normally distributed were examined using non-parametric tests. The data are presented as mean and standard deviation unless otherwise specified.
For survival analysis, Kaplan Meier survival curves were generated, and a log rank test was used to assess for statistically significant differences between the groups.
Results
Of 133 patients attending the dialysis clinic, 103 consented to participation between May 2006 and May 2007. 10 patients relocated prior to completion of the echocardiogram, and 3 patients were excluded due to technically inadequate echocardiograms. Thus, the cohort consisted of 90 consecutively consented and enrolled patients.
Clinical characteristics
The baseline characteristics of the patients are shown in . The majority of patients were male (66%), with an average age of 58 ± 15 years. 64% of the patients were African American, 18% were Hispanic, and 18% Caucasian. The most frequent cause of ESRD requiring HD was a combination of diabetes and hypertension (33%) followed by hypertension alone (30%), other causes (28%), and lastly diabetes alone (9%). For HD access, the majority of patients had arteriovenous grafts (AVGs) (44%) followed by arteriovenous fistulas (AVFs) (40%) and catheters (16%). The average time on HD at the time of echocardiogram was 3.7 ± 2.8 years.
Table 1 Baseline clinical characteristics
Pulmonary hypertension
42 patients (47%) met the def inition of PH of whom 18 patients (20%) had more severe PH. Patients with PH were older, and there was a trend for a female predominance in those with more severe PH; however, this did not reach statistical significance (). Between the 3 groups, no significant differences were noted in the distribution of different ethnicities, type of access, years on HD, incidence of diabetes, hypertension, or history of coronary artery disease. Patients with PH did have a significantly lower body mass index (BMI) (P < 0.02). Laboratory parameters such as haptoglobin, hemoglobin and LDH (potential indicators of hemolysis) were not significantly different between the 3 groups (data not shown).
Echocardiographic parameters are demonstrated in . As expected, patients with PH had larger right atrial (RA) and right ventricular (RV) sizes, higher RAP and a trend towards decreased RV function. Left ventricular (LV) size did not change with increasing TR jet velocity; however, a trend towards more LV hypertrophy was noted. The mean LV ejection fraction (LVEF) in all patients was 58.4% ± 11% (range 22%–70%). With increasing TR jet velocity, a significant decrease in LV systolic function was noted. However, even in patients with TR ≥3 m/s, mean LVEF was preserved at 53.2% ± 14.7%. Patients with PH had significantly higher estimates of left sided filling pressures. Consistent with these findings, there was a trend towards larger left atrial size with increasing PAP (). A pericardial effusion was more frequently present in patients with higher PAP (0% in group 1, 8% in group 2, and 33% in group 3). The occurrence of significant valvular disease was rare in all groups.
Table 2 Echocardiographic parameters
Echocardiographic data were available for the calculation of pulmonary capillary wedge pressure (PCWP) in 70 patients. Patients with PH had significantly higher PCWP (). Correlation analysis revealed a significant correlation between PAP and PCWP (Pearson correlation coefficient = 0.5, P = 0.001).
Clinical outcome
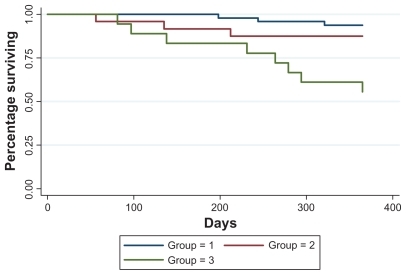
At 12 months, a total of 14 deaths (15.6%) had occurred. Mortality was significantly higher in patients with PH (26%) compared with patients without PH (6%), and this was driven largely by increased mortality in patients with more significant PH (TR ≥ 3 m/s). By 12 months, mortality was 44% (8/18) in group 3 versus 6% (3/48) and 12.5% (3/24) in groups 1 and 2, respectively (P = 0.001). The Kaplan Meier survival curves are shown in .
Figure 1 Kaplan Meier survival estimates based on presence, absence, and degree of PH by TR jet: group 1 = no TR, and TR < 2.5 m/s; group 2 = TR 2.5–3.0 m/s; group 3 = TR > 3.0 m/s (P = 0.0004). At 12 months, mortality was 44% (8/18) in group 3 versus 6% (3/48) and 12.5% (3/24) in groups 1 and 2, respectively (P = 0.001). The P = 0.0004 is based on the time to event analysis and signifies an overall difference between the 3 curves/groups. P = 0.001 is based on a chi-square test for the number of deaths by 12 months between the 3 groups.
In exploratory analysis comparing survivors to non-survivors (), there was no significant difference in age, race, gender, BMI, access type, and incidence of diabetes or hypertension, or history of coronary artery disease between the 2 groups. However, patients who died had been on HD for a significantly longer duration than survivors. In addition, patients who died had larger RA and RV sizes, worse RV function, more severe TR, and significantly higher RAP and PAP. There was no significant difference in LV size between the 2 groups, but non-survivors did show a trend towards lower LVEF than survivors. Moreover, PCWP was significantly higher in non-survivors ().
Table 3 Analysis by survivors and non-survivors
Discussion
Previously reported data has demonstrated that PH occurs in about 25%–45% of patients with ESRD on HD.Citation1–Citation3 However, these analyses were retrospective, based on patients undergoing echocardiography for clinical indications (preselection bias) and were performed in non-US countries (). This study is the first prospective evaluation of PH in dialysis patients in the US. Our study revealed that PH is highly prevalent in dialysis patients (47%), with 20% of the patients having echocardiographic evidence of more severe PH. This study further demonstrates that the presence of PH is associated with a significant increase in mortality with a 12-month mortality of 44% in patients with more severe PH. This is in relation to annual mortality rates of approximately 20% in dialysis patients in the US.Citation9 Not surprisingly, this study provides evidence that the development of PH in dialysis patients may reflect the impact of chronic volume overload and chronically elevated left heart filling pressures.
Table 4 Review of studies evaluating pulmonary hypertension in patients with end stage renal disease
Several mechanisms have been proposed for the development of PH in dialysis patients, and ultimately, a combination of multiple factors likely contributes to its development. PH may evolve due to the inability of the pulmonary circulation to accommodate increased cardiac output (resulting from increased volume, anemia, arteriovenous fistula) or as a result of increased pulmonary vascular stiffness due to endothelial dysfunction (decreased nitric oxide).Citation3,Citation5,Citation6 In the current study, neither type of access nor anemia were associated with PH. The only echocardiographic parameters that were significantly associated with PH in the current study were PCWP and LV function. 63% of patients had echocardiographic evidence of elevated PCWP, and an elevated PCWP was associated with elevated RAP and PAP. Moreover, correlation analysis revealed a significant correlation between PAP and PCWP, suggesting that elevated PCWP may be a major contributor to PAP elevation.
Although there was a significant decrease in LV function with increasing PAP, the mean LVEF in patients with PH was still preserved (mean LVEF 53.2% ± 14.7%). Similarly, in subjects with higher estimates of PCWP, there was only a trend towards decreased LVEF. These findings suggest that diastolic heart failure with preserved LV systolic function may be the mechanism for elevated filling pressures in a significant proportion of patients. The elevated filling pressures in turn may contribute to the development of PH. In our study population, volume retention appeared to contribute more towards elevated filling pressures than severity of diastolic dysfunction. This is reflected by the observation that the increase in filling pressures was driven more by an increase in mitral E wave velocity rather than a decrease in tissue Doppler E velocity. However, the potential importance of multifactorial diastolic dysfunction, an increasingly frequent cause of PH in the non-dialysis patient population, should not be discounted as a contributor to PH and its outcome in the dialysis population.
Two previous studies also suggest a causal relationship between PCWP and PAP. Havlucu et al followed 25 dialysis and 23 pre-dialysis patients for 6 months. At the end of 6 months, left atrial diameter (LAD) (reflection of left-sided filling pressures), vena cava index (VCI) (reflection of RAP), and systolic PAP improved in the dialysis patients, whereas in pre-dialysis patients, VCI did not change and LAD and PAP actually increased. The authors concluded that effective HD and volume control (as reflected by decrease in LAD and VCI) may have resulted in improved PAP.Citation14
In another study, Barberato et al compared dialysis patients with normal and pseudonormal mitral inflow patterns, the latter reflecting elevated left-sided filling pressures. Interestingly, although mean LVEF was significantly lower in the pseudonormal group, it was still preserved on average (73% ± 7% versus 65% ± 10%, P < 0.01). Moreover, systolic PAP was significantly higher in the pseudonormal group (38 ± 11 mmHg versus 20 ± 6 mmHg, P = 0.01).Citation15 These results further support the findings in the current study that elevated filling pressures in dialysis patients are associated with preserved LV function and elevated PAP.
In ESRD patients, even in the presence of preserved LV systolic function, diastolic abnormalities with impaired LV relaxation and increased ventricular stiffness are not infrequent.Citation16–Citation18 In our study population, 77% of the dialysis patients had echo evidence of abnormal diastolic function. Diastolic dysfunction in dialysis patients can be due to multiple factors. Left ventricular hypertrophy mainly as a result of hypertension is prevalent in dialysis patients and is strongly associated with impaired diastolic function. Furthermore, a large number of dialysis patients have diabetes, which by itself has been associated with increased myocardial stiffness and diastolic dysfunction. Other factors that may contribute to increased ventricular stiffness in ESRD patients include uremia and upregulation of various neurohormones.Citation19
In dialysis patients, the impact of PH on clinical outcomes has not previously been prospectively studied. While this is a cross-sectional study and may have suffered from survivor bias, given that the mortality was higher in the patients with PH, the survivor bias should predispose to an underestimation of the frequency of PH in the US dialysis patient population. In a recently published retrospective database review of HD patients receiving echocardiograms for clinical indications, 127 dialysis patients were evaluated, and the prevalence of PH was found to be 29%. Of the 37 patients with PH, 17 had PH at initiation of HD, and 20 patients developed PH after HD initiation. Survival was significantly higher in patients without PH. The 1-, 3-, and 5-year mortality in patients with and without PH were 21.4% versus 3.5%, 57.1% versus 21.2%, and 74.8% versus 33.6% respectively.Citation1 Similar findings were noted in the current study: at 12 months follow-up, mortality was 26% in patients with PH and 6% in patients without PH. Interestingly, with respect to all-cause hospitalizations (including emergency room visits), no significant difference was found amongst the 3 groups. Thus, morbidity by this definition amongst dialysis patients appears to be very high irrespective of PAP.
In the current study, when comparing survivors with non-survivors, PAP and PCWP were significantly higher in non-survivors. On the one hand, this may reflect the progression of diastolic heart failure with elevated PCWP eventually resulting in PH, which then carries a poor long-term prognosis. Alternatively, PH may have developed as a result of other causes, may not necessarily be associated with elevated PCWP, and may thus independently be a poor prognostic marker.
No relation was found between the nature of the dialysis access and the presence of PH. This is not unexpected. There is a clear rationale for aggravation of heart failure in patients with established PH or diastolic dysfunction due to the higher output imposed by a chronic graft of fistula. However, there is a very low likelihood that a 25%–30% increase in basal cardiac output over a relatively short period of time (years as opposed to decades) would be likely to predispose to the high flow induced changes in the pulmonary vascular bed leading to PH. The development of PH is remarkably infrequent (about 4%) in patients with common congenital heart disease such as ASD.
Limitations
We included patients in whom PAP could not be measured due to insufficient TR in the normal group (group 1). We did ensure that these patients did not have any other echocardiographic signs indicating the presence of PH. Secondly, we used a TR jet cutoff of ≥2.5 m/s for the classification of PH. Assuming a RAP of 10 mmHg, this would equate to a PAP of ≥35 mmHg. In the setting of lower RAP, these patients may have been misclassified as having higher PAP. Although these 2 scenarios may have led to misclassification by potentially including PH patients in the normal group and normal patients in the PH group, we were still able to identify a difference in prognosis between the groups. Due to the small number of overall events, a multivariable regression analysis could not be performed to evaluate the extent to which PH independently contributed to mortality. Thus, although PH is associated with higher mortality, our study was not able to ascertain causality versus association between PH and mortality or whether PH is merely an indicator of sicker patients. We believe, however, that this approach in this cross-sectional analysis conservatively provides a lower estimate of the prevalence and impact of PH in chronic ESRD patient on dialysis.
Conclusion
In this first prospective study of PH in US dialysis patients, at a single center, we found that PH is highly prevalent and is associated with echocardiographic evidence of elevated left-sided filling pressures and higher mortality. The clinical importance of PH appears to be as a marker of disease severity, as a potential target of therapy, and as an important prognostic indicator. While multiple factors may contribute to the development of PH in dialysis patients our results suggest that chronic volume overload, and/or diastolic dysfunction may contribute to the development of PH, which is associated with poorer survival. This suggests that the presence of PH should be incorporated in the decision making for renal transplantation, since PH is associated with worse survival, and appears to improve after renal transplantation.Citation2
The results of the current study demonstrating the frequency and impact of PH in the US dialysis population underline the importance of adequate volume removal, and potentially the importance of future therapies targeting PH and diastolic dysfunction.
Support and financial disclosure declaration
Funding for this study was obtained from the Frueauff Foundation, a nonprofit, non-industry organization, which supports limited medical and scientific studies. In addition, as stated in the acknowledgments, Biosound provided an echo machine free of charge to undertake the external echocardiograms which were done at the dialysis units.
Conflict of interest
Dr Frost has received funding for FDA approved multicenter studies in pulmonary hypertension from Bayer, Actelion, Gilead, Lilly, United Therapeutics, Novartis and Pfizer. She is on the speaker bureau and/or has received honoraria for participation in advisory boards on PH from Gilead, Pfizer, United Therapeutics and Actelion. The other authors declare no conflicts of interest.
Acknowledgments
This project was supported by a grant from the Frueauff Foundation. The authors wish to thank: Biosound for providing the ultrasound machine; Fresenius and the physicians, nurses, and patients, and Dr Wadi N Suki, Medical Director at the Medical Center Kidney Clinic, Houston, Texas for their cooperation in this study.
References
- YiglaMFruchterOAharonsonDPulmonary hypertension is an independent predictor of mortality in hemodialysis patientsKidney Int20097596997519212417
- YiglaMNakhoulFSabagAPulmonary hypertension in patients with end-stage renal diseaseChest20031231577158212740276
- AbdelwhabSElshinnawySPulmonary hypertension in chronic renal failure patientsAm J Nephrol20082899099718635926
- ThambyrajahJLandrayMJMcGlynnFJJonesHJWheelerDCTownendJNAbnormalities of endothelial function in patients with predialysis renal failureHeart20008320520910648498
- MorrisCRGladwinMTKatoGJNitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disordersCurr Mol Med2008862063218991648
- OkuraHTakatsuYHigh-output heart failure as a cause of pulmonary hypertensionIntern Med1994333633657919625
- BarakMKatzYMicrobubbles: pathophysiology and clinical implicationsChest20051282918293216236969
- FathiRIsbelNHaluskaBCaseCJohnsonDWMarwickTHCorrelates of subclinical left ventricular dysfunction in ESRDAm J Kidney Dis2003411016102512722036
- USRDS 2008 Annual Data Report [report on the Internet]United States Renal Data System Available from: www.usrds.org/adr.htm2009
- QuinonesMAOttoCMStoddardMWaggonerAZoghbiWARecommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of EchocardiographyJ Am Soc Echocardiogr20021516718411836492
- LangRMBierigMDevereuxRBRecommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of CardiologyJ Am Soc Echocardiogr2005181440146316376782
- NaguehSFAppletonCPGillebertTCRecommendations for the evaluation of left ventricular diastolic function by echocardiographyJ Am Soc Echocardiogr20092210713319187853
- HortonKDMeeceRWHillJCAssessment of the right ventricle by echocardiography: a primer for cardiac sonographersJ Am Soc Echocardiogr20092277679219560657
- HavlucuYKursatSEkmekciCPulmonary hypertension in patients with chronic renal failureRespiration20077450351017505128
- BarberatoSHPecoits-FilhoRUsefulness of left atrial volume for the differentiation of normal from pseudonormal diastolic function pattern in patients on hemodialysisJ Am Soc Echocardiogr20072035936517400114
- MallGHutherWSchneiderJLundinPRitzEDiffuse intermyocardiocytic fibrosis in uraemic patientsNephrol Dial Transplant1990539442109283
- MallGRambausekMNeumeisterAKollmarSVetterleinFRitzEMyocardial interstitial fibrosis in experimental uremia – implications for cardiac complianceKidney Int1988338048112968479
- LondonGMParfreyPSCardiac disease in chronic uremia: pathogenesisAdv Ren Replace Ther199741942119239425
- LondonGMLeft ventricular alterations and end-stage renal diseaseNephrol Dial Transplant200217 Suppl 1293611812909
- AminMFawzyAHamidMAElhendyAPulmonary hypertension in patients with chronic renal failure: role of parathyroid hormone and pulmonary artery calcificationsChest20031242093209714665485
- Mahdavi-MazdehMAlijavad-MousaviSYahyazadehHAzadiMYoosefnejadHAtaiipoorYPulmonary hypertension in hemodialysis patientsSaudi J Kidney Dis Transpl20081918919318310865
- TarrassFBenjellounMMedkouriGHachimKBenghanemMGRamdaniBDoppler echocardiograph evaluation of pulmonary hypertension in patients undergoing hemodialysisHemodial Int20061035635917014511
- YiglaMFruchterOAharonsonDPulmonary hypertension is an independent predictor of mortality in hemodialysis patientsKidney Int200975969975619212417