Abstract
Short-term duodenal administration of n-3 polyunsaturated fatty acid (PUFA)-rich seal oil may improve gastrointestinal complaints in patients with subjective food hypersensitivity, as well as joint pain in patients with inflammatory bowel disease (IBD). The aim of the present explorative pilot study was to investigate whether 10-day open treatment with seal oil, 10 mL self-administrated via a nasoduodenal tube 3 times daily, could also benefit nongastrointestinal complaints and quality of life (QoL) in patients with subjective food hypersensitivity. Twenty-six patients with subjective food hypersensitivity, of whom 25 had irritable bowel syndrome (IBS), were included in the present study. Before and after treatment and 1 month posttreatment, patients filled in the Ulcer Esophagitis Subjective Symptoms Scale (UESS) and the Gastrointestinal Symptom Rating Scale (GSRS) for gastrointestinal symptoms and subjective health complaints (SHC) inventory for nongastrointestinal symptoms in addition to short form of the Nepean dyspepsia index (SF-NDI) for evaluation of QoL. Compared with baseline, gastrointestinal, as well as nongastrointestinal, complaints and QoL improved significantly, both at end of treatment and 1 month posttreatment. The consistent improvements following seal oil administration warrant further placebo-controlled trials for confirmation of effect.
Introduction
Although about 20% of the general population report having adverse reactions to food, food allergy is medically confirmed in only 1%–2% of adults.Citation1,Citation2 Patients with subjective food hypersensitivity present with unexplained gastrointestinal complaints, which they self-attribute to the ingestion of specific foods. Usually, the gastrointestinal complaints comply with the Rome-II criteria for irritable bowel syndrome (IBS).Citation3,Citation4 Nongastrointestinal complaints are prevalent, and health-related quality of life (QoL) is often considerably impaired.Citation3,Citation5
Subjective health complaints (SHC) are major health problems as they are the most frequent sources for long-term sickness and permanent inability to work.Citation6 The pathogenesis of subjective food hypersensitivity, as of SHC in general, is not well understood. Central sensitization, in which cognitive as well as somatic sensitization contribute to amplification of the complaints, is a widely accepted explanatory model.Citation7 Compared to the general population, patients with subjective food hypersensitivity report more frequent and severe SHC.Citation5 As usual, in case of multiple unexplained complaints from various organ systems, psychological mechanisms are suspected. Thus, cognitive sensitization has been suggested as a much more important cause of subjective food hypersensitivity than immunological sensitization.Citation5 This assumption is supported by findings of increased prevalence of anxiety and depression in patients with IBS and subjective food hypersensitivityCitation8 and the fact that anxiety and depression often present with somatic rather than emotional symptoms.Citation9,Citation10 However, psychological factors are not major predictors of symptom severity in patients with subjective food hypersensitivity.Citation11
By using a SHC inventory, Lind et al found musculoskeletal pain and fatigue to be common among patients with subjective food hypersensitivity.Citation5 Indeed, subjective food hypersensitivity with IBS-like symptoms, joint pain, and fatigue appear to be increasingly recognized worldwide.Citation12 Long-term per oral administration of long-chain n-3 polyunsaturated fatty acids (PUFAs)-rich fish oil is known to relieve joint pain in patients with rheumatoid arthritis,Citation13 an effect supposed to be in part due to inhibition of eicosanoid synthesis. The most abundant PUFA in human cell membrane phospholipids, the n-6 fatty acid arachidonic acid (AA, 2:4n-6), is liberated upon stimulation and generally gives rise to more proinflammatory eicosanoids via the cyclooxygenase and lipooxygenase pathways. The long-chain n-3 PUFAs, especially eicosapentaenoic acid (EPA; 20:5n-3), cause a shift in cyclooxygenase and lipooxygenase pathways, producing less proinflammatory mediators.Citation14 Intriguingly, Clarke et al recently found elevated plasma AA and prostaglandin E2 (PGE2) levels in patients with IBS compared to healthy controls.Citation15 Seal oil is slightly less rich in the n-3 long-chain PUFAs EPA and docosahexaenoic acid (DHA; 22:6n-3), but contains more of docosapentaenoic acid (DPA; 22:5n-3), compared to fish oil.Citation16 In recent studies, short-term duodenal administration of seal oil,Citation17,Citation18 but not n-6 fatty acid-rich soy oil,Citation19,Citation20 attenuated joint pain with prolonged effects in patients with inflammatory bowel disease (IBD).Citation19 Compared with soy oil, short-term seal oil administration via nasoduodenal tube also relieved gastrointestinal symptoms in patients with subjective food hypersensitivity.Citation20
The aim of the present explorative pilot study was to investigate whether 10-day open duodenal administration of seal oil could attenuate also nongastrointestinal symptoms and improve QoL in patients with subjective food hypersensitivity. In case of positive effects, further placebo-controlled trials would be warranted.
Material and methods
Screening of patients
Consecutive outpatients referred to Haukeland University Hospital due to various unexplained gastrointestinal complaints self-attributed to specific food items were eligible for inclusion in the study. The patients were carefully examined to exclude organic diseases such as peptic ulcer, Helicobacter pylori infection, celiac disease, IBD, and parasitic infections as previously described.Citation21 Pregnant or lactating women were also excluded. The allergological examination included skin prick tests using 22 common food items and inhalants (ALK, Abello, Hørsholm, Denmark), blood samples for determination of both serum total IgE and food-specific IgE levels (ImmunoCap-System, Phadia, Uppsala, Sweden), and, when indicated, open and double-blind provocation tests. Twenty-six of 68 (38%) screened patients were willing to participate in the present study. The majority of the patients were women (F/M, 23/3), and the mean age was 47 years, with range 26–88 years.
Study design
Refined seal oil (Rieber Skinn A/S, Bergen, Norway) from adult harp seals (Pagophilus groenlandicus) was administered by a nasoduodenal feeding tube, which was inserted at start of the 10-day intervention period. The tube (Freka® Feeding Tube, Fresenius Kabi, GmbH, Hesse, Germany) was positioned with its tip to the lower duodenum by aid of fluoroscopy. As in our prior studies,Citation17–Citation20 the patients self-administered 10 mL of seal oil via the tube, 3 times/day for 10 days, while they were instructed not to alter their background diet. This amount of seal oil is equivalent to a daily intake of approximately 2.4 g EPA, 1.1 g DPA, and 2.6 g DHA, that is, 6.1 g of long-chain n-3 PUFA, which is approximately the double dose of that required to achieve anti-inflammatory effect in rheumatoid arthritis (2.7 g EPA and DHA day−1).Citation13
Before and after seal oil treatment and at day 30 posttreatment, the patients filled in previously validated Norwegian versions of questionnaires for SHC, the short form of the Nepean dyspepsia index (SF-NDI) for assessment of QoL, and two questionnaires regarding gastrointestinal complaints, namely, the Gastrointestinal Symptom Rating Scale (GSRS) and the Ulcer Esophagitis Subjective Symptoms Scale (UESS).
The Regional Ethical Research Committee approved the study, which was performed in accordance with the Helsinki Declaration, and all participants gave written informed consent before inclusion in the study.
Experimental oil
The refined seal oil used in the present study was approved according to current legislations on contaminants. The fatty acid composition in the oil was analyzed by gas liquid chromatography (Trace GC 2000) according to previously described methods,Citation17,Citation22 with some modifications; the fatty acids were esterified in 20% boron fluoride (BF3) in methanol, and sample parallels were analyzed. The level of fat soluble vitamins ACitation23,Citation24 and ECitation25 was analyzed by high-performance liquid chromatography, and the lipid peroxidation was analyzed by thiobarbituric acid reactive substancesCitation26,Citation27 (). The seal oil was added with a combination of natural and synthetic tocopherols, the latter being dl-α tocopheryl acetate. The oil was protected with nitrogen on top in bottles and stored in a refrigerator during study, otherwise in −20°C freezer.
Table 1 Fatty acid profile (g/100 g), vitamins A and E and thiobarbituric acid reactive substances in seal oil
Subjective health complaint inventory
The SHC inventory includes 29 items concerning somatic and psychological complaints.Citation28 The questionnaire contains five subscales: musculoskeletal pain (migraine, headache, arm pain, shoulder pain, neck pain, upper back pain, lower back pain, and leg pain), gastrointestinal complaints (gas discomfort, stomach discomfort, gastritis/ulcer, heartburn, diarrhea, constipation, and stomach pain), allergy (allergies, breathing difficulties, eczema, and asthma), pseudoneurology (tiredness, sleep problems, dizziness, heat flushes, extra heartbeats, sadness/depression, and anxiety), and flu (cold/flu and coughing). The scores were determined by rating each item by a four-point graded Likert scale ranging from 0 (not at all), 1 (a little), 2 (quite a lot) to 3 (severely). The questionnaire has been tested with satisfactory validity and reliability outcome.Citation28
Gastrointestinal symptom rating scale
The questionnaire includes 15 items, which are grouped into five subscales: abdominal pain syndrome (abdominal pain/discomfort, sucking sensation in the epigastrium, nausea, and vomiting), reflux syndrome (heartburn and acidic regurgitation), indigestion (borborygmi, abdominal distension, eructation, and increased flatus), diarrhea (increased passage of stools, loose stools, and urgent need for defecation), and constipation (decreased passage of stools, hard stools, and feeling of incomplete evacuation). The scores were determined by rating each item by a seven-point graded Likert scale with descriptive anchors (1, no symptoms at all; 2, minimal symptoms; 3, mild symptoms; 4, moderate symptoms; 5, rather serious symptoms; 6, serious symptoms; and 7, very serious symptoms). A Norwegian version of the GSRS was used in the present study.Citation29
Ulcer Esophagitis Subjective Symptoms Scale
The UESS was developed to examine the symptoms frequently experienced by patients with peptic ulcer and esophagitis. The questionnaire includes nine items, which are grouped into four subscales: abdominal discomfort (abdominal pain and sucking sensation), reflux discomfort (acid regurgitation and heartburn), intestinal discomfort (abdominal distension and borborygmi), and sleep dysfunction (difficulty falling asleep, insomnia, and rested waking up).Citation30 A Norwegian version of the UESS was used in the present study.Citation29 The scores were determined by rating each item by a 100-mm horizontal visual analog scale ranging from 0 (very well) to 100 (very poor). This Norwegian version of visual analog scale has previously been validated in terms of reliability, validity, and sensitivity.Citation31
Short form of the Nepean dyspepsia index
The SF-NDI is a 10-item questionnaire with five subscales measuring the influence of dyspepsia on domains of health-related QoL, namely, tension, interference with daily activities, altered eating/drinking habits, knowledge/control over disease symptoms, and interference with work/study, and each subscale contains two items.Citation32 The scores were determined by measuring each item by a five-point graded Likert scale ranging from 1 (not at all or not applicable), 2 (a little), 3 (moderately), 4 (quite a lot) to 5 (extremely). The total sum score for QoL ranges from 10 to 50, and the sum score of each of the five subscales ranges from 2 to 10. Higher scores indicate poorer QoL. The 10-item SF-NDI has been validated in patients with functional dyspepsia,Citation32 as well as in patients with subjective food hypersensitivity.Citation3 The questionnaire gives consistent outcome, and the stability of the test–retest results suggest that the chance of spontaneous regression of symptoms reported by the SF-NDI in patients with subjective food hypersensitivity is small.
Statistics
Data were analyzed and displayed using the GraphPad Prism statistical software package (GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, CA, USA). Results are presented as individual values and as mean ± standard error of mean (SEM). Differences in total sum scores and total subscale scores were evaluated by paired student t-tests. All tests were two-tailed, and P values < 0.05 were regarded statistically significant.
Results
Patient characteristics at baseline
Most patients reported adverse reactions to three or four food items: cow’s milk (54%), diverse fruits (38%), and raw vegetables (35%) being most common (). All patients had negative tests for Helicobacter pylori infection, celiac disease, and parasitic infections. Double-blind placebo-controlled food challenge was positive in three patients having non-IgE-mediated allergy or nonallergic food hypersensitivity. None of the patients had IgE-mediated food allergy as confirmed by both skin prick tests, food-specific IgE levels in serum, and double-blind placebo-controlled food challenge with the same food item. Twenty-five of the 26 patients had IBS according to the Rome II criteria.
Table 2 Food items suspected by patients to cause food hypersensitivity
Subjective health complaints
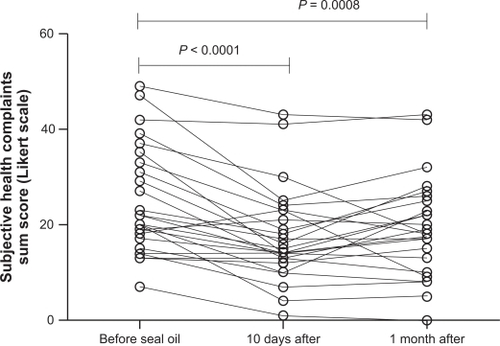
Total sum score of the SHC inventory was 24.3 ± 2.2 before seal oil treatment, 17.4 ± 1.9 after 10 days, taking 30 mL seal oil/day, and 20.1 ± 2.0 one month posttreatment. There was a significant difference in the total sum score between before and after 10-day seal oil treatment (P < 0.0001) and between before and 30 days after the seal oil treatment was ended (P = 0.0008) ().
Figure 1 Total sum score for SHC in patients with self-reported food hypersensitivity (n = 26), measured before and after seal oil treatment, and 1 month posttreatment. Individual values are displayed, and P values are indicated.
Subscale scores for SHC were highest for musculoskeletal and gastrointestinal complaints. As compared with pretreatment, the musculoskeletal pain score was significantly decreased after 10-day seal oil treatment from 7.1 ± 0.9 to 4.6 ± 0.7 (P < 0.01). The gastrointestinal complaint score was significantly decreased from 6.9 ± 0.6 to 5.0 ± 0.6 (P < 0.01) 30 days after seal oil treatment. Subscale sum score for pseudoneurology was also significantly decreased, both at 10 days (P < 0.01) and at 30 days posttreatment (P <0.05) ().
Table 3 SHC : Mean score ± SEM for total sum and subscales before seal oil, 10 days after seal oil, and 1 month posttreatment with seal oil
The five most frequently reported complaints were tiredness (92%), diarrhea (92%), gas discomfort (85%), stomach pain (81%), and headache (81%) (). Each patient reported at least four complaints. Five to ten complaints were reported by six patients (23%), 11–15 complaints by seven patients (27%), whereas 12 patients reported more than 15 complaints (46%).
Table 4 Number (percentage) of individuals with complaints for each item of the SHC inventory before seal oil, 10 days after seal oil, and 1 month posttreatment with seal oil
Quality of life
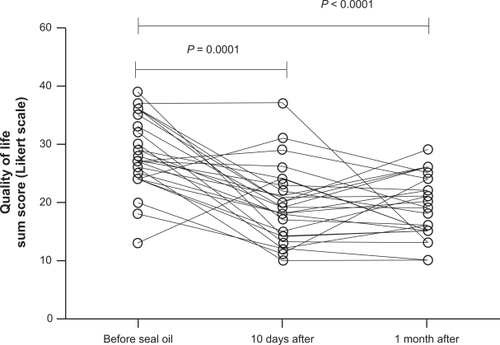
Total sum score of the SF-NDI was 28.2 ± 1.2 before the seal oil treatment, 19.5 ± 1.3 after 10-day seal oil treatment, and 19.9 ± 1.3 one month after completion of seal oil treatment. The decrease from baseline was significant both at 10 days (P < 0.0001) and at 30 days posttreatment (P = 0.0008) ().
Figure 2 Total sum score for SF-NDI in patients with self-reported food hypersensitivity (n = 26), measured before and after seal oil treatment, and 1 month posttreatment. Individual values are displayed, and P values are indicated.
The six subscale scores were all significantly reduced after 10 days’ treatment. The highest subscale score was for eating/drinking (7.1 ± 0.4), and the lowest score was for knowledge/control (4.7 ± 0.3) ().
Table 5 QoL on SF-NDI: Mean score ± SEM for total sum and subscales before seal oil, 10 days after seal oil, and 1 month posttreatment with seal oil
Abdominal complaints
Total sum scores for GSRS before the seal oil treatment was 35.1 ± 2.3, after 10-day seal oil treatment was 26.4 ± 2.7, and 30 days after completion of seal oil treatment was 26.5 ± 2.8. The decrease from baseline was significant both at 10 days (P = 0.007) and at 30 days posttreatment (P = 0.02) ().
Figure 3 Total sum score for GSRS A) and UESS B) in patients with self-reported food hypersensitivity (n = 26), measured before and after seal oil treatment, and 1 month posttreatment. Individual values are displayed and P values are indicated.
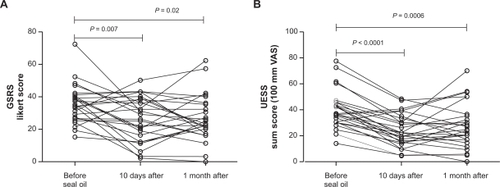
Total sum score for UESS before the seal oil treatment was 39.1 ± 2.7, after 10 days with seal oil treatment was 24.0 ± 2.4, and 30 days after completion of seal oil treatment was 28.3 ± 3.3. The decrease from baseline was significant both at 10 days (P < 0.0001) and at 30 days post-treatment (P = 0.0006) ().
Compared with baseline, the abdominal pain syndrome and the diarrhea subscale scores on the GSRS were significantly decreased both at 10 days and at 1 month posttreatment (abdominal pain syndrome: P < 0.05; diarrhea: P < 0.01), and the indigestion subscale score was significantly decreased at 10 days (P < 0.05) (). Likewise, the intestinal discomfort and sleep dysfunction subscale scores on the UESS were significantly decreased both at 10 days and at 1 month after completion of seal oil treatment (P < 0.01), and the abdominal discomfort subscale score was significantly decreased after 10 days (P < 0.05) (). The most reported complaints on the GSRS were indigestion, diarrhea, and abdominal complaints. On the UESS, sleep dysfunction, abdominal complaints, and intestinal complaints had the highest scores. Reflux symptoms had lowest scores on both questionnaires.
Table 6 Mean score (±SEM) for subscales in GSRS and UESS before seal oil, 10 days after seal oil, and 1 month posttreatment with seal oil
Discussion
The present study concerns patients who attribute their IBS-like complaints to the ingestion of food. Besides the avoidance of certain food items, a range of therapies, such as dietary fibers, antispasmodics, antidepressants, 5-HT3 antagonists, 5-HT4 agonists, probiotics, and cognitive therapies, are used in IBS. But in general, the effect of the treatment is modest and inconsistent. Although a biopsychosocial model is well accepted, considerable disagreement exists as to whether the treatment should be focused on the somatic or on the psychological aspects of the condition.Citation33 Indeed, we recently showed that 90% of the variance in symptoms severity in patients with subjective food hypersensitivity is not explained by psychological factors.Citation11 Thus, further exploration of possible nonpsychological treatments is well justified. Interestingly, following 10-day intraduodenal administration of seal oil in the present study, gastrointestinal, as well as nongastrointestinal, symptoms were significantly attenuated and QoL was consistently improved. The results thus corroborate our previous findings of beneficial effects of similarly administrated seal oil on gastrointestinal complaints in patients with subjective food hypersensitivity and on joint pain in patients with IBD.Citation19,Citation20
As in a prior study, our patients scored high on both gastrointestinal and nongastrointestinal complaints on the SHC inventory.Citation3,Citation5 The high scores for gastrointestinal complaints were indeed expected, being the main cause for seeking medical help. The high scores on musculoskeletal complaints, tiredness, sleep problems, and anxiety show that nongastrointestinal complaints are highly relevant problems for patients with subjective food hypersensitivity. Thus, it may be valuable to assess SHC and QoL in future treatment studies in these patients. Although tube administration is an invasive and cumbersome procedure, the treatment was remarkably well tolerated and largely without side effects. The therapeutic benefit observed following this short-term therapy suggests that a rapid effect is achieved by administrating seal oil directly into the duodenum using feeding tube.Citation17–Citation20,Citation34 No direct comparative study of oral versus duodenal administration of marine oils for pain relief exists, but it is worth noticing that seal oil orally administrated for 14 days showed no significant effect in patients with IBD or psoriatic arthritis.Citation35,Citation36 Thus, it is possible that pain relief by marine oils is achieved more rapidly by intraduodenal administration compared to oral administration, and an effect within 10 days may be possible by tube administration only. This form of administration was, therefore, chosen in this explorative pilot study. The use of nasoduodenal tube also ensures compliance regarding intake of the oil.
Indications of immune activation have been shown both in patients with IBSCitation37,Citation38 and in patients with subjective food hypersensitivity.Citation21,Citation39 The anti-inflammatory effect of long-chain PUFA is supposed to be brought about by modulation of the amount and types of eicosanoids produced via the cyclooxygenase and lipooxygenase enzyme pathways. Consistently, plasma levels of proinflammatory PGE2 was reduced in response to 10-day treatment with duodenal administered seal oil in patients with IBD-related joint pain.Citation18 Other effects may be eicosanoid-independent mechanisms through actions upon intracellular signalling, transcription factor activity, and gene expression.Citation40–Citation42 The vagus nerve is increasingly recognized to be involved in control of immune responses. Ingestion of high amounts of dietary fat induces release of cholecystokinin that binds to cholecystokinin-receptors on vagal afferents and inhibits the release of proinflammatory cytokines like tumor necrosis factor-α and interleukin-6 from immune-activated macrophages, through acetylcholine receptor binding. Based on these findings, high-fat enteral nutrition has been suggested as potentially therapeutic in various inflammatory disorders.Citation43
Intestinal dysbiosis has recently been suggested as a key pathogenetic mechanism in patients with IBS.Citation44 Animal models indicate that the intestinal microbiota can influence brain functions, via the gut–brain axis, and conceivably shape behavior and mood. In man, enteric infections, antibiotic usage, and stress may disturb the indigenous gut flora and predispose to IBS.Citation45 Consistently, we have previously shown that ingestion of lactulose, an unabsorbable, but fermentable carbohydrate, may replicate the gastrointestinal symptoms in patients with subjective food hypersensitivity,Citation46 and others have shown that reduced intake of dietary carbohydrate may benefit patients with IBS.Citation47 Bloating and perception of increased gas production are indeed common complaints of patients with subjective food hypersensitivity. Intestinal gases, such as hydrogen, methane, and carbon dioxide, are produced by colonic microbial fermentation. Intriguingly, EPA capsules for 7 days have been found to reduce total breath hydrogen excretion after challenge with lactitol, another unabsorbable but fermentable carbohydrate.Citation48 EPA contains five double bonds, which potentially can be saturated during bacteria metabolism, and thus offer a salvage route for excess hydrogen.Citation48 Whether tube administered seal oil, rich in EPA, could influence microbial metabolism is yet not known.
The major limitation of the present pilot study is the lack of control. Placebo effects are indeed known to be strong in patients with subjective complaints.Citation49 However, as seal oil previously improved gastrointestinal symptoms in this patient group compared with soy oil,Citation20 we wanted to first perform an open pilot study to examine if positive effects on also nongastrointestinal symptoms could be anticipated. Other limitations were lack of diet surveillance and no biological (eg, fatty acid profile in blood for validation of intake) or objective clinical effect measures.
Conclusion
In conclusion, gastrointestinal, as well as nongastrointestinal symptoms, and QoL of patients with subjective food hypersensitivity all improved significantly following short-term, duodenal administration of seal oil. The apparent effect could be mediated through the well-known anti-inflammatory effects of n-3 PUFAs, but other mechanisms including effects on the intestinal microflora and placebo effects might also be involved. For confirmation of results, further studies with placebo are warranted.
Acknowledgements
The authors are indebted to the patients for their participation.
Disclosure
The authors declare that they have no competing interests.
References
- JansenJJKardinaalAFMHuijbersGVlieg-BoerstraBJMartensBPOckhuizenTPrevalence of food allergy and intolerance in the adult Dutch populationJ Allergy Clin Immunol1994934464568120272
- OsterballeMHansenTMortzCGHøstABindslev-JensenCThe prevalence of food hypersensitivity in an unselected population of children and adultsPediatr Allergy Immunol20051656757316238581
- ArslanGLindROlafssonSFlorvaagEBerstadAQuality of life in patients with subjective food hypersensitivity: applicability of the 10-item short form of the Nepean dyspepsia indexDig Dis Sci20044968068715185878
- AzizQVisceral hypersensitivity: fact or fictionGastroenterology200613166167016890617
- LindRArslanGEriksenHRSubjective health complaints and modern health worries in patients with subjective food hypersensitivityDig Dis Sci2005501245125116047467
- IhlebaekCEriksenHRUrsinHPrevalence of subjective health complaints (SHC) in NorwayScand J Public Health200230202911928829
- UrsinHEriksenHRSensitization, subjective health complaints, and sustained arousalAnn N Y Acad Sci200193311912912000015
- LillestølKBerstadALindRFlorvaagELiedGATangenTAnxiety and depression in patients with self-reported food hypersensitivityGen Hosp Psychiatry201032424820114127
- Munk-JorgensenPAllgulanderCDahlAAPrevalence of generalized anxiety disorder in general practice in Denmark, Finland, Norway, and SwedenPsychiatr Serv2006571738174417158488
- CraytonJWAdverse reactions to foods: relevance to psychiatric disordersJ Allergy Clin Immunol1986782432503522711
- LindRLiedGALillestølKValeurJBerstadADo psychological factors predict symptom severity in patients with subjective food hypersensitivity?Scand J Gastroenterol2010457–883584320433401
- GweeKAPost-infectious irritable bowel syndrome, an inflammation-immunological model with relevance for other IBS and functional dyspepsiaJ Neurogastroenterol Motil201016303420535323
- ClelandLGJamesMJProudmanSMFish oil: what the prescriber needs to knowArthritis Res Ther2006820221016542466
- ChenCCOX-2’s new role in inflammationNat Chem Biol20106640140220479749
- ClarkeGFitzgeraldPHennessyAAMarked elevations in proinflammatory polyunsaturated fatty acid metabolites in females with irritable bowel syndromeJ Lipid Res2010511186119219965606
- BroxJOlaussenKØsterudBA long-term seal- and cod-liver-oil supplementation in hypercholesterolemic subjectsLipids20013671311214732
- ArslanGBrunborgLAFrøylandLBrunJGValenMBerstadAEffects of duodenal seal oil administration in patients with inflammatory bowel diseaseLipids20023793594012530551
- BjørkkjærTAraujoPMadlandTMBerstadAFrøylandLA randomized double blind comparison of short-term duodenally administrated whale and seal blubber oils in patients with inflammatory bowel disease and joint painProstaglandins Leukot Essent Fatty Acids20098142543219713092
- BjørkkjærTBrunborgLAArslanGReduced joint pain after short-term duodenal administration of seal oil in patients with inflammatory bowel disease: comparison with soy oilScand J Gastroenterol2004391088109415545167
- GregersenKLindRBjørkkjærTFrøylandLBerstadAEffects of seal oil on meal-induced symptoms and gastric accommodation in patients with subjective food hypersensitivity: a pilot studyClin Med Gastroenterol200813341
- ArslanGKahrsGELindRFrøylandLFlorvaagEBerstadAPatients with subjective food hypersensitivity: the value of analyzing intestinal permeability and inflammation markers in gut lavage fluidDigestion200470263515297775
- LieOLambertsenGFatty acid composition of glycerolphospholipids in seven tisues of cod (Gadus morhua), determined by combined high-performance liquid chromatography and gas chromatographyJ Chromatogr19915651191291874861
- NöllGNHigh-performance liquid chromatographic analysis of retinal and retinol isomersJ Chromatogr A19967212472598611941
- MorenMOpstadIHamreKA comparison of retinol, retinal and retinyl ester concentrations in larvae of Atlantic halibut (Hippoglossus hippoglossus L.) fed Artemia or zooplanktonAquac Nutr200410253259
- CENFoodstuffs – determination of vitamin E by high performance liquid chromatograpy – measurement of aplha-, beta-, gamma-, and delta-tocopherols (prEN 12822)2000
- HamreKNæssTEspeMHolmJCLieØA formulated diet for Atlantic halibut (Hippoglossus hippoglossus, L.) larvaeAquac Nutr20017123132
- SchmedesAHølmerGA new thiobarbituric acid (TBARS) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidationJAOCS198966813817
- EriksenHRA scoring system for subjective health complaints (SHC)Scand J Public Health199927637210847674
- OlafssonSHatlebakkJGBerstadAPatients with endoscopic gastritis and/or duodenitis improve markedly following eradication of Helicobacter pylori, although less so than patients with ulcersScand J Gastroenterol2002371386139412523587
- DimenäsEGliseHHallerbäckHHernqvistJSvedlundJWiklundIQuality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens?Scand J Gastroenterol1993286816878210982
- NRRK http://www.nrrk.no/modules/module_123/proxy.asp?D=2&C=634&I=2668
- TalleyNJVerlindenMJonesMQuality of life in functional dyspepsia: responsiveness of the Nepean dyspepsia index and development of a new 10-item short formAliment Pharmacol Ther20011520721611148439
- GroverMHerfarthHDrossmanDAThe functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease-irritable bowel syndromeClin Gastroenterol Hepatol20097485318848909
- BjørkkjærTBrunJGValenMShort-term duodenal seal oil administration normalised n-6 to n-3 fatty acid ratio in rectal mucosa and ameliorated bodily pain in patients with inflammatory bowel diseaseLipids Health Dis20065616549021
- MadlandTMBjørkkjærTBrunborgLAFrøylandLBerstadABrunJGSubjective improvement in patients with psoriatic arthritis after short-term oral treatment with seal oil. A pilot study with double blind comparison to soy oilJ Rheumatol20063330731016465662
- BrunborgLAMadlandTMLindRAArslanGBerstadAFrøylandLEffects of short-term oral administration of dietary marine oils in patients with inflammatory bowel disease and joint pain: a pilot study comparing seal oil and cod liver oilClin Nutr20082761462218374458
- ÖhmanLLindmarkACIsakssonSB-cell activation in patients with irritable bowel syndrome (IBS)J Neurogastroenterol Motil200921644650
- KindtSvan OudenhoveLBroekaertDImmune dysfunction in patients with functional gastrointestinal disordersJ Neurogastroenterol Motil200921389398
- ArslanGØdegaardSElsayedSFlorvaagEBerstadAFood allergy and intolerance: response to intestinal provocation monitored by endosonographyEur J Ultrasound200215293612044850
- StulnigTMImmunomodulation by polyunsaturated fatty acids: mechanisms and effectsInt Arch Allergy Immunol200313231032114707462
- SimopoulosAPThe role of fatty acids in gene expression: health implicationsAnn Nutr Metab1996403033119087307
- RobinsonDRUrakazeMHuangRDietary marine lipids suppress continuous expression of interleukin-1 beta gene transcriptionLipids199631S23S318729089
- LuyerMDGreveJWHadfouneMJacobsJADejongCHBuurmanWANutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerveJ Exp Med20052021023102916216887
- CollinsSMBercikPThe relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and diseaseGastroenterology20091362003201419457424
- SpillerRGarsedKPostinfectious irritable bowel syndromeGastroenterology20091361979198819457422
- ValeurJMorkenMHNorinEMidtvedtTBerstadACarbohydrate intolerance in patients with self-reported food hypersensitivity: comparison of lactulose and glucoseScand J Gastroenterol2009441416142319883270
- ShepherdSJParkerFCMuirJGGibsonPRDietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidenceClin Gastroenterol Hepatol2008676577118456565
- ThompsonLSpillerRCImpact of polyunsaturated fatty acids on human colonic bacterial metabolism: an in vitro and in vivo studyBr J Nutr1995747337418541279
- BenningaMAMayerEAThe power of placebo in pediatric functional gastrointestinal diseaseGastroenterology20091371207121019717127