Abstract
Background
Waterpipe smoking is a global health problem and a serious public concern. Little is known about the effects of waterpipe smoking on oral health. In the current study, we examined the alterations of oral microbial flora by waterpipe smoking.
Methods
One hundred adult healthy subjects (59 waterpipe smokers and 41 non-smokers) were recruited into the study. Swabs were taken from the oral cavity and subgingival regions. Standard culturing techniques were used to identify types, frequency, and mean number of microorganisms in cultures obtained from the subjects.
Results
It was notable that waterpipe smokers were significantly associated with a history of oral infections. In subgingiva, Acinetobacter and Moraxella species were present only in waterpipe smokers. In addition, the frequency of Candida albicans was higher in the subgingiva of waterpipe smokers (p = 0.023) while the frequency of Fusobacterium nucleatum was significantly lower in the subgingiva of waterpipe smokers (p = 0.036). However, no change was observed in other tested bacteria, such as Campylobacter species; Viridans group streptococci, Enterobacteriaceae, and Staphylococcus aureus. In oral cavity and when colony-forming units were considered, the only bacterial species that showed significant difference were the black-pigmented bacteria (p < 0.001).
Conclusion
This study provides evidence indicating that some of the oral microflora is significantly altered by waterpipe smoking.
Introduction
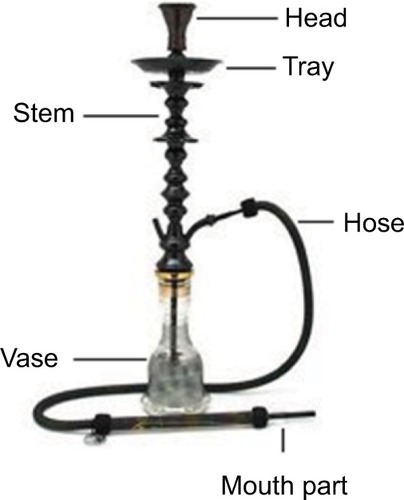
Waterpipe is a way of tobacco consumption in which the smoke passes through the water before it is inhaled.Citation1 The use of waterpipes is increasing all over the world, especially among young people and women.Citation2 A waterpipe machine has four major parts: a head, stem, vase, and hose (). Smoking using this machine includes the use of flavored and hydrated, tobacco known as “moassel.” A charcoal is placed on top of the tobacco to provide the heat needed to burn the moassel.Citation1 The bottom of the head has holes in it that passes the produced smoke to the stem, which is submerged in water that half-fills the vase. The hose is not submerged, exits from the bowl’s top, and ends with a mouthpiece, from which the user inhales.Citation1
Figure 1 A waterpipe machine. The major components of a waterpipe machine are labeled and include the head, stem, vase, and hose.
The health effects of cigarette smoking are well documented; however, knowledge regarding the impact of waterpipe smoking on body health is still lacking.Citation3 Previous literature has shown that smoke produced by a waterpipe contains a similar toxicant profile to that produced by cigarettes with different magnitude. For example, the tar produced by a single episode of waterpipe is about five times that produced by a single cigarette.Citation4 Similarly, exposure to carbon monoxide is at least several folds higher during waterpipe smoking compared with that of cigarette smoking.Citation5 Furthermore, the polycyclic aromatic hydrocarbons in waterpipe smoke are many times more than that of cigarette smoke.Citation6 In addition, the style of waterpipe smoking results in a dramatically higher exposure volume to smoke, more tobacco consumption per smoking event, and longer smoke inhalation periods.Citation7 Finally, tobacco in waterpipes is usually mixed with sugar, glycerol, and flavors, this mixture is burned by charcoal.Citation1 Thus, it is expected that waterpipe smoking will have a distinct effect on oral microbial flora.
The effects of cigarette smoking on oral health show that cigarette smoking is associated with oral cancer, periodontal disease, oral infections, and interference with the taste and modulation of normal flora.Citation8,Citation9 Several studies have also investigated the effect of smoking on oral microbiota and showed significant differences in the subgingival bacteria between smokers and non-smokers,Citation10 For example, Zambon reported that smokers harbored significantly higher levels of Bacteroides forsythus subgingivally.Citation11 In addition, the prevalence of several oral pathogens such as Prevotella nigrescens, Prevotella intermedia, Porphyromonas gingivalis, and Tannerella was significantly higher in smokers than in non-smokers.Citation12 Regarding waterpipe smoking, few studies have examined effects of waterpipe smoking on oral health. A recent study has shown a strong association between waterpipe smoking and periodontal disease.Citation13 In addition, waterpipe smoking has been shown to significantly increase potentially malignant oral mucosal lesionsCitation14 and lower lip squamous cell carcinoma and keratoacanthoma.Citation15 Moreover, waterpipe smoking has been shown to induce DNA damage in buccal cells.Citation16
Several bacterial species were identified in the human oral cavity including many anaerobic periodontal pathogens which are associated with periodontal infections such as P. gingivalis, Tannerella forsythia, P. intermedia, Eikenella corrodens, Campylobacter rectus, Aggregatibacter actinomycetemcomitans, Treponema denticola, and Fusobacterium nucleatum.Citation17 The frequency of these pathogens in the oral cavity has been shown to be altered by cigarette smoking.Citation18 Since the profile of toxicants and behavior of smoking are different between waterpipe and cigarette smoking, in this study, we investigated the effect of waterpipe smoking on the profile of normal flora in the oral cavity and subgingiva which is still undefined and unclear. The results of this study might be used to derive policies and interventions that target waterpipe smokers.
Materials and methods
Subjects
Fifty-nine healthy waterpipe smokers were recruited to participate in the study. As a control, 41 healthy non-smokers that matched the smokers group in gender and age were also recruited from the same geographical area. Smokers and their matched controls were recruited from customers of waterpipe cafes in Irbid city, which is the largest urban population north of Jordan. Usual customers of waterpipe cafes are young adults of both genders, who are either waterpipe smokers or waterpipe non-smokers who accompany their smoking friends, relatives, or family members to these places. Those who used tobacco products other than waterpipe were excluded from the study. Additionally, participants with previous history of oral diseases/infections or who were taking medications during the past 2 months were also excluded from the study. This study was approved by the Institutional Review Board of Jordan University of Science and Technology (Approval number 152/2012), and written informed consent was obtained from all subjects according to Institutional Review Board approval.
Sample collection
Subgingival samples were collected by inserting and rotating absorbent sterile paper-points (Meta Biomed Co Ltd, Cheongju, South Korea) for 10–15 seconds between the front upper and lower teeth to get high quantities of bacteria. Subjects with bleeding gums were excluded. In addition, any participant with a history of oral infection in the past 2 months was excluded. Oral cavity samples (teeth, tongue, and cheeks) were collected using sterile cotton transport swabs. Occasionally, bleeding occurred while taking the sample after inserting and rotating the sterile paper points (one for lower part and one for the upper part of teeth). In this case, the sample was discarded and sampling was repeated from a non-bleeding site. Samples were transported under anaerobic conditions using liquid dental transport medium (LDTM) (Anaerobe Systems, Morgan Hill, CA, USA). All samples were processed for microbiology techniques within 1–2 hours.
Culture conditions
Isolation of microorganisms was carried out by methods previously reported.Citation19 Isolated strains were characterized using standard microbiological methods as described in Clinical and Laboratory Standards Institute (CLSI) ML35-A2Citation20 document (). Each sample was vortexed at high speed for 60 seconds and then subjected to a series of 10-fold dilutions (up to 10–4) by using sterile Dulbecco’s phosphate buffered saline. Thereafter, aliquots of 100 μL from each different dilution were spread onto different differential and selective media including: crystal violet erythromycin (CVE), Wolinella agar, MacConkey agar, mannitol salt agar (MSA), kanamycin–vancomycin laked blood-2 (KVLB-2), tryptic soy agar supplemented with hemin 5 mg/mL and vitamin K3 (Menadione) 0.5 μg/mL, Mitis Salivarius agar supplemented with tellurite solution, and Sabouraud dextrose agar (SDA). For each microbial species, colony-forming units (CFUs/mL) were recorded for each plate by CFU enumeration assay. Total counts were determined on Columbia blood agar, aerobically and anaerobically. The following media were inoculated and incubated anaerobically at 37°C for 7–10 days by using the Oxoid™ Anaerobic Atmosphere Generation System, AnaeroGen™ 2.5 L Sachets: 1; ThermoFisher Scientic, Waltham, MA, USA) CVE agar (trypticase soy agar, yeast extract 5 g/L, sodium chloride 5 g/L, glucose 2 g/L, tryptophan 0.2 g/L, crystal violet 5 mg/L, erythromycin 4 mg/L, defibrinated sheep blood 50 mL/L) was used to assess F. nucleatum;Citation21 2) Kanamycin Vancomycin Laked Blood-2 (KVLB-2: Kanamycin 75 μg/mL, Vancomycin 2 μg/mL, laked blood) supplemented with hemin 5 μg/mL, vitamin K1 1 μg/mL), and trypticase soy agar supplemented with (hemin 5 mg/mL, Vitamin K3 0.5 μg/mL, sheep blood) were used to assess the black-pigmented P. intermedia and P. gingivalis, respectively; and 3) Wolinella agar (trypticase soy agar, vancomycin 9 μg/mL, ferrous sulfate 0.2 g/L, sodium thiosulphate 0.3 g/L, sodium fumarate 3 g/L, sodium formate 2 g/L) was used for the isolation of C. rectus.Citation22
Table 1 Culture media used in the current study
MSA was used to identify aerobic species such as Staphylococcus aureus and S.epidermidis. Yeast cells Candida albicans were identified by SDA. Species of Enterobacteriaceae family were identified on MacConkey agar and depending on the following tests: Gram stain, citrate test, motility test, urease test, indole test, oxidase test, catalase test, and triple sugar iron (TSI) agar. Mitis Salivarius Agar with 1% potassium tellurite was used to detect oral viridans streptococci, which consists of Streptococcus mutans, S. salivarius, and S. mitis.
For the determination of total aerobic and anaerobic bacteria counts for each microbial species, CFUs/mL were recorded for each plate by using the CFU enumeration assay. Bacteria were grown on Columbia blood agar under: 1) aerobic condition: 35°C ± 2°C for 18–72 hours under appropriate atmospheric conditions; and 2) anaerobic condition: 37°C for 7–10 days by using AnaeroGen 2.5 L sachets.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 17). Comparison of frequency of bacteria and pathogens between waterpipe smokers and control groups was conducted using the Mann–Whitney U-test (as data were not normally distributed), and chi-square test. CFU values were expressed as mean ± standard error of the mean (SEM) and were compared between different groups using the Mann–Whitney U-test (as data was also not normally distributed). Significant differences were examined at p < 0.05. Power analysis was carried out using G power version 3.0.10 (Franz Faul, Universtat Kiel, Germany). Sample size analysis was performed at 80% and 5% α-level of significance.
Results
The characteristics of study participants are shown in . The mean age of the waterpipe group was 23.98 ± 2.77 years versus 24.14 ± 4.37 years in the control group (p = 0.8). Males represented 74.5% of the waterpipe group and 65.9% of the control group. The average smoke sessions in waterpipe group per week were 4.98 ± 2.12. It was notable that waterpipe smokers were significantly associated with a history of oral infections (p = 0.01). shows the distribution of bacterial species isolated from subgingiva of participants. The most abundant bacteria from examined species were viridans streptococci, C. rectus and F. nucleatum in waterpipe users (range 84%–94%) and in non-smokers (range: 85%–97%). Acinetobacter and Moraxella species were present only in waterpipe smokers with a frequency of 5.1% and a mean of 31.7. The frequency of F. nucleatum was significantly lower in subgingiva of waterpipe smokers (p = 0.036, ) while the frequency of C. albicans was higher in waterpipe smokers (p = 0.023, ). Finally, the profile of the remaining examined bacteria was similar between the two groups (p > 0.05).
Figure 2 Frequency of some microbes in the waterpipe smokers isolated from the subgingiva of participants. Significant changes in the frequency of (A) Fusobacerium nucleatum and (B) Candida albicans in the subgingiva of participants. *Indicates significant changes (p < 0.05) using the chi-square test.
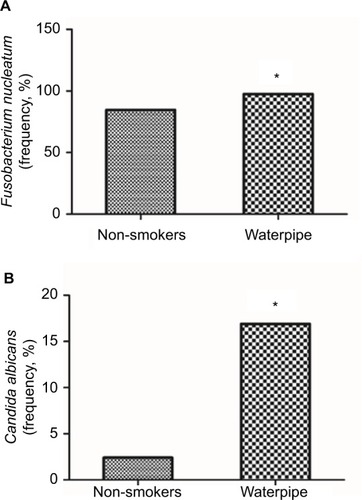
Table 2 Characteristics of participants
Table 3 Frequency and CFUs of isolates of detected microorganisms from subgingival plaque of participants
shows the distribution of bacterial species isolated from the oral cavity of participants. The most abundant bacteria from examined species were viridans streptococci, Campylobacter spp. F. nucleatum and black-pigmented bacteria in waterpipe users (range 94%–100%) and in non-smokers (range: 92%–96%). From these abundant bacteria, the mean of CFUs of black-pigmented bacteria was significantly lower (p = 0.001, ) in waterpipe smokers. Finally, no significant variations were detected in the distribution or mean of the rest of examined bacteria between the two groups (p > 0.05).
Figure 3 Frequency of some microbes in the waterpipe smokers isolated from the oral cavity of the participants. Significant changes in the frequency of black-pigmented bacteria. *Indicates significant changes (p < 0.05) using the chi-square test.
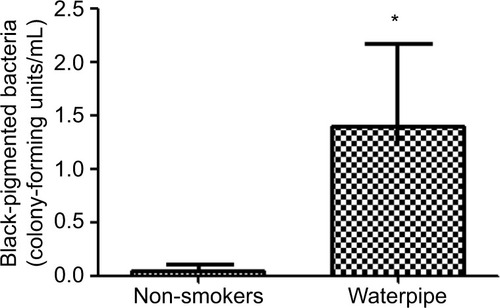
Table 4 Frequency and CFUs of isolates of detected microorganisms from oral cavity of participants
Discussion
The popularity of waterpipe smoking is growing in the Eastern Mediterranean and throughout the world, including the USA and other Western countries, especially among youth.Citation23 This spread is due, in part, to the use of tobacco that is sweetened and flavoredCitation24 and the misperception that the waterpipe “filters” the smoke, rendering it less harmful and less dependent than cigarette smoke.Citation25 While prevalence increases, science lags behind: little is known about harmful effects of the waterpipe on different body organs and whether or not it causes dependence.
The current study was performed to investigate the effect of waterpipe tobacco smoking (WTS) on oral and subgingival microbial flora. Variations were shown in the microbial profile of waterpipe smokers as compared to that of non-smokers, with significant differences in the prevalence and abundance of health-compatible organisms.
The limited information concerning the waterpipe effects on health could represent a major reason for its massive spread globally.Citation26 It has been reported that waterpipe smokers inhale similar toxicants to that of cigarettes smokingCitation4 including polycyclic aromatic hydrocarbons,Citation6 carbon monoxide, heavy metals, and aldehydes.Citation5 In addition, WTS has been shown to increase DNA damage in the lymphocytes and buccal mucosa cells of the users.Citation27 Lately, waterpipe tobacco smoking was reported to interfere with respiratory and vascular functions,Citation28 and to enhance oral diseases.Citation13 The results reported in the current study showing changes in oral microflora are in accordance with the above findings. This is particularly true because most of the bacteria that appeared or were enhanced among waterpipe smokers, can be pathogenic to human.Citation29,Citation30
Current results indicated the appearance of Acinetobacter and Moraxella species in subgingiva of waterpipe smokers. Both of these bacterial species are a common cause of human respiratory diseases. For example, a common Acinetobacter species is Acinetobacter baumannii, which can cause community-acquired pneumonia.Citation29,Citation31 Another common Moraxella species is Moraxella catarrhalis, which causes upper and lower respiratory infections, sinusitis and otitis media in susceptible humans.Citation32,Citation33 Notably, for both former mentioned species, tobacco consumption is a listed risk factor for infections.Citation29
The results of this study showed increased frequency of C. albicans in the subgingiva of waterpipe smokers. C. albicans is a part of the normal oral microflora;Citation34 however, any increase in its number will lead to oral Candida infection, which is known as oral thrush.Citation30 In fact, it has been reported that cigarette smoking increases the incidence of Candida infections in healthy humans.Citation35 Additionally, it was shown that exposure to tobacco smoke enhances the virulence of C. albicans.Citation36
In this study, black-pigmented bacteria such as P. gingivalis and P. intermedia had higher CFUs in isolates from the oral cavity of waterpipe smokers. These species are common in the oral cavity and are associated with periodontitis.Citation37 Another bacterial species that was reduced in the gingival flora of waterpipe smokers was F. nucleateum, which is associated with limited pathogenesis.Citation38 In fact, F. nucleatum is one of the most abundant anaerobic species in the oral cavity, in both diseased and healthy individuals. It may normally present in the healthy human oral cavity or considered as periodontal pathogenic. F. nucleatum proportions may be higher in current cigarette smokers.Citation39 However, in a study from Jordan, F. nucleatum was less prevalent among cigarettes smokers.Citation40 Notably, toxicant profile of waterpipe smoke has been shown to be different from that of cigarette smoke in terms of quantity and types. This could explain such variations observed in the current study as compared to cigarette literature.
Cigarettes smoking was also found to cause alteration in subgingival and oral bacterial profiles.Citation41 For example, periodontitis in smokers is associated with greater depletion of beneficial bacteria such as Veillonella, Neisseria, and Streptococcus species. On the other hand, the abundance of harmful bacteria such as Parvimonas, Campylobacter, Treponema, Fusobacterium, and Bacteroides was greatly enhanced in smokers.Citation41,Citation42 Only a few studies assessed the effect of WTS on periodontal disease using different outcome measurement such as periodontal bone height loss, plaque index, and gingivitis showing a statistically significant association of periodontal disease with WTS.Citation43 This is in support of the findings of the current study.
It is worth mentioning that the waterpipe group showed significantly higher history of oral infection than the control group. This is expected as a previous study showed the presence of a spectrum of pathogenic bacteria in the hoses of waterpipe smokers.Citation44 In addition, waterpipe smoking has been shown to be associated with periodontal disease,Citation45 and oral cancer.Citation46 Moreover, cigarette smoking has also been shown to be associated with oral infection.Citation47 Since the sample of the current study was random, the higher history of oral infection in the waterpipe group supported the imbalance in microbial flora induced by this type of smoking.
Public health policy specific to WTS is lacking in many countries.Citation48,Citation49 For example, in Jordan legislation requires health warnings on all tobacco products, but these warnings appear on cigarette packs only and not on WTS tobacco or waterpipes. The same is true in the US, where WTS is not yet regulated nationally. The current study highlights the importance of adopting strong policy regarding waterpipes. The knowledge presented in this study might also be used in interventions that target this type of smoking.
One of the limitations of the study was that a good fraction of oral microflora is uncultivable, and cultural techniques cannot differentiate between close bacteria such as Campylobacter spp., the use of other cultivation-independent approaches such as DNA hybridization, q-PCR, and 16S rRNA gene sequencing would be valuable in future investigations. Finally, it is recommended in future investigations to associate altered pathogens by waterpipe smoking with oral diseases by examining their resistance to commonly used antibiotics.
Conclusion
The study provided preliminary evidence for the effect of waterpipe smoking on oral microbiota.
Acknowledgments
The abstract of this paper was presented at the 2nd World Congress on Pediatric Care and Pediatric Infectious Diseases and the International conference on Pediatric Care and Pediatric Infectious Diseases as an abstract presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in J Infect Dis Ther 2016, 4:4(Suppl).
This work was supported by a grant from the Deanship of Research at Jordan University of Science and Technology (grant number 152/2012 to MAKS and OFK).
Disclosure
The authors report no conflicts of interest in this work.
References
- ShihadehAInvestigation of mainstream smoke aerosol of the argileh water pipeFood Chem Toxicol200341114315212453738
- JawadMAbassJHaririAWaterpipe smoking: prevalence and attitudes among medical students in LondonInt J Tuberc Lung Dis201317113714023232013
- MaziakWTalebZBBahelahRThe global epidemiology of waterpipe smokingTob Control201524Suppl 1i3i1225298368
- SepetdjianEShihadehASalibaNAMeasurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smokeFood Chem Toxicol20084651582159018308445
- ShihadehASalehRPolycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipeFood Chem Toxicol200543565566115778004
- ShihadehAAzarSAntoniosCHaddadATowards a topographical model of narghile water-pipe cafe smoking: a pilot study in a high socioeconomic status neighborhood of Beirut, LebanonPharmacol Biochem Behav2004791758215388286
- CobbCWardKDMaziakWShihadehALEissenbergTWaterpipe tobacco smoking: an emerging health crisis in the United StatesAm J Health Behav201034327528520001185
- HaukiojaAAsuntaMSöderlingESyrjanenSPersistent oral human papillomavirus infection is associated with smoking and elevated salivary immunoglobulin G concentrationJ Clin Virol201461110110625011603
- SherwinGBNguyenDFriedmanYWolffMSThe relationship between smoking and periodontal disease. Review of literature and case reportN Y State Dent J20137965257
- EggertFMMcLeodMHFlowerdewGEffects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival creviceJ Periodontol20017291210122011577953
- ZambonJJPeriodontal diseases: microbial factorsAnn Periodontol1996118799259118283
- KononenEPajuSPussinenPJPopulation-based study of salivary carriage of periodontal pathogens in adultsJ Clin Microbiol20074582446245117567788
- BibarsARObeidatSRKhaderYMahasnehAMKhabourOFThe effect of waterpipe smoking on periodontal healthOral Health Prev Dent201513325325925197731
- WarnakulasuriyaSWaterpipe smoking, oral cancer and other oral health effectsEvid Based Dent2011122444521701545
- El-HakimIEUthmanMASquamous cell carcinoma and keratoacanthoma of the lower lip associated with “Goza” and “Shisha” smokingInt J Dermatol199938210811010192158
- Al-AmrahHJAboznadaOAAlamMZElAssouliMZMujallidMIElAssouliSMGenotoxicity of waterpipe smoke in buccal cells and peripheral blood leukocytes as determined by comet assayInhal Toxicol2014261489189625357232
- D’ErcoleSCatamoGPiccolominiRDiagnosis in periodontology: a further aid through microbiological testsCrit Rev Microbiol2008341334118259979
- RiepBEdesi-NeussLClaessenFAre putative periodontal pathogens reliable diagnostic markers?J Clin Microbiol20094761705171119386852
- D’ErcoleSCatamoGTripodiDPiccolominiRComparison of culture methods and multiplex PCR for the detection of periodontopathogenic bacteria in biofilm associated with severe forms of periodontitisNew Microbiol200831338339118843894
- Clinical and Laboratory Standards Institute (CLSI)Abbreviated Identification of Bacteria and Yeast, Approved guidlines2nd edCLSI document ML35-A2Wayne, PACLSI2008
- WalkerCBRatliffDMullerDMandellRSocranskySSMedium for selective isolation of Fusobacterium nucleatum from human periodontal pocketsJ Clin Microbiol1979106844849521483
- KammaJJNakouMBaehniPCClinical and microbiological characteristics of smokers with early onset periodontitisJ Periodontal Res1999341253310086883
- PrimackBASidaniJAgarwalAAShadelWGDonnyECEissenbergTEPrevalence of and associations with waterpipe tobacco smoking among U.S. university studentsAnn Behav Med2008361818618719977
- RastamSWardKDEissenbergTMaziakWEstimating the beginning of the waterpipe epidemic in SyriaBMC Public Health200443215294023
- KandelaPNargile smoking keeps Arabs in WonderlandLancet20003569236117511030308
- AklEAJawadMLamWYCoCNObeidRIraniJMotives, beliefs and attitudes towards waterpipe tobacco smoking: a systematic reviewHarm Reduct J2013101223816366
- KhabourOFAlsatariESAzabMAlzoubiKHSadiqMFAssessment of genotoxicity of waterpipe and cigarette smoking in lymphocytes using the sister-chromatid exchange assay: a comparative studyEnviron Mol Mutagen201152322422820740646
- AlomariMAKhabourOFAlzoubiKHShqairDMEissenbergTCentral and peripheral cardiovascular changes immediately after waterpipe smokingInhal Toxicol2014261057958725144473
- DexterCMurrayGLPaulsenITPelegAYCommunity-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesisExpert Rev Anti Infect Ther201513556757325850806
- HebeckerBNaglikJRHubeBJacobsenIDPathogenicity mechanisms and host response during oral Candida albicans infectionsExpert Rev Anti Infect Ther201412786787924803204
- DoughariHJNdakidemiPAHumanISBenadeSThe ecology, biology and pathogenesis of Acinetobacter spp.: an overviewMicrobes Environ201126210111221502736
- de VriesSPBootsmaHJHaysJPHermansPWMolecular aspects of Moraxella catarrhalis pathogenesisMicrobiol Mol Biol Rev200973338940619721084
- Perez VidakovicsMLRiesbeckKVirulence mechanisms of Moraxella in the pathogenesis of infectionCurr Opin Infect Dis200922327928519405217
- CannonRDChaffinWLOral colonization by Candida albicansCrit Rev Oral Biol Med199910335938310759414
- MuzurovicSHukicMBabajicESmajicRThe relationship between cigarette smoking and oral colonization with Candida species in healthy adult subjectsMed Glas (Zenica)201310239739923892865
- AlanaziHSemlaliAPerraudLChmielewskiWZakrzewskiARouabhiaMCigarette smoke-exposed Candida albicans increased chitin production and modulated human fibroblast cell responsesBiomed Res Int2014201496315625302312
- TakeuchiKFurutaMTakeshitaTSerum antibody to Porphyromonas gingivalis and periodontitis progression: the Hisayama StudyJ Clin Periodontol2015
- ParahitiyawaNBScullyCLeungWKYamWCJinLJSamaranayakeLPExploring the oral bacterial flora: current status and future directionsOral Dis201016213614519627515
- EickSRamseierCARothenbergerKBraggerUBuserDSalviGEMicrobiota at teeth and implants in partially edentulous patients. A 10-year retrospective studyClin Oral Implants Res201627221822525827437
- KarasnehJAAl HabashnehRAMarzoukaNAThornhillMHEffect of cigarette smoking on subgingival bacteria in healthy subjects and patients with chronic periodontitisBMC Oral Health20171716428327165
- BrookIThe impact of smoking on oral and nasopharyngeal bacterial floraJ Dent Res201190670471021558542
- ShchipkovaAYNagarajaHNKumarPSSubgingival microbial profiles of smokers with periodontitisJ Dent Res201089111247125320739702
- NattoSBaljoonMAbanmyABergstromJTobacco smoking and gingival health in a Saudi Arabian populationOral Health Prev Dent20042435135716296253
- MasadehMMHusseinEIAlzoubiKHKhabourOShakhatrehMAGharaibehMIdentification, characterization and antibiotic resistance of bacterial isolates obtained from waterpipe device hosesInt J Environ Res Public Health20151255108511525985311
- ObeidatSRKhabourOFAlzoubiKHPrevalence, social acceptance, and awareness of waterpipe smoking among dental university students: a cross sectional survey conducted in JordanBMC Res Notes2014783225421621
- WaziryRJawadMBalloutRAAl AkelMAklEAThe effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysisInt J Epidemiol2017461324327075769
- ChenPCPanCCKuoCLinCPRisk of oral nonmalignant lesions associated with human papillomavirus infection, betel quid chewing, and cigarette smoking in Taiwan: an integrated molecular and epidemiologic studyArch Pathol Lab Med20061301576116390239
- JawadMEl KadiLMugharbilSNakkashRWaterpipe tobacco smoking legislation and policy enactment: a global analysisTob Control201524Suppl 1i60i6525550418
- WardKDSiddiqiKAhluwaliaJSAlexanderACAsfarTWaterpipe tobacco smoking: The critical need for cessation treatmentDrug Alcohol Depend2015153142126054945
