Abstract
The body defense mechanism has evolved to protect animals from invading pathogenic microorganisms and cancer. It is able to generate a diverse variety of cells and molecules capable of specifically recognizing and eliminating a limitless variety of foreign invaders. These cells and molecules act together in a dynamic network and are known as the immune system. Innate mucosal immunity consists of various recognition receptor molecules, including toll-like receptors, NOD-like receptors, and RIG-I-like receptors. These recognition receptor molecules recognize various invading pathogens effectively, and generate an immune response to stop their entry and neutralize their adverse consequences, such as tissue damage. Furthermore, they regulate the adaptive response in cases of severe infection and also help generate a memory response. Most infections occur through the mucosa. It is important to understand the initial host defense response or innate immunity at the mucosal surface to control these infections and protect the system. The aim of this review is to discuss the effects and functions of various innate mucosal agents and their importance in understanding the physiological immune response, as well as their roles in developing new interventions.
Keywords:
Introduction
The mucous membrane that covers the digestive and urogenital tracts, the respiratory canal, the eye conjunctiva, the inner ear, and layers of most of the exocrine glands, is a strong component of the immune system, with very specialized mechanical and chemical barriers that either prevent the entry of foreign bodies or facilitate their degradation through a series of chemical processes, most of which are yet to be fully clarified. The mucosa prevents colonization and invasion by foreign pathogens, and prevents any aggravated response of the immune system to such pathogens that could harm the organism. This is a rather local but specialized version of the immune system, and is well interlinked with the lymphatic system, and thus is known as mucosa-associated lymphatic tissue or, in a more functional sense, the mucosal immune system. There are various subsets of this broad term, including gut-associated lymphatic tissue, bronchus-associated lymphatic tissue, and nasal-associated lymphatic tissue. The mucosal immune system has two parts, ie, the innate system and the adaptive system. The innate system comprises various recognition molecules and natural killer cells, while the adaptive system comprises various antigen-presenting cells and the T and B lymphocytes.Citation1
The recognition of foreign bodies at the mucosal surface takes place in a number of ways. Cell-intrinsic recognition involves the dendritic cell recognizing cytosolic pathogen-associated microbial patterns (PAMPs) using various pattern recognition receptors, followed by the presentation of pathogens, either by the major histocompatibility complex (MHC) Class I pathway (via rough endoplasmic reticulum) or the MHC Class II pathway (via autophagy). Signaling by pattern recognition receptors induces costimulatory molecules and cytokines that activate T cells. In cell-intrinsic recognition, the MHC I pathway depends on an abundance of antigens, while the MHC II pathway depends on autophagy machinery. Cell-extrinsic recognition can recognize either pathogenic or infected cells. On encountering an infected cell, pattern recognition receptors induce the secretion of interferons (IFNs) and dendritic cell-activating cytokines. This is followed by MHC Class I or II antigen presentation. If toll-like receptors are involved in recognition, they induce costimulatory molecules and cytokines, thus activating the T cell response. On an encounter, pathogen toll-like receptors recognize the pathogen and move, along with the pathogen, into the cell phagosome where antigen processing and presentation occurs only via the MHC Class II pathway, followed by secretion of T cell activation moleculesCitation2–Citation4 (see ).
Figure 1 Pathogen recognition by dendritic cells in mucosa by toll-like receptors and RLR and their processing via major histocompatibility complex I and II pathways in a macrophage.
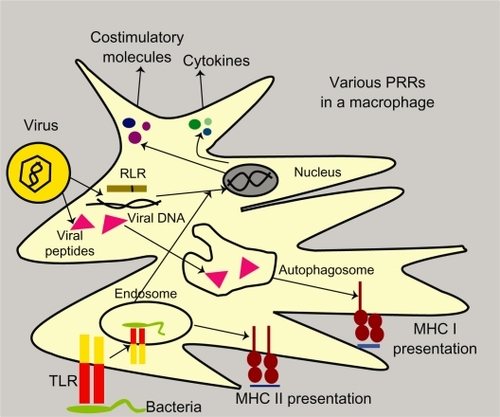
The mucosal immune system is unique, being characterized by the presence of specific types of lymphocytes which are not found in the bloodstream and have a very different type of immune reaction, with the presence of the secretory antibody, immunoglobulin (Ig)A.Citation5 Another characteristic feature of this system is the ability to differentiate, via pattern recognition receptors, between a pathogenic particle and a nonpathogenic one.Citation6 Toll-like receptors, a class of pattern recognition receptors, are specialized antigen- and adjuvant-sensing molecules found on almost all mucosal antigen-presenting cells. They not only recognize foreign pathogens, but also commensals, and thus maintain a steady-state environment inside the gut. Similar molecules, like NOD-like receptors and Type C lectins, also provide an immediate response to pathogens and tissue damage by recruiting innate immune cells, like macrophages and neutrophils, thus triggering tissue repair and switching over to adaptive immune mechanisms.Citation7
The gastrointestinal tract is the largest mucosal surface in all mammalian systems, and most pathogens try to invade the body system through the gut mucosa. Intestinal epithelial cells comprise the most vital part of the gut mucosa and regulate the various gut processes, including digestion, secretion, absorption, and defense. Their main function is the transport of polymeric immunoglobulins produced by plasma cells in the lamina propria to the intestinal lumen via a receptor-mediated pathway and the recognition of foreign matter via pattern recognition receptors.Citation8 They also have an important role in antigen presentation, and have the dual ability to present both Class I and Class II antigens and also secrete immunologically active proteins in response to proinflammatory cytokines, thus preventing any damage to the intestinal lumen and mucosa.Citation9 In addition, epithelial cells express CD14 molecules that bind to lipopolysaccharides and also control the interaction between commensals and the mucosa.Citation10
The second class of cells in the mucosa basically comprises T lymphocytes expressing CD8, CD45RO, integrins, and perforins.Citation11 Another specialized class of cells, called M cells, found in the mucosal membrane, help absorb antigens and then transport them to Peyer’s patches, followed by antigen activation of T cells.Citation12
Innate immunity cells release proinflammatory cytokines that subsequently activate the components of the adaptive immune system. Specialized epithelial and intraepithelial cells also form a part of the mucosa, and maintain a balance by self-renewal, and also maintain a balance between the commensal colonies residing in the mucosa. Migration of T and B lymphocytes from the lymph nodes to the common mucosa has been observed, and is related to activation, recirculation, and activation of lymphocytes, and also the development of memory lymphocytes. Such movements are controlled by specific integrin-type molecules and chemokines secreted in the mucosa, including CC chemokine ligand 25.Citation13,Citation14 Regulatory T cells secrete lymphokines, including interleukin (IL)-10 and transforming growth factor beta (TGF-β), that result in oral immunosuppression/tolerance, thus preventing any immune response to food particles during ingestion.Citation15 Similar mechanisms are hypothesized to work in the colorectal immune response, which has no effect on the small intestine, and in the nasal immune response in the upper respiratory tract mucosa that is exposed to large amounts of nasal and lacrimal secretions.
The populations of various intra-epithelial T lymphocytes increase gradually, but markedly, in both germ-free and normal mice after birth upon colonization by commensal microbiota, and are thus important in controlling their growth and interaction with cells in the local environment of the gut mucosa.Citation11
A study of bronchus-associated lymphatic tissue in patients with lung carcinoma showed T cells surrounding B cell follicles, and most CD4 T cells also exhibited CD45RO, and thus had memory responses as well. All the B cells exhibited α4 integrin and L-selectin, while only 43% and 20% of the total population of T cells showed α4 integrin and L-selectin, respectively. In lung mucosa-associated disease, there is a strong combined mucosal and systemic immune response regulated by CD4-expressing TH2 cells and TH1 cells in patients with asthma and tuberculosis, respectively.Citation16 L-selectin, a receptor molecule prominent in the head and neck mucosa and in the lymph nodes, regulates the trafficking of lymphocytes to these sites. Another set of molecules, known as α-defensins, regulates the mucosal immune system of the gut and prevents microbial invasion at the epithelial surface. These are of six types, ie, human neutrophil peptides 1, 2, 3, and 4, and human defensins 5 and 6. Their counterparts in murine gut-associated lymphatic tissue are known as cryptidins. Human defensin 5 is expressed in disorders like gastritis, ulcerative colitis, and Crohn’s disease. Human neutrophil peptides 1, 2, and 3 are strongly expressed in all types of inflammatory bowel disease. Activation of α-defensins and cryptidins requires a colocalized molecule known as metalloproteinase matrilysin.Citation17
In metalloproteinase matrilysin-mutant mice, there is a prominent absence of cryptidins and accumulation of cryptidin precursors. Such mice are much more susceptible to ingested pathogens than wild-type varieties.
Human defensins not only prevent invasion by pathogens, but also trigger the influx of different populations of T lymphocytes for further adaptive responses. C type lectins, like RegIIIγ, are expressed in the intestinal mucosa, have antimicrobial properties, interact with bacteria via a peptidoglycan mechanism, and aid the recruitment of symbiotic bacteria in the intestine.Citation18
Mucosal immunity is directly involved in a number of infectious diseases and allergies. In the upper respiratory tract, where active influx of IgA and IgM occurs in response to any immune aggravation, with some locally produced IgG, Waldeyer’s ring exhibits adhesion molecules and various chemokine receptors expressed in B lymphocytes present there, due to a higher possibility of airborne allergens, which are more frequent.Citation19
A general hypothesis that can be made from all the studies done to date at the mucosa is that innate immunity is the true regulator of the immune system. This hypothesis is at the basis of almost all mucosal disease, as well as mucosal vaccine development.
Mucosal diseases
Because the mucosal surface serves as a portal for many infectious diseases, almost all types of pathogens, ie, bacteria, virus, fungi, and parasites, cause disease at the mucosa while trying to invade the body. Some common mucosal diseases caused by a variety of pathogens are described.
The main disease of concern affecting the intestinal mucosa is inflammatory bowel disease, in particular, Crohn’s disease and ulcerative colitis. Crohn’s disease is a transmural inflammation of the bowel at the terminal ileum and right colon preferentially, but sometimes also involves the colon, rectum, and peritoneal area. The main symptoms are diarrhea, abdominal pain, and weight loss, often accompanied by extradigestive manifestations, including fever, ulcers, arthralgia, and erythema nodosum. About 20% of people with Crohn’s disease have a blood relative with some form of inflammatory bowel disease, often a brother or sister, and sometimes a parent or child. Crohn’s disease can occur in people of all age groups, but is more often diagnosed in people aged 20–30 years. Ulcerative colitis causes inflammation and ulcers in the top layer of the lining of the large intestine. Ulcerative colitis primarily affects the colonic mucosa, and the extent and severity of colon involvement are variable. In its most limited form, it may be restricted to the distal rectum, while in severe cases, the entire colon is involved. Both genders are equally affected. In Western Europe and in the US, ulcerative colitis has an incidence of approximately 6–8 cases per 100,000 and an estimated prevalence of approximately 70–150 per 100,000. The leading initial symptom of ulcerative colitis is diarrhea with blood and mucus, sometimes with pain. Fever and weight loss are less frequent. Extraintestinal symptoms can be an initial manifestation or can occur later in the course of the disease. In proctitis, obstipation can very occasionally be the initial symptom. Eighty percent of patients have only proctitis or proctosigmoiditis, and only 20% have extensive colitis. The etiology of both Crohn’s disease and ulcerative colitis remains unknown, although very recent studies have hypothesized multiple factors leading to the disease, ranging from tobacco smoking to malnutrition or unhealthy eating habits.Citation20,Citation21
Helicobacter pylori is a Gram-negative bacterium that colonizes the gastric mucosa and causes chronic gastritis and gastric ulcers. The bacterium adheres strongly to the surface of gastric epithelial cells without actually invading them. H. pylori infection is relatively common worldwide, although less than one-quarter of infected individuals progress to the development of gastric disease. H. pylori infection is common in the US. About 20% of people younger than 40 years of age and half of those over 60 years of age have the disease. However, most infected individuals do not develop ulcers. Whether or not an individual proceeds to a disease state may be influenced by any combination of host, bacterial, and environmental factors. H. pylori weakens the protective mucous coating of the stomach and duodenum, which allows acid to get through to the sensitive lining beneath. Both the acid and the bacteria irritate the lining and cause an ulcer. Serious problems may occur, including perforation (when the ulcer burrows through the stomach or duodenal wall), bleeding (when acid or the ulcer breaks a blood vessel), and obstruction (when the ulcer blocks the path of food trying to exit the stomach).Citation22
Diarrhea is the second leading cause of childhood morbidity and mortality worldwide, and each year almost two million children under five years of age die from severe gastroenteritis. Rotaviruses are the single leading cause of diarrhea-related deaths in this age group. It is estimated that each year, amongst children younger than five years of age, rotaviruses account for 114 million episodes of diarrhea, 25 million clinic visits, 2.4 million hospital admissions, and more than 500,000 deaths worldwide. About 99% of rotavirus-associated deaths occur in middle- and low-income countries, predominantly affecting infants in the first year of life living in socioeconomically deprived rural regions of Africa and Asia. In India alone, rotaviruses cause more than 120,000 deaths, 400,000 hospital admissions, 5 million clinic visits, and 25 million episodes of diarrhea annually. One in 250 children from the low-income countries of Africa and Asia die from rotavirus gastroenteritis before the age of five years. After an incubation period of 1–3 days, rotavirus gastroenteritis typically begins abruptly with fever and vomiting, followed by watery diarrhea. Less commonly, it may present with vomiting and diarrhea only, or even with either symptom alone with or without fever. Symptoms usually last for 3–8 days, with dehydration, and electrolyte and acid-base disturbance as the most serious complications. Compared with other enteric pathogens, children hospitalized with rotavirus gastroenteritis are more likely to have high fever, vomiting, and dehydration. Noroviruses are important pathogens in both sporadic cases and outbreaks of gastroenteritis in humans. Noroviruses can affect individuals of all ages in a variety of settings. The most common acute symptoms are diarrhea and nausea followed by vomiting, abdominal pain, fever, and fecal incontinence. Various nonspecific symptoms are also reported on the first day of illness, with anorexia being most frequent, followed by thirst and lethargy, then headache and vertigo.Citation23–Citation26
Entamoeba histolytica is a pathogenic organism that is widespread throughout the world, but is especially common in developing countries, where many people do not have access to clean water. The parasite causes invasive disease in over 50 million people and an estimated 100,000 deaths per year, making it a leading cause of parasitic death in humans. Infection with E. histolytica can lead to asymptomatic colonization, amoebic colitis, or disseminated extraintestinal disease. The infectious cycle of E. histolytica begins with ingestion of the cyst, a nondividing, quadrinucleate form that is able to survive in the environment. After ingestion, the cyst undergoes excystation in the small intestine to produce the proliferative trophozoite form. Trophozoites colonize the colon, adhering to the mucous layer by means of lectin, which binds colonic mucins. Disease results when this mucous layer is penetrated and the trophozoites attack the intestinal epithelial cells after entering the mucous layer due to a protective, chitin-containing cell wall.Citation27–Citation29
Influenza is a highly contagious acute respiratory disease caused by infection of the host respiratory tract by the influenza virus. The global morbidity and mortality burden of influenza is considerable, with an estimated one million deaths annually worldwide. In temperate regions, there are clear seasonal variations in the occurrence of influenza, with a marked peak in the cold winter months. In contrast, seasonality is less well-defined in tropical regions, where there is high background influenza activity throughout the year, with epidemics occurring in the intermediate months between the influenza season in temperate countries of the Northern and Southern hemispheres. The major limitation of current influenza vaccines is that they are strain-specific and thus ineffective against new variants of the viruses resulting from genetic changes that cause antigenic drifts in hemagglutinin or antigenic shifts to another hemagglutinin subtype. Administration of vaccines is also limited by the potential for sensitivity to the egg-propagated vaccine and by the fact that, as a preventative measure, vaccination cannot provide a means of immediate treatment or prophylaxis.Citation30,Citation31
Human papilloma virus (HPV) infections occur all over the world. HPV is associated with a plethora of clinical conditions, ranging from innocuous lesions to cervical cancer. HPV infects the skin and mucosa, and may induce the formation of both benign and malignant tumors. The infection starts when the virus penetrates the new host through micro injury. The development of the incubation phase into active expression depends on three factors, ie, cell permeability, virus type, and host immune status. Mucosal HPV infects and replicates in the mucous membranes (genital, oral, and conjunctival papillomas), and induces epithelial proliferation. In recent years, HPV has become one of the most common sexually transmitted diseases in both men and women worldwide. Cervical cancer is also one of the most common cancers in women. Recurrent respiratory papillomatosis is a benign, often multifocal neoplasm. A potentially fatal manifestation of HPV infection, recurrent respiratory papillomatosis is characterized by multiple warty excrescences on the mucosal surface of the respiratory tract.Citation32,Citation33
Molecules of mucosal immunity
Mucosal immune regulation is activated and induced by various pathways utilizing a variety of biomolecules, including lectin, selectins, integrins, NOD-like receptors, pyrin domain-containing NOD-like receptors (NALPs), and retinoic acid-inducible protein-1 (RIG-I)-like receptors, with the most important being the toll-like receptors.
Mammalian toll-like receptors are a class of pattern recognition receptors that play a major role in the initiation of innate mucosal immune responses and the following adaptive immune responses to microbial pathogens. Activation of the toll-like receptor signaling pathway leads to the expression of numerous genes regulating host defense, including inflammatory cytokines, chemokines, antigen-presenting molecules, and costimulatory molecules. These evolutionarily conserved receptors, homologs of the Drosophila toll gene, recognize highly conserved structural motifs only expressed by PAMPs. PAMPs are very different from host cell-surface molecular patterns, and toll-like receptors have unique recognition ability for PAMPs. TLR1, TLR2, TLR4, and TLR6 recognize lipid microbial ligands, TLR3, TLR7, TLR8, and TLR9 recognize nucleotide ligands, TLR5 recognizes protein ligands, and uropathogenic organisms are recognized by TLR11.Citation1
Toll-like receptors are Type 1 transmembrane proteins characterized by an extracellular domain containing leucine-rich repeats and a cytoplasmic tail that contains a conserved region called the toll/IL-1 receptor domain. The structure of the extracellular domain of TLR3 was recently revealed by crystallography studies as being a large horse shoe shape. Stimulation of toll-like receptors by PAMPs initiates signaling cascades that involve a number of proteins, including myeloid differentiation primary response gene (88), toll/IL-1 receptor domain-containing adapter-inducing IFN-β, and IL receptor-associated kinase.Citation1 These signaling cascades lead to the activation of transcription factors, such as activator protein-1, nuclear factor kappa B (NFκB), and IFN regulatory factors, inducing the secretion of proinflammatory cytokines and effector cytokines that direct the adaptive immune response. IL receptor-associated kinase-4 then activates IL receptor-associated kinase-1 by phosphorylation. Both IL receptor-associated kinase-1 and IL receptor-associated kinase-4 leave the myeloid differentiation primary response gene (88)-toll-like receptor complex and associate temporarily with TNF receptor-associated factor (TRAF)6, leading to its ubiquitination. Bcl10 and MALT1 form oligomers that bind to TRAF6, and promote TRAF6 self-ubiquitination. Following ubiquitination, TRAF6 forms a complex with TGF-β activated kinase (TAK)2/TAK3/TAK1, inducing TAK1 activation. TAK1 is then coupled to the inhibitor of kappa B kinase gamma complex, which includes the scaffold protein, nuclear factor kappa B essential modulator, leading to the phosphorylation of IκB and subsequent nuclear localization of NFκB. Activation of NFκB triggers the production of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), IL-1, and IL-12, which direct the adaptive immune response.Citation34–Citation36
In one study, it was found that the expression pattern and function of toll-like receptors in different murine B cell subsets including follicular, marginal zone, B-1, and Peyer’s patches, were different. B cell subsets express all known toll-like receptors, except TLR5 and TLR8. Strong proliferation and antibody secretion was recorded on stimulation by different toll-like receptor ligands, particularly ligands to TLR1 and 2, TLR2 and 6, and TLR4, 7, and 9. Marginal zone B cells have a similar pattern of toll-like receptor expression and response. However, B-1b cells do not respond strongly to TLR2 ligands. In contrast, Peyer’s patch B cells respond strongly to TLR2, 6, and 7 ligation, but poorly to ligands for TLR9 and 4. Follicular B cells that stimulate the T cell-dependent immune response express and secrete antibodies to IgG3 and IgM in response to a variety of toll-like receptor agonists in vitro. An experiment done with cells of gut-associated lymphatic tissue revealed strong expression of TLR9 specifically in Peyer’s patches and mesenteric lymph nodes. In the Peyer’s patches they were prominent on dendritic cells, B cells, follicle-associated epithelium, and M cells. TLR9 confers a wide range of innate immune defenses against foreign oligonucleotides derived from bacteria.Citation37
It was found that there was a high upregulation of costimulatory molecules in Shigella dysenteriae porin, such as C80 and CD86, followed by a marked increase in IgA secretion. These cells were found to express TLR2 and TLR6 for the detection of porin, and can thus be directly correlated with mucosal regulation in the case of Shigella infection.Citation38 Induction of TLR2 by synthetic Pam3 CysSK4 in intestinal mucosa demonstrated a tight junction-associated barrier assembly preventing stress-induced damage via the induction of Akt-mediated cell survival using the myeloid differentiation primary response gene (88). Inflammatory stress-like colitis results in breakage of tight junctions in intestinal cells, while treatment with TLR2-PCSK4 effectively restores tight junction integrity and suppresses mucosal inflammation, thus providing a way to use toll-like receptors to modulate mucosal injury.Citation39 Shigella infection induces the production of NFκB and IL-8 in intraepithelial cells and is mediated by NOD1.Citation40 It has also been established that Type I fimbriae on Shigella are detected by toll-like receptors.Citation41
Ethanol intake causes great stress to the intestinal mucosa and damages epithelial cells. A study in C57BL/6J mice with ethanol injury demonstrated the expression of TLR4 following cyclo-oxygenase-2 and prostaglandin E2 expression, that was further followed by expression of macrophage inflammatory protein-2. Thus TLR4 can have a protective role in ethanol poisoning of the gut.Citation42 It was demonstrated in a murine study of Helicobacter-dependent colitis that mice lacking TLR4 showed low expression of IL-10 and thus had dysregulation in T cell functioning and lack of homeostasis in mucosal cells.Citation43 In another study, using V-TLR4 transgenic mice, it was demonstrated that TLR4 has a role in the recruitment of B cells in the lamina propria of the mucosa and also upregulates the expression of IgA and IgA-mediated class switching of B cells.Citation44 Expression of TLR5 and TLR9 in the apices of gastric epithelial cells due to H. pylori infection was detected, although this expression was lost in H. pylori gastritis.Citation45 TLR5 was shown to detect H. pylori flagellin, although this reaction has low immunogenicity.Citation46 Similarly, heat shock protein 60 of H. pylori induces activation of TLR2 and 4, resulting in upregulation of NFκB and IL-8 secretion in gastric epithelial cells.Citation47
Elevation of TLR4 expression in epithelial cells of the colon has been observed in ulcerative colitis and Crohn’s disease, and Asp299Gly and Thre399Ile polymorphisms have been correlated with development of these diseases.Citation48 Upregulation of TLR2 expression in colitis has been observed in mice.Citation27 It has also been found in inflammatory bowel disease that flagellin-specific antibodies are present in high amounts in serum, which links this condition with TLR5.Citation49 Intestinal myofibroblasts were shown to express TLR2 and TLR4 in response to lipopolysaccharide and lipoteichoic acid, and were linked to the development of fibrosis related to Crohn’s disease.Citation50 Various toll-like receptors of the enteric mucosa are known to work alongside NOD2, and control inflammatory bowel disease with excessive expression of NFκB.Citation51 In the event of E. histolytica infection, neutrophil influx to the infection site followed by IL-8 expression was identified a long time ago, and later found to be a TLR2-and TLR4-dependent mechanism induced by lipopeptide phosphoglycan from E. histolytica.Citation52,Citation53
Commensal-dependent colitis is a condition that mostly arises due to deficiency of the anti-inflammatory and immune tolerance pathways. In a study carried out in IL-10-mutant and IL-2-mutant mice, it was observed that in the absence of toll-like receptor signaling pathways, both these mutant strains developed commensal-dependent colitis due to a possible lack of innate immune regulation.Citation54
TLR4 was shown to be the immediate responder to Salmonella infection while TLR2 was linked to a later response.Citation55 Salmonella flagellin has been shown to induce TLR5 activation in intestinal epithelial cells.Citation56 Studies in TLR4-mutant mice have confirmed the role of TLR4 in infection control and production of TNF-α and several other chemokines.Citation57 Salmonella-susceptible mice were shown to be deficient in TLR5, and this was linked to the SPI2 pathogenicity island and SopE2 guanine exchange factor in Salmonella.Citation58,Citation59 Salmonella typhi was shown to inhibit TLR4 and TLR5 responses, and such infection were unable to produce IL-8 or neutrophil activation.Citation60 Resolution of primary Salmonella infection, the major causative agent of typhoid fever, resulting in enteric bleeding from Peyer’s patches, was demonstrated to be controlled by the expression of a toll/IL-1R domain-containing adaptor protein through downstream signaling via TLR1, 2, 4, and 6.Citation61
A common cause of enteric diarrhea is Escherichia coli, the lipopolysaccharide of which is readily recognized by TLR4 of intestinal epithelial cells.Citation62 In the event of infection by pathogenic strains, it was found that TLR5 induced activation of NFκB and IL-8 production by intraepithelial cells.Citation63 E. coli Type 2 enterotoxin was shown to activate TLR2 through an interaction of its B subunit.Citation64 It is also established that fimbriae on E. coli activates TLR2 and TLR4 and causes inflammation, followed by IL-8 secretion.Citation65
It has been shown that TLR9-deficient mice have upregulation in the population of CD4Foxp3 regulatory T cells (T-regs) and a reduction in the expression of IL-17- and IFN-γ-producing effector T cells. In such strains of mice, engagement of TLR9 from hematopoietic-derived cells controlled the T-reg population and gut priming for the immune response to Encephalitozoon cuniculi infection.Citation66 Studies of total parenteral feeding showed upregulation of various toll-like receptors in the intestinal mucosa to prevent bacteria-mediated septic shock arising from IFN-γ-mediated epithelial cell apoptosis due to lack of enteric feeding. The major toll-like receptors involved were TLR4, TLR5, TLR7, and TLR9.Citation67 A toll-like receptor study in goats (Capra hircus) revealed expression of all toll-like receptors in peripheral blood mononuclear cells, as well as lung mucosal lymphocytes, with high amounts of TLR10. TLR3, 4, and 10 were expressed at lower levels in the uterine and jejunal mucosa, while the mucosa of the uterus and skin expressed high TLR6 levels.Citation68
The detection of viral influenza has been linked to TLR3, 7, and 8 for a long time. A case study in rat H3N2 influenza showed stimulation of Type 1 IFN, TNF-α, IL-1, and IFN-γ by TLR7 and TLR8. Prophylactic administration of these toll-like receptors suppresses viral titers in the lung with local IFN secretion, hinting at the potential prophylactic and/or therapeutic use of these toll-like receptors.Citation69 Another study of Haemophilus influenza in mice demonstrated the role of TLR4 in intranasal immunization by activating the TH1 response followed by mucosal IgA and IgG secretion, all of which were absent in TLR4-mutant mice.Citation70 It was also shown in another study that cigarette smoke mixed with double-stranded DNA induces expression of RANTES (regulated on activation normal T cell expressed and secreted) in patients suffering from chronic rhinitis, followed by activation of TLR3, leading to high levels of human β-defensin 2, while cell activation was also upregulated.Citation71 Nasal mucosa inflammation has been reported to be mediated by TLR4 via a local TH1 and TH2 response and upregulation of IL-10. This TLR4-mediated activation is induced by lipopolysaccharide and expression of the TLR4 receptor by CD3 T cells.Citation72 Smooth muscle cells in human airways have been shown to express all toll-like receptors. TNF-α and double-stranded RNA were found to be the most potent inducers of TLR2 and TLR3, and along with these two cytokines, IFN-γ helped activation of TLR4. Toll-like receptor activation led to the release of IL-8 and eotaxin. When Dexamethasone is administered with IFN-γ and TNF-α, a strong TLR2 expression is induced. Together, TLR2, 3, and 4 were found to be major activators of chemokine release and mucosal tolerance in human airways.Citation73 Epithelial nasal polyp cells were found to express TLR3 strongly on induction by double-stranded RNA and then mediated very strong proinflammatory responses via the secretion of RANTES, IP-10, IL-8, and granulocyte-macrophage colony-stimulating factor. Similar studies with lipopolysaccharide also mediated similar responses but were comparatively weaker.Citation74 A study of bovine nasal-associated lymphatic tissue infected with foot and mouth disease virus demonstrated high expression of TLR4 mRNA from the dorsal soft palate cells in the acute stages of the disease, along with strong expression of mRNA for IFN-α, with some expression of IFN-γ, IL-1α, TNF-α, and IL-2.Citation75
A study of airway epithelial cells in cystic fibrosis showed high expression of TLR2 in diseased cells on induction and mediated proinflammatory responses in the airway mucosa.Citation76 Stimulation of lung mucosal TLR2 and TLR6 by MALP-2 showed secretion of IL-8 and macrophage inflammatory protein-Iβ and enhances phagocytosis.Citation77 Patients suffering from chronic rhinosinusitis with nasal polyps exhibited high expression of TLR2 followed by induction of high quantities of macrophage inflammatory protein-α, RANTES, and granulocyte-macrophage colony-stimulating factor.Citation78 The virulence of Group B Streptococcus is mainly due to the PBP1a protein required for cell wall synthesis and encoded by the ponA gene. An experiment in TLR2-mutant mice using two different strains of Group B Streptococcus, ie, wild-type and ponA-mutant, revealed that TLR2-mutant mice were more susceptible to infection by both strains of Group B Streptococcus, indicating the involvement of TLR2 in the immune response to Group B Streptococcus infection.Citation79 TLR2 and TLR4 show strong expression in the middle ear of the rat, and thus have a specific role in the detection of the airborne pathogens that usually invade the ear mucosa and lead to otitis.Citation80 It was found that oral mucosal Langerhans cells express TLR4, and stimulation of TLR4 showed expression of coinhibitory molecules, such as B7-H1 and B7-H3, and downregulation of the costimulatory molecule, CD86, while there was a high increase in IL-10 secretion followed by the induction of TGF-β1, Foxp3, IFN-γ, and IL-2 in T cells. Thus, TLR4 has great importance in oral tolerance via Langerhans cells.Citation81
It has been established that toll-like receptors have a role in activating both the cellular and humoral immune response in Candida albicans infection, along with C type lectin receptors.Citation82 Gingival epithelial cells showed expression of TLR2, TLR4, and TLR6, with the production of IL-6, and IL-8, and human β-defensins 1 and 2 in the oral mucosa. In the same study, it was found that a quorum-sensing molecule, farnesol, that regulates the virulence of C. albicans, had a synergistic effect in the production of these cytokines, along with TLR2, TLR4, and TLR6.Citation83 TLR2 and soluble CD14 expression was found to be unregulated in the oral mucosa in diseases such as oral lichen planus and burning mouth syndrome. These molecules are present in saliva, and thus, can be used as biomarkers to give an indication of such diseases. However, the expression of TLR2 was found to be lowered in oral mucosa epithelial cells.Citation84
Papillomavirus is a great threat to the mucosal membrane because it invades the human body through the mucosa and is a potential cause of cancer at a number of sites, including the vulva, uterus, intestine, and anus. TLR4 can be used as a biomarker to study papillomavirus infection. In a bovine study, infection with the E7 and E2 strains of bovine papillomavirus-1 was demonstrated to reduce expression of TLR4 in fibroblasts significantly.Citation85 The human fallopian tube is also lined by mucosa and exhibits mucosal tolerance against several viral infections. Epithelial cells of the fallopian tube express TLR1-9. These cells, when treated with a TLR3 agonist, showed upregulation of IL-8, TNF-α, human β-defensin 2, and IFN-β, and also induced TLR2, TLR3, and TLR7.Citation86 Viral infection caused by rotavirus, calicivirus, and adenovirus of the gut is usually sensed by TLR3 and TLR8. Surface TLR3 has been shown to interact with rotaviral double-stranded RNA. Single-stranded RNA viruses are usually detected by TLR7 and TLR8.Citation87,Citation88
TLR2 expressed in trophoblast cells of the human placenta has been linked to disorders such aschorioamnionitis. Localized expression of TLR2 in cytotrophoblast and syncytiotrophoblast mucosal cells and decidual stromal cells was shown to be decreased in chorioamnionitis.Citation89 Recent research has also shown a link between TLR5 expressed in gut mucosa and obesity-related metabolic disorders. TLR5-deficient mice exhibited hyperphagia, hyperlipidemia, insulin resistance, and hyperadiposity.Citation90 In one study, Staphylococcus aureus-mediated allergic conjunctivitis of the eye was linked to TLR2, with high production of IL-4, IL-5, IL-13, and eotaxin, mediated by a TH2 response, while TLR2-mutant mice failed to exhibit any of these responses.Citation91 In a study of TLR4 in the vaginal mucosa of pregnant women, it was found that a single nucleotide polymorphism in TLR4 (896 A > G) resulted in a TLR4 variant causing high vaginal pH, leading to infection by Gardnerella vaginalis and some anaerobic Gram-negative rods, while women without this polymorphism were immune against such infection.Citation92 A study of vaginal Trichomonas vaginalis infection indicated the activation of TLR4 via secretion of proinflammatory cytokines, in particular TNF-α. This resulted in a heavy influx of leukocytes to the infection sites.Citation93
A general conclusion that can be drawn from the aforegoing observations is that all the different pattern recognition receptors and molecules of innate mucosal immunity are the first to encounter and recognize a pathogen or antigen, and they further induce proinflammatory cytokine production, leading to adaptive immunity.
NOD-like receptors constitute a recently identified family of intracellular pattern recognition receptors, which contains more than 20 members in mammals. NOD-like receptors are characterized by tripartite-domain organization, with a conserved nucleotide binding oligomerization domain and leucine-rich repeats. NOD1 and NOD2 detect specific motifs within peptidoglycans. NOD1 senses D-γ-glutamyl-meso-DAP dipeptide which is found in the peptoglycans of all Gram-negative and some Gram-positive bacteria, whereas NOD2 recognizes the muramyl dipeptide structure found in almost all bacteria.Citation94,Citation95 NOD1 and NOD2 signal via receptor-interacting serine/threonine-protein kinase 2 (RIPK2). RIPK2 mediates ubiquitination of the inhibitor of kappa B kinase gamma, leading to activation of the receptor activator of NFκB and the production of inflammatory cytokines, including TNF-α and IL-6. In addition to the NFκB pathway, stimulation of NOD1 and NOD2 induces the activation of mitogen-activated protein kinases. Studies have shown the involvement of caspase-recruiting domain-containing protein (CARD)9 in the selective control of NOD2-dependent p38 and c-Jun N-terminal kinase signaling.Citation96–Citation98 Genetic variations in NOD2 are associated with Crohn’s disease.Citation99 Several NOD1 polymorphisms are linked with the development of atopic eczema and asthma.Citation100 NOD2 is required for the expression of an antimicrobial peptide, cryptidin, found in the gastrointestinal mucosa.Citation98 NOD1-deficient mice are highly susceptible to infection with H. pylori, whereas NOD2-deficient mice are more susceptible to oral infection with Listeria monocytogenes.Citation101
NOD2 deficiency results in abnormal development and function of Peyer’s patches in mice. This phenotype is Peyer’s patch-specific and not apparent after birth but progresses with time, having been observed for up to 52 weeks. Peyer’s patches in NOD2-deficient mice are larger than in controls, with increased numbers of both M cells and CD4+ T cells. Knockout mice exhibit increased translocation of yeast and bacteria across Peyer’s patches and higher concentrations of TNF-α, IFN-γ, IL-12, and IL-4.Citation102
Pyrin domain-containing NOD-like receptors play a key role in the regulation of caspase-1 by forming a multiprotein complex known as the “inflammasome”. Caspase-1 participates in the processing and subsequent release of proinflammatory cytokines, including IL-1β and IL-18. At least two types of NALP inflammasomes have been identified, ie, the NALP1 inflammasome comprising NALP1, nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing protein 1, CARD7, ASC (apoptosis-associated speck-like protein containing a CARD), caspase-1 and caspase-5, and the NALP3 inflammasome containing NALP3 (NLRP3, cryopyrin, cold-induced auto-inflammatory syndrome 1), ASC, cardinal, and caspase-1. Activation of caspase-1 induced by NALP3 appears to be toll-like receptor-independent, whereas secretion of mature IL-1β seems to require two stimuli involving the toll-like receptor and NALP3. The first stimulus, a toll-like receptor ligand, such as lipopolysaccharide, triggers the generation of pro-IL-1β, while the second, a stimulus such as adenosine triphosphate, induces oligomerization and inflammasome assembly. A murine variant of NALP1 (NALP1b) was shown to respond to the anthrax toxin, suggesting an engagement of the NALP1 inflammasome in the immune response to Bacillus anthracis infection. NALP3 mediates caspase-1 activation in response to a wide variety of bacteria, including L. monocytogenes and S. aureus.Citation103–Citation105
RIG-I-like receptors (also known as Ddx58) contain RIG-I-like helicases, which are a family of cytoplasmic RNA helicases that are prominent in antiviral responses. RIG-I and melanoma differentiation-associated gene 5 (MDA-5) sense double-stranded RNA, a replication intermediate for RNA viruses, leading to the production of Type IIFNs in infected cells.Citation106,Citation107 Upon recognition of double-stranded RNA, they are recruited by the adaptor IPS-1 to the outer membrane of the mitochondria, leading to the activation of several transcription factors, including IFN regulatory factors 3 and 7, as well as NFκB. IFN regulatory factors 3 and 7 control the expression of Type I IFNs, while NFκB regulates the production of inflammatory cytokines. Interferon regulatory factor 3 and 7 activation involves TRAF3, NAK-associated protein 1, TANK (TRAF family member-associated NFκB activator), and the protein kinase, TANK-binding kinase 1 (TBK1) or IκB kinase epsilon.Citation108–Citation110 Experiments in RIG-I- and MDA-5-deficient mice have demonstrated that conventional dendritic cells, macrophages, and fibroblasts isolated from these mice have impaired IFN induction after RNA virus infection.Citation111 RIG-I disruption in mice resulted in reduced body weight caused by severe damage and inflammatory infiltration into the colonic mucosa. The number of Peyer’s patches was significantly reduced in RIG-I-deficient mice, and RIG-I was found to control the transcriptional activity of G αi2, a negative regulator of T cell responses, which may mediate the modulatory effects of RIG-I.Citation112,Citation113
The β-defensins are a family of antimicrobial peptides that are diversely expressed on mucosal surfaces, and in airways and submucosal gland epithelia. These small cationic peptides are products of individual genes and demonstrate broad-spectrum activity against bacteria, fungi, and some enveloped viruses. Their expression in airway epithelia is mostly induced by bacterial peptides or proinflammatory cytokines. B-defensins also act as chemokines for adaptive immune cells, including immature dendritic cells and T cells via the CCR6 receptor, and provide a link between innate and adaptive immunity.Citation114 Lipocalin is a more recently identified mucosal molecule that responds to bacterial enterobactin and inhibits the colonization of Klebsiella pneumoniae followed by induction of IL-8 from cultured respiratory cells.Citation115 This is followed by a strong influx of neutrophils to the site of infection. Specialized molecules known as cathelicidins, a type of defensin, also harbor the apical granules of epithelial cells and have shown antimicrobial activity in preventing the entry of larger pathogens. They also act as a bridge between innate and adaptive immunity by chemotactically attracting dendritic cells and T cells in the presence of severe immune aggravation.Citation116
A case study of avian infectious bronchitis revealed potent TH1 adaptive immunity accompanied by IL-β activation after primary immunization, with strong activation of T cells and IgA upregulation, and a local memory response governed by IgG at the bronchial mucosal surface after second immunization.Citation117 TGF-β in the gut is known to suppress mucosal inflammation and to heal damaged mucosa by upregulating the deposition of extracellular matrix in the mucosal mesenchymal cells. It was found that in smoking-related chronic obstructive pulmonary disease, the population of mucosal mast cells showed a large increase, with altered expression of TGF-β, CD88, and renin. Conversely, in acute necrotizing pancreatitis, the total mast cell population in the gut was found to decrease. Antigen-processing in the gut is a well orchestrated process whereby M cells lacking the MHC Class II pathway take up foreign antigens through their irregular microvilli, transport them to follicular areas where they are processed by dendritic cells, and then IgA-secreting plasma cells are directed to produce IgA. Dimeric secretory immunoglobulin IgA prevents bacteria from adhering to the mucosa and their further penetration into local cells. IgA has resistance to proteolysis and helps transport bactericidal chemicals like lactoferrin and lactoperoxidase to the bacterial surface.Citation118 A study of trachoma infection of the eye mucosa revealed a high concentration of proinflammatory cytokines, such as TNF-α, which were instrumental in attracting and activating neutrophils, and low levels of TH1 and TH2 cytokines were observed in chronic trachoma. Conversely, prolonged production of TNF-α and IL-β together was associated with a reduction in the IL-1Ra inhibitory mechanism. Furthermore, there was the IL-2 class of cytokines were highly involved, including IL2, IL2-R, and IL-15.Citation119
Conclusion
Innate mucosal immunity, although not well-studied to date, plays a significant role in pathogen trafficking in the body. It recognizes pathogens using its various molecules, and triggers cascades of signals to eliminate the foreign body, induce tissue repair, and further trigger the adaptive immune response. Innate mucosal immunity has its own special memory stores in the form of pattern recognition receptors which are heritable and highly specialized. Each pattern recognition receptor has the capability to distinguish a PAMP and, unlike the adaptive response, is genetic and evolutionarily conserved, thus making mucosal immunity the primary line of defense in almost all mammalian body systems. Similarly, mucosal vaccines trigger an innate immune response as a starting signal, which leads to adaptive memory, giving rise to specific immunity against diseases.
After skin, mucosal surfaces are the largest area of host-pathogen interaction in mammalian systems. The prospect of using the mucosal route for vaccination is increased by the fact that almost all infection either begins at or encounters this route. Most gastrointestinal, respiratory, urinary, and sexually transmitted diseases use the mucosal route for primary and subsequent infection. Vaccines are being developed to prevent the pathogen from attaching or colonizing the mucosal surface or their subsequent penetration and replication. The primary role of vaccines is to provide strong stimulation of IgA that further controls the adaptive immune response. Due to bystander suppression, mucosal tolerance can be activated using T-regs to produce suppressive cytokines.Citation120 This is primarily seen in human systems to prevent self-antigenic responses. It has been observed that nasal vaccines stimulate T-regs to produce IL-10, while oral vaccines stimulate T-regs to produce TGF-β. Increased doses had been shown to deplete the population of killer T cells and even higher doses cause their apoptosis.Citation121
Innate mucosal immunity and mucosal vaccine development based on pattern recognition receptors has been a neglected area of research for years. However, recent research has given an idea of the involvement of innate mucosal immunity in different defense mechanisms, and has paved the way for ongoing research in this area. Mucosal pattern recognition receptor pathways, pattern recognition receptor structure-activity relationships, development of new mucosal adjuvant and pattern recognition receptor mimetics, are currently hot topics in mucosal immunity research. In this review, we have attempted to establish the importance of innate immunity which may lead to the development of future therapeutics for better disease management and control.
Acknowledgements
The authors would like to thank the Department of Atomic Energy, Science and Technology and the Department of Biotechnology, Government of India, for financial assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- MesteckyJMucosal Immunology3rd edSan Diego, CAAcademic Press2005
- StetsonDBConnections between antiviral defense and autoimmunityCurr Opin Immunol20092124425019497722
- BlanderJMMedzhitovRToll-dependent selection of microbial antigens for presentation by dendritic cellsNature200644080881216489357
- StetsonDBKoJSHeidmannTMedzhitovRTrex1 prevents cell-intrinsic initiation of autoimmunityCell200813458759818724932
- PhaliponACardonaAKraehenbuhlJPEdelmanLSansonettiPJCorthesyBSecretory component: A new role in secretory IgA-mediated immune exclusion in vivoImmunity20021710711512150896
- AkiraSHemmiHRecognition of pathogen-associated molecular patterns by TLR familyImmunol Lett200385859512527213
- MedzhitovRJanewayCJrInnate immune recognition: Mechanisms and pathwaysImmunol Rev2000173899710719670
- StroberWFussIJBlumbergRSThe immunology of mucosal models of inflammationAnnu Rev Immunol20022049554911861611
- BlandPWMucosal T cell-epithelial cell interactionsChem Immunol19987140639761946
- FundaDPTuckovaLFarreMAIwaseTMoroITlaskalova-HogenovaHCD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: Lipopolysaccharide activation of epithelial cells revisitedInfect Immun2001693772378111349042
- HaydayATheodoridisERamsburgEShiresJIntraepithelial lymphocytes: Exploring the Third Way in immunologyNat Immunol20012997100311685222
- FujihashiKKatoHvan GinkelFWA revisit of mucosal IgA immunity and oral toleranceActa Odontol Scand20015930130811680650
- StaggAJKammMAKnightSCIntestinal dendritic cells increase T cell expression of alpha4beta7 integrinEur J Immunol2002321445145411981833
- MoraJRBonoMRManjunathNSelective imprinting of gut-homing T cells by Peyer’s patch dendritic cellsNature2003424889312840763
- EricssonASvenssonMAryaAAgaceWWCCL25/CCR9 promotes the induction and function of CD103 on intestinal intraepithelial lymphocytesEur J Immunol2004342720272915368288
- KawamataNXuBNishijimaHExpression of endothelia and lymphocyte adhesion molecules in bronchus-associated lymphoid tissue (BALT) in adult human lungRespir Res2009109719845971
- WilsonCLOuelletteAJSatchellDPRegulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defenseScience199928611311710506557
- HeatherLCColeMALygateCAFatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heartCardiovasc Res20067243043717034771
- HeritagePLBrookMAUnderdownBJMcDermottMRIntranasal immunization with polymer-grafted microparticles activates the nasal-associated lymphoid tissue and draining lymph nodesImmunology1998932492569616375
- SheilBShanahanFO’MahonyLProbiotic effects on inflammatory bowel diseaseJ Nutr20071373 Suppl 2819S824S17311981
- ZhangSZZhaoXHZhangDCCellular and molecular immunopathogenesis of ulcerative colitisCell Mol Immunol20063354016549047
- RamakrishnanKSalinasRCPeptic ulcer diseaseAm Fam Physician2007761005101217956071
- CandyDCARotavirus infection: A systemic illness?Plos Med20074614615
- HyserJMEstesMKRotavirus vaccines and pathogenesis: 2008Curr Opin Gastroenterol200925364319114772
- GrimwoodKLambertSBRotavirus vaccines: Opportunities and challengesHum Vaccin20095576918838873
- GollerJLDimitriadisATanAKellyHMarshallJALong-term features of norovirus gastroenteritis in the elderlyJ Hosp Infect20045828629115564004
- SinghJCCruickshankSMNewtonDJToll-like receptor-mediated responses of primary intestinal epithelial cells during the development of colitisAm J Physiol Gastrointest Liver Physiol2005288G514G52415499080
- PrittBSClarkCGAmebiasisMayo Clin Proc2008831154115918828976
- SallesJMMoraesLASallesMCHepatic amebiasisBraz J Infect Dis200379611012959680
- ViboudCAlonsoWJSimonsenLInfluenza in tropical regionsPLoS Med20063e8916509764
- D’HeillySJJanoffENNicholPNicholKLRapid diagnosis of influenza infection in older adults: Influence on clinical care in a routine clinical settingJ Clin Virol20084212412818289930
- JungWWChunTSulDStrategies against human papillomavirus infection and cervical cancerJ Microbiol20044225526615650698
- OsenWJochmusIMullerMGissmannLImmunization against human papillomavirus infection and associated neoplasiaJ Clin Virol200019757811091150
- AdachiOKawaiTTakedaKTargeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated functionImmunity199891431509697844
- SunLDengLEaCKXiaZPChenZJThe TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytesMol Cell20041428930115125833
- KeatingSEMaloneyGMMoranEMBowieAGIRAK-2 participates in multiple toll-like receptor signalling pathways to NFkappaB via activation of TRAF6 ubiquitinationJ Biol Chem2007282334353344317878161
- GururajanMJacobJPulendranBToll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsetsPLoS One20072e86317848994
- RayAKarmakarPBiswasTUp-regulation of CD80-CD86 and IgA on mouse peritoneal B-1 cells by porin of Shigella dysenteriae is Toll-like receptors 2 and 6 dependentMol Immunol2004411167117515482852
- CarioEGerkenGPodolskyDKToll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier functionGastroenterology20071321359137417408640
- GirardinSETournebizeRMavrisMCARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneriEMBO Rep2001273674211463746
- SnellingsNJTallBDVenkatesanMMCharacterization of Shigella type 1 fimbriae: Expression, FimA sequence, and phase variationInfect Immun199765246224679169792
- ZhangYChenHYangLToll-like receptor 4 participates in gastric mucosal protection through Cox-2 and PGE2Dig Liver Dis20104247247620018573
- MatharuKSMizoguchiECotonerCAToll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient miceGastroenterology20091371380139019596011
- ShangLFukataMThirunarayananNToll-like receptor signalling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propriaGastroenterology200813552953818522803
- SchmausserBAndrulisMEndrichSExpression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infectionClin Exp Immunol200413652152615147355
- GewirtzATYuYKrishnaUSIsraelDALyonsSLPeekRMJrHelicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunityJ Infect Dis20041891914192015122529
- TakenakaRYokotaKAyadaKHelicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cellsMicrobiology2004150Pt 123913392215583145
- FranchimontDVermeireSEl HousniHDeficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299 gly polymorphism is associated with Crohn’s disease and ulcerative colitisGut20045398799215194649
- LodesMJCongYElsonCOBacterial flagellin is a dominant antigen in Crohn diseaseJ Clin Invest20041131296130615124021
- OtteJMRosenbergIMPodolskyDKIntestinal myofibroblasts in innate immune responses of the intestineGastroenterology20031241866187812806620
- MaedaSHsuLCLiuHNod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processingScience200530773473815692052
- MaldonadoCTrejoWRamirezALipophosphopeptidoglycan of Entamoeba histolytica induces an antiinflammatory innate immune response and downregulation of toll-like receptor 2 (TLR-2) gene expression in human monocytesArch Med Res2000314 SupplS71S7311070229
- Maldonado-BernalCKirschningCJRosensteinYThe innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4Parasite Immunol20052712713715910421
- Rakoff-NahoumSHaoLMedzhitovRRole of toll-like receptors in spontaneous commensal-dependent colitisImmunity20062531932916879997
- LemboAKalisCKirschningCJDifferential contribution of Toll-like receptors 4 and 2 to the cytokine response to Salmonella enterica serovar Typhimurium and Staphylococcus aureus in miceInfect Immun2003716058606214500530
- Andersen-NissenESmithKDStrobeKLEvasion of Toll-like receptor 5 by flagellated bacteriaProc Natl Acad Sci U S A20051029247925215956202
- Vazquez-TorresAVallanceBABergmanMAToll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: Importance of the Kupffer cell networkJ Immunol20041726202620815128808
- HapfelmeierSStecherBBarthelMThe Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanismsJ Immunol20051741675168515661931
- HuangFCWerneALiQGalyovEEWalkerWACherayilBJCooperative interactions between flagellin and SopE2 in the epithelial interleukin-8 response to Salmonella enterica serovar typhimurium infectionInfect Immun2004725052506215321998
- RaffatelluMChessaDWilsonRPDusoldRRubinoSBaumlerAJThe Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosaInfect Immun2005733367337415908363
- JerkeSSrinivasanAMcSorleySJExpression of Toll/IL-1R domain-containing adaptor protein (TIRAP) is detrimental to primary clearance of Salmonella and is not required for the generation of protective immunityImmunol Lett2008116647118096248
- BackhedFNormarkSSchwedaEKOscarsonSRichter-DahlforsAStructural requirements for TLR4-mediated LPS signalling: A biological role for LPS modificationsMicrobes Infect200351057106314554246
- SteinerTSNataroJPPoteet-SmithCESmithJAGuerrantRLEnteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cellsJ Clin Invest20001051769177710862792
- HajishengallisGTappingRIMartinMHToll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxinsInfect Immun2005731343134915731031
- AsaiYOhyamaYGenKOgawaTBacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2Infect Immun2001697387739511705912
- HallJABouladouxNSunCMCommensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responsesImmunity20082963764918835196
- IkedaTHiromatsuKHotokezakaMChijiiwaKUp-regulation of intestinal toll-like receptors and cytokine expressions change after TPN administration and a lack of enteric feedingJ Surg Res201016024425219524259
- TirumurugaanKGDhanasekaranSRajGDRajaAKumananKRamaswamyVDifferential expression of toll-like receptor mRNA in selected tissues of goat (Capra hircus)Vet Immunol Immunopathol201013329630119748133
- HammerbeckDMBurlesonGRSchullerCJAdministration of a dual toll-like receptor 7 and toll-like receptor 8 agonist protects against influenza in ratsAntiviral Res20077311116959331
- HiranoTKodamaSMoriyamaMKawanoTSuzukiMThe role of Toll-like receptor 4 in eliciting acquired immune responses against nontypeable Haemophilus influenzae following intranasal immunization with outer membrane proteinInt J Pediatr Otorhinolaryngol2009731657166519765832
- YaminMHolbrookEHGraySTCigarette smoke combined with Toll-like receptor 3 signalling triggers exaggerated epithelial regulated upon activation, normal T-cell expressed and secreted/CCL5 expression in chronic rhinosinusitisJ Allergy Clin Immunol20081221145115318986692
- TulicMKFisetPOManoukianJJRole of toll-like receptor 4 in protection by bacterial lipopolysaccharide in the nasal mucosa of atopic children but not adultsLancet20043631689169715158630
- SukkarMBXieSKhorasaniNMToll-like receptor 2, 3, and 4 expression and function in human airway smooth muscleJ Allergy Clin Immunol200611864164816950283
- WangJMatsukuraSWatanabeSAdachiMSuzakiHInvolvement of Toll-like receptors in the immune response of nasal polyp epithelial cellsClin Immunol200712434535217602875
- ZhangZBashiruddinJBDoelCHorsingtonJDurandSAlexandersenSCytokine and Toll-like receptor mRNAs in the nasal-associated lymphoid tissues of cattle during foot-and-mouth disease virus infectionJ Comp Pathol2006134566216423571
- MuirASoongGSokolSToll-like receptors in normal and cystic fibrosis airway epithelial cellsAm J Respir Cell Mol Biol20043077778314656745
- PabstRDurakDRoosALuhrmannATschernigTTLR2/6 stimulation of the rat lung: Effects on lymphocyte subsets, natural killer cells and dendritic cells in different parts of the air-conducting compartments and at different agesImmunology200912613213918565128
- LaneAPTruong-TranQASchleimerRPAltered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polypsAm J Rhinol20062013814416686375
- AsplinIRCarlDJWaySSJonesALRole of Toll-like receptor 2 in innate resistance to Group B StreptococcusMicrob Pathog200844435117851030
- SongJJChoJGWooJSLeeHMHwangSJChaeSWDifferential expression of toll-like receptors 2 and 4 in rat middle earInt J Pediatr Otorhinolaryngol20097382182419303147
- AllamJPPengWMAppelTToll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cellsJ Allergy Clin Immunol200812136837418036651
- FerwerdaGNeteaMGJoostenLAvan der MeerJWRomaniLKullbergBJThe role of Toll-like receptors and C-type lectins for vaccination against Candida albicansVaccine20102861462219887129
- JohnPSAndreaECeciliaE586 toll-like receptor 3 (TLR3) signaling improves intestinal mucosal defense against pathogensGastroenterology2009136A93A94
- SrinivasanMKodumudiKNZuntSLSoluble CD14 and toll-like receptor-2 are potential salivary biomarkers for oral lichen planus and burning mouth syndromeClin Immunol2008126313717916440
- YuanZQBennettLCampoMSNasirLBovine papillomavirus type 1 E2 and E7 proteins down-regulate toll like receptor 4 (TLR4) expression in equine fibroblastsVirus Res201014912412720109504
- GhoshMSchaeferTMFaheyJVWrightJAWiraCRAntiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C)Fertil Steril2008895 Suppl1497150617669408
- EdelmannKHRichardson-BurnsSAlexopoulouLTylerKLFlavellRAOldstoneMBDoes Toll-like receptor 3 play a biological role in virus infections?Virology200432223123815110521
- GlassRINoelJAndoTThe epidemiology of enteric caliciviruses from humans: A reassessment using new diagnosticsJ Infect Dis2000181Suppl 2S254S26110804134
- RindsjoEHolmlundUSverremark-EkstromEPapadogiannakisNScheyniusAToll-like receptor-2 expression in normal and pathologic human placentaHum Pathol20073846847317239937
- Vijay-KumarMAitkenJDCarvalhoFAMetabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5Science201032822823120203013
- ChungSHNamKHKweonMNStaphylococcus aureus accelerates an experimental allergic conjunctivitis by Toll-like receptor 2-dependent mannerClin Immunol200913117017719358332
- GencMRVardhanaSDelaneyMLRelationship between a toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant womenEur J Obstet Gynecol Reprod Biol200411615215615358455
- ZariffardMRHarwaniSNovakRMGrahamPJJiXSpearGTTrichomonas vaginalis infection activates cells through toll-like receptor 4Clin Immunol200411110310715093558
- ChamaillardMHashimotoMHorieYAn essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acidNat Immunol2003470270712796777
- GirardinSEBonecaIGCarneiroLANod1 detects a unique muropeptide from gram-negative bacterial peptidoglycanScience20033001584158712791997
- KobayashiKInoharaNHernandezLDRICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systemsNature200241619419911894098
- InoharaNKosekiTLinJAn induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signalling pathwaysJ Biol Chem2000275278232783110880512
- KobayashiKSChamaillardMOguraYNod2-dependent regulation of innate and adaptive immunity in the intestinal tractScience200530773173415692051
- OguraYBonenDKInoharaNA frameshift mutation in NOD2 associated with susceptibility to Crohn’s diseaseNature200141160360611385577
- HysiPKabeschMMoffattMFNOD1 variation, immunoglobulin E and asthmaHum Mol Genet20051493594115718249
- EckmannLSensor molecules in intestinal innate immunity against bacterial infectionsCurr Opin Gastroenterol2006229510116462163
- TohnoMUedaWAzumaYMolecular cloning and functional characterization of porcine nucleotide-binding oligomerization domain-2 (NOD2)Mol Immunol20084519420317559936
- LucianoFKrajewskaMOrtiz-RubioPNur77 converts phenotype of Bcl-B, an antiapoptotic protein expressed in plasma cells and myelomaBlood20071093849385517227826
- BoydenEDDietrichWFNalp1b controls mouse macrophage susceptibility to anthrax lethal toxinNat Genet20063824024416429160
- MartinonFPetrilliVMayorATardivelATschoppJGout-associated uric acid crystals activate the NALP3 inflammasomeNature200644023724116407889
- YoneyamaMFujitaTFunction of RIG-I-like receptors in antiviral innate immunityJ Biol Chem2007282153151531817395582
- KatoHSatoSYoneyamaMCell type-specific involvement of RIG-I in antiviral responseImmunity200523192816039576
- KawaiTTakahashiKSatoSIPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon inductionNat Immunol2005698198816127453
- SahaSKPietrasEMHeJQRegulation of antiviral responses by a direct and specific interaction between TRAF3 and CardifEMBO J2006253257326316858409
- SasaiMShingaiMFunamiKNAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN inductionJ Immunol20061778676868317142768
- YoneyamaMKikuchiMMatsumotoKShared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunityJ Immunol20051752851285816116171
- WangXLiMZhengHInfluenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferonJ Virol200074115661157311090154
- WangYZhangHXSunYPRig-I-/- mice develop colitis associated with downregulation of G alpha i2Cell Res20071785886817893708
- RohrlJYangDOppenheimJJHehlgansTSpecific binding and chemotactic activity of mBD4 and its functional orthologue hBD2 to CCR6-expressing cellsJ Biol Chem20102857028703420068036
- BachmanMAMillerVLWeiserJNMucosal lipocalin 2 has proinflammatory and iron-sequestering effects in response to bacterial enterobactinPLoS Pathog20095e100062219834550
- SchauberJGalloRLAntimicrobial peptides and the skin immune defense systemJ Allergy Clin Immunol200812226126618439663
- GuoXRosaAJChenDGWangXMolecular mechanisms of primary and secondary mucosal immunity using avian infectious bronchitis virus as a model systemVet Immunol Immunopathol200812133234317983666
- BevinsCLMartin-PorterEGanzTDefensins and innate host defence of the gastrointestinal tractGut19994591191510562592
- SkworTAAtikBKandelRPAdhikariHKSharmaBDeanDRole of secreted conjunctival mucosal cytokine and chemokine proteins in different stages of trachomatous diseasePLoS Negl Trop Dis20082e26418628987
- ZhaoriGSunMOgraPLCharacteristics of the immune response to poliovirus virion polypeptides after immunization with live or inactivated polio vaccinesJ Infect Dis19881581601652839578
- WuHYWeinerHLOral toleranceImmunol Res20032826528414713719