Abstract
Background:
Sulfur mustard can cause several long-term complications in the organs of individuals exposed to this toxic gas, and among these, pulmonary sequelae are the most important. More than 25 years after the Iran–Iraq war, thousands of Iranians are suffering from the chronic respiratory complications of sulfur mustard. Currently, based on several clinical findings, bronchiolitis obliterans is confirmed as the major diagnosis in these patients. Numerous studies have revealed that this disorder is strongly associated with oxidative stress due to excessive production of harmful reactive substances and decreased levels of endogenous antioxidants. Metallothioneins (MTs) are a group of low molecular weight sulfhydryl-rich intra-cellular proteins, and several isoforms have been identified in humans. MT-1A is an inducible and important MT isoform, which is transcriptionally activated by a variety of stress stimuli, such as free radicals.
Methods:
MT-1 mRNA expression and protein levels in endobronchial biopsy samples from 24 sulfur mustard-exposed patients and 15 unexposed control cases were evaluated by semi-quantitative reverse transcriptase polymerase chain reaction, real-time reverse transcriptase polymerase chain reaction, and immunohistochemistry.
Results:
mRNA- MT-1A expression levels in sulfur mustard-exposed patients were upregulated compared with normal samples. Protein expression was also markedly higher in controls than in sulfur mustard-exposed patients.
Conclusion:
Upregulation of MT-1A mRNA in patients who have been exposed to sulfur mustard seems to be due to oxidative stress, which is induced in an attempt to ameliorate this harmful situation by reestablishment of homeostasis, but depletion of its protein might be due to secondary consequences of sulfur mustard toxicity, which are as yet not understood.
Introduction
Respiratory system sequelae are the most important long-term complications in patients exposed to sulfur mustard. Thousands of Iranian civilians and veterans are still suffering from delayed respiratory difficulties caused by inhalation of this toxic gas during the Iraq conflict with Iran more than 25 years ago.Citation1,Citation2
Cough, dyspnea, excessive sputum production, and hemoptysis are among the most prominent chronic pulmonary symptoms in sulfur mustard-injured patients.Citation3,Citation4 Nowadays, bronchiolitis obliterans is part of the diagnosis in these patients.Citation5 However, a number of differences have been found between this kind of bronchiolitis obliterans and other kinds, such as that following lung transplantation, and there is no progression pattern, rare fibrosis, differing severity, and less obliteration.Citation6 Investigations have shown that the pulmonary disorder in patients with chronic sulfur mustard exposure is strongly associated with oxidative stress, caused by an imbalance between excessive creation of reactive oxygen and/or nitrogen species and depletion of endogenous antioxidants,Citation7 along with the release of several kinds of inflammatory mediators from a number of cell types.Citation8,Citation9 A recent study has shown that serum glutathione levels are significantly lower in patients exposed to sulfur mustard than in controls, but the level of malondialdehyde is significantly higher in sulfur mustard victims.Citation10 More recently, in a study of airway biopsy samples, we demonstrated that expression of lipocalin-2 at the mRNA level was significantly higher in sulfur mustard-injured patients in comparison with controls, but there was no significant difference in the expression of lipocalin-2 protein between patients and controls.Citation11 These data confirm that oxidative stress due to excessive production of harmful reactive substances and decreased levels of endogenous protective antioxidants is a phenomenon in these patients that plays a pivotal role in the pathology of late pulmonary complications of sulfur mustard exposure. However, the exact mechanisms still need to be elucidated.
Metallothioneins (MTs) are a group of low molecular weight (6–7 kDa), sulfhydryl-rich intracellular proteins with 61 to 68 amino acid residues, initially recognized by Margoshes and Vallee in 1957 as cadmium-binding proteins in equine kidney tissue. In human beings, several MT isoforms, from MT-1 through MT-4, have been identified.Citation12 MT-1 is an inducible MT isoform encoded by a group of more than 10 functional genes, amongst which MT-1A is a broadly disseminated MT isoform in the human body. Its transcription is activated by a variety of stress stimuli, including metals, glucocorticoids, a number of proinflammatory cytokines, and reactive oxygen species.Citation13,Citation14 Intracellular metal homeostasis, heavy metal detoxification, and scavenging of a wide variety of compounds, including hydroxyl radicals, superoxide, hydrogen peroxide, and nitric oxide, are among the accepted roles of MT-1, although the exact molecular mechanisms involved are not fully understood.Citation15,Citation16
A number of studies have demonstrated coexpression of MT-1 and lipocalin-2 in some illnesses, including oxidative stress-mediated lung injuries.Citation17 The present study aimed to assess the expression of MT-1A at the mRNA and protein levels in bronchial biopsy samples of sulfur mustard-injured patients in comparison with controls. Our results may pave the way to new treatments for the chronic respiratory complications encountered in these patients.
Material and methods
Study design
Twenty-four sufferers of chronic respiratory sequelae from exposure to sulfur mustard during the 1980–1988 Iran–Iraq war were enrolled as the sulfur mustard-exposed group and 15 unexposed individuals as the control group. All of the subjects were male. Contact with sulfur mustard was confirmed by documents from the Iranian military health services at the time of exposure. These victims all developed pulmonary symptoms immediately after contact with sulfur mustard, without any symptom-free periods. The study was approved by the ethics committee of the Baqiyatallah University of Medical Sciences. The procedures conformed to the guiding principles of the Declaration of Helsinki, and all subjects signed informed consent forms for participation in the study. Potential subjects having other interactive criteria, as well as those involving other chronic pulmonary diseases (eg, asthma), autoimmune disease (eg, rheumatoid arthritis), lung cancer, diabetes mellitus, acute infective bronchitis, or pneumonia, were excluded. Drug addicts, elderly people (>65 years old), smokers, organ transplant recipients, and patients with a history of occupational pulmonary exposure to other toxic agents were also excluded. Age, gender, and pulmonary function test data for the two groups are shown in .
Table 1 Characteristics and pulmonary function test results of SM-injured patient and control group
All subjects were anesthetized by inhalation of 2% aerosolized lidocaine and intravenous midazolam, and slept lightly throughout the procedure. Bronchoscopy was carried out using a flexible fiberoptic bronchoscope (BF1T; Olympus, Tokyo, Japan) passed through the airway to reach the segmental and subsegmental carinae, and endobronchial biopsy specimens were taken from these regions using bronchoscopic forceps (Olympus). Supplemental oxygen was given throughout the procedure, and oxygen saturation was checked at regular intervals by a pulse oximeter until the subjects regained consciousness.
Two biopsy samples were taken from each patient, and were immediately and separately immersed in Tripure isolation reagent (Roche, Mannheim, Germany) and formalin (Merck, Darmstadt, Germany). The samples in Tripure were stored at −80°C until RNA extraction, and the formalin samples were kept at 4°C for immunohistochemistry.
Reverse transcriptase polymerase chain reaction analysis of MT-1A gene expression
We have already described the reverse transcriptase polymerase chain reaction procedure used in this study.Citation11 In brief, all the RNA contained in the airway biopsy specimens was harvested in Tripure isolation reagent in accordance with the manufacturer’s protocol and kept at −80°C during the procedure. The RNA extracted was evaluated by Nanodrop spectrophotometer (ND-1000; Wilmington, DE), and its quality was confirmed by electrophoresis in 1% agarose gel (Cinnagen, Tehran, Iran). Aliquots of 500 ng of isolated RNA were utilized as templates for cDNA synthesis by SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions.
Semiquantitative reverse transcriptase polymerase chain reaction for the MT-1A gene was carried out using equal amounts of synthesized cDNA, in a final reaction volume of 25 μL. All reagents and recombinant Taq DNA polymerase were obtained from Cinnagen, and the reactions were done in a master cycler thermal cycler. Specific primers for MT-1A and β-actin (as a housekeeping gene) were designed using primer3 software (http://frodo.wi.mit.edu/) and ordered from Bioneer (Daejeon, South Korea, see ). The polymerase chain reaction conditions comprised primary denaturation at 94°C for 5 minutes, followed by 30 polymerase chain reaction cycles comprising denaturation at 94°C for 30 seconds, annealing at 59°C (both genes at the same temperature) for 30 seconds, extension at 72°C for 60 seconds, followed by 5 minutes of terminal extension at 72°C. Finally, the polymerase chain reaction products were electrophoretically separated in 2% agarose gel and dyed with ethidium bromide (Cinnagen). Bands were visualized under ultraviolet light in gel documentation (Bio-RadLaboratories, Hercules, CA).
Table 2 Sequence and features of PCR
Quantitative real-time reverse transcriptase polymerase chain reaction was then performed in a Rotor-Gene RG 3000 (Corbett Research, Mortlake, Australia). The amplification procedure, run in triplicate for each sample, used SYBR Green Premix (Takara Holdings, Inc, Shiga, Japan) according to the manufacturer’s instructions. Quantitative polymerase chain reaction criteria comprised initial denaturation at 94°C for 1 minute, followed by 40 amplification cycles, including denaturation at 94°C for 20 seconds, annealing at 59°C for 30 seconds, and extension at 72°C for 30 seconds. β-actin gene expression was used to normalize threshold cycle values (Ct) of the target gene, MT-1A, and provided us with a control for relative quantitative evaluation of the abundance of transcripts using the 2−ΔΔCT method.
Immunohistochemistry
We have described the immunohistochemistry procedures used in this study in detail elsewhere.Citation18 Briefly, 10 airway biopsy samples from sulfur mustard-injured patients and 10 specimens from unexposed controls were examined. All samples were fixed in 4% formalin (Merck) and then immersed in phosphate-buffered saline (Takara Holdings, Inc) containing 30% sucrose (Wako, Osaka, Japan). Water-embedded sections 15 μm thick were prepared by cryostat (Histo-line, Milan, Italy), and incubated at 4°C with primary antibody at a dilution of 1:200 in phosphate-buffered saline for 12 hours. The primary antibody was a mouse monoclonal antibody raised against the human MT-1 isoform (Abcam, Cambridge, UK). Next, the sections were incubated with biotinylated antimouse secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and diluted to 1:200 for immunostaining. The sections were visualized using the mouse ABC staining system (Santa Cruz Biotechnology), and using 3,3′-diaminobenzidine as the substrate.
Statistical analysis
Data were calculated as mean ± the standard deviation of fold-changes in MT-1A gene expression in three independent experiments. SPSS software (v 15.0; SPSS, Inc, Chicago, IL) was used for the statistical analyses. Student’s t-test was used for the evaluation of differences in gene expression between the sulfur mustard-injured group and the unexposed group, and P < 0.05 was considered to be statistically significant.
Results
In total, 39 subjects participated in this study, comprising 24 sulfur mustard-injured patients and 15 normal unexposed control individuals. The average age of the sulfur mustard-injured patients and the unexposed controls was not significantly different (42.9 versus 43.6 years, respectively, P = 0.83, see ).
The results of pulmonary function testing are shown in . Although forced vital capacity in the control group was higher than in sulfur mustard-injured cases, the difference was not statistically significant (P = 0.11). On the other hand, forced expiratory volume in 1 second (FEV1) in the sulfur mustard group was significantly lower than in the controls (P = 0.007). Moreover, FEV1/forced vital capacity also differed between the two groups, being significantly higher in the controls (P = 0.001). Residual volume was significantly elevated in sulfur mustard-injured patients in comparison with controls (P = 0.43).
We initially used a semiquantitative reverse transcriptase polymerase chain reaction to elucidate whether there were any variations in MT-1A gene expression among the control samples, and our results revealed no significant differences (data not shown).
Next, we examined the expression of MT-1A in the sulfur mustard-injured patients. Because the controls had expressed identical levels of the gene, all of them were used for comparison with the results in the sulfur mustard-injured group. Our data revealed that MT-1A mRNA is upregulated in sulfur mustard-injured patients (). The expression of MT-1A was also quantitatively evaluated by real-time reverse transcriptase polymerase chain reaction. The results showed that expression of this gene at the mRNA level is 4.0 ± 2.60 times higher in sulfur mustard-injured patients in comparison with control samples (P = 0.001, see ).
Figure 1 MT-1A gene expression in SM-injured patients and in unexposed control cases. Gene expressions were measured by semiquantitative reverse transcriptase polymerase chain reaction, and were upregulated in SM-injured patients (lanes 3–12). Normal samples (lanes 1 and 2), SM-injured patients samples (lanes 3–13).
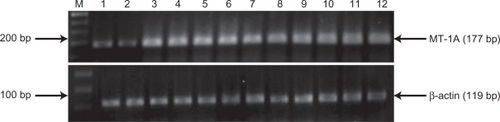
Table 3 Increases in MT1A expression in sulfur mustard-exposed patients in comparison with control group
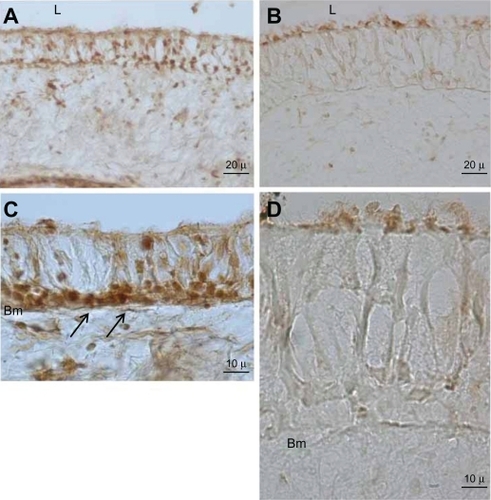
Immunohistochemistry was employed for evaluation and localization of MT-1 protein expression in airway biopsy samples from the two groups. It was immediately observed that the thickness of the bronchial epithelium layer in sulfur mustard-exposed patients was about twice that in normal control biopsies. In the control group, strong MT-1 immunoreactivity was seen in airway epithelium cells, especially in the basal (germinal) layer ( and ). In contrast, in sulfur mustard-injured specimens, very weak MT-1 protein expression was seen in the bronchial epithelial cells, especially at the luminal side of the brush border cells, indicating very low MT-1 protein expression ( and ).
Figure 2 Immunohistochemical staining for MT-1A protein in bronchial epithelium. Left side micrographs reveal the expression of MT-1A in the basal cell layer of control group airway epithelium (arrow, A and C). Right side micrographs show MT-1A expression in SM-injured airway epithelium (B and D). Note the immunoreactivity of MT-A1 in the control group is higher and wider than in the SM-injured samples. In the high and same magnification the epithelium layer thickness in SM-injured is much thicker than control group (C and D).
Discussion
Bronchiolitis obliterans is the major long-term pulmonary complication in Iranian victims of sulfur mustard exposure,Citation5 but the exact molecular mechanisms involved in the symptoms observed need to be clarified. Air trapping in expiratory high-resolution computed tomography scans (data not shown) was the most common finding in our sulfur mustard-exposed patients. Pulmonary function test results showed an airflow obstruction pattern highly indicative of bronchiolitis obliterans as the major chronic pulmonary disorder in these patients.
Moreover, our semiquantitative reverse transcriptase polymerase chain reaction results, confirmed by quantitative real-time reverse transcriptase polymerase chain reaction, indicate that expression of MT-1A at the mRNA level was significantly elevated in bronchial biopsy samples from the sulfur mustard-exposed group in comparison with the unexposed controls. As far as we are aware, very little is known about the precise molecular mechanisms involved in the structural modifications and pathophysiological symptoms of late complications of sulfur mustard exposure, and there are no published reports concerning MT-1A expression and its interaction with the delayed respiratory difficulties arising from sulfur mustard exposure.
A number of studies have shown that oxidative stress plays an important role in the pathogenesis of various lung diseases, including chronic obstructive pulmonary disease and asthma.Citation20–Citation21 Consistent with these findings, Ghanei et alCitation22 have hypothesized that the long-term pulmonary complications of sulfur mustard exposure are neutrophil-dominant and/or lymphocyte-dominant disorders. It has also been shown that oxidative stress due to excessive production of reactive substances and depletion of endogenous antioxidants plays an important role in the pathogenesis of these delayed pulmonary sequelae.Citation10,Citation23 The recent finding that sulfur mustard increases endogenous production of reactive oxygen species suggests that reactive oxygen species are likely to be involved in the toxicity induced by this chemical warfare agent.Citation7,Citation8
The antioxidant activity of MT-1 was first demonstrated by Thornalley and Vasak,Citation24 and it has since been proposed that MT-1 is involved in protection against damage caused by oxidative stress. Although the exact mechanism of the free radical scavenging action of MT-1 is not fully described, it has been postulated that MT-1 cysteine residues can react directly as thiolate groups with a number of reactive substances, as well as with hydroxyl radicals, peroxyl radicals, superoxide hydrogen peroxide, and nitric oxide.Citation25–Citation27
Pitt et alCitation28 demonstrated that overexpression of MT decreases the sensitivity of pulmonary endothelial cells to oxidative damage. They showed that pulmonary artery endothelial cells in sheep transfected by both human and mouse MT were more resistant to tert-butyl hydroperoxide, an oxidant-generating substance, and were also more resistant to hyperoxia. They suggested that MT can defend this cell type from attack by a variety of pro-oxidants. In another study, Wesselkamper et alCitation29 showed that MT-1/2 transgenic mice had more resistance to nickel-induced acute lung injury than did MT-1/2 knockout mice, implying that MT can improve survival and inhibit progression of acute lung injury. More recently, an in vivo study by Helal and HelalCitation30 demonstrated that intraperitoneal administration of MT can protect rats against carmustine-provoked lung injury because of its antioxidant attributes and its ability to suppress tumor necrosis factor alpha.
Upregulation of MT-1A mRNA in the airways of sulfur mustard-injured patients, in addition to oxidative stress, could be due to inflammation. Some in vivo experiments have demonstrated that a number of pro-inflammatory cytokines, as well as tumor necrosis factor alpha, interleukin (IL)-1, IL-6, and interferon gamma can induce MT gene expression.Citation31–Citation33 Depending on the site, stimulus direction, and type of pathophysiologic situation, MT can have diverse anti-inflammatory roles.Citation34 Emad and EmadCitation35 evaluated levels of several cytokines in bronchoalveolar lavage fluid from sulfur mustard-exposed patients and demonstrated that IL-8, IL-1β, IL-6, IL-12, and tumor necrosis factor alpha levels in sulfur mustard-injured patients were all significantly higher than in controls, and suggested that neutrophilic pulmonary inflammation is possibly the mechanism underlying the long-term pulmonary complications of sulfur mustard exposure. Molecular level investigation of the existence of inflammation in the airways of sulfur mustard-injured patients is underway in our laboratory, and preliminary, as yet unpublished data show that tumor necrosis factor alpha expression at the mRNA level is significantly upregulated in sulfur mustard-exposed patients in comparison with controls, suggesting that inflammatory processes are involved in this chronic pulmonary complication. Consistent with these findings, Takano et alCitation36 have shown that MT-1/2 knockout mice are more vulnerable to acute pulmonary inflammatory injury mediated by intratracheal instillation of lipopolysaccharide than wild-type mice. Additionally, in line with these findings, a complementary DNA microarray study based on acute inflammatory murine lung damage induced by lipopolysaccharide and diesel exhaust particles revealed that MT-1 gene expression at the mRNA level is significantly upregulated in mice exposed to these irritants.Citation17 Similarly, a more recent study showed that MT plays an important role in ozone-induced pulmonary inflammation, and showed that lung inflammation is significantly greater in MT-1/2 knockout than wild-type mice.Citation37 However, despite numerous investigations of the possible contribution of MT-1 to inflammatory lung injuries, its exact role still needs to be elucidated.
Paradoxically, our immunohistochemistry results revealed that, in contrast with the upregulation of MT-1A mRNA expression in airway biopsy samples from sulfur mustard-injured patients, its protein level was higher in control subjects. Several MT-1 immunoreactive cells were seen in the bronchial epithelia of our control cases, and immunoreactivity was more intense in cells adjacent to the basement membrane. This finding is in accordance with that of Courtade et al,Citation38 who evaluated the expression of MT in the normal human lung, and observed positively stained pleural endothelial cells and basal cells from the bronchial epithelium. In contrast, in our chemically-injured cases, only a very weak expression of MT-1 was observed on the luminal side of the bronchial epithelium. We have also already shown an inconsistency between mRNA and protein expression of lipocalin-2 and heme oxygenase in the bronchial epithelium of sulfur mustard-exposed patients compared with unexposed cases.Citation11,Citation39,Citation40 We hypothesize that this discrepancy between mRNA and protein expression of MT-1A may be caused by translational inefficiency and/or posttranslational regulation. Interestingly, it has been revealed that, despite a huge amount of evidence strongly suggesting that the expression of MT genes is transcriptionally regulated, new data have also indicated that posttranslational modulations also have effects on MT gene expression.Citation14 This discrepancy may be due to a change in the expression of microRNAs, which are posttranscriptional regulators that bind to complementary sequences on target messenger RNA transcripts, usually resulting in translational repression and gene silencing.Citation41
Intriguingly, it has been shown that administration of N-acetylcysteine can significantly improve clinical symptoms in sulfur mustard-exposed patients,Citation19 and it has also been demonstrated that N-acetylcysteine, as an antioxidant, provides thiol groups which are essential for serum glutathione production.Citation39 In this way, N-acetylcysteine can compensate for decreased expression of MT-1 protein, but, in spite of this improvement, no cure has as yet been achieved in sulfur mustard-injured patients.
Conclusion
This study shows that although the expression of MT-1 at the mRNA level was significantly increased in the bronchial epithelium of sulfur mustard-exposed patients, expression of MT-1 protein was significantly higher in the airways of our controls. This upregulation of MT-1 mRNA seems to be due to oxidative stress and depletion of the endogenous antioxidants which exist in the lungs of patients who have inhaled sulfur mustard, and may reflect an attempt to ameliorate this harmful situation by reestablishment of homeostasis. The contrasting depletion of its protein may be a result of secondary effects of sulfur mustard toxicity. Therefore, further studies clarifying the mechanisms involved in the long-term outcome of sulfur mustard exposure are warranted.
Acknowledgements
We thank the members of our laboratory at the Chemical Injury Research Center, Baqiyatallah University of Medical Sciences for their contributions to this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- GhaneiMAslaniJKhateriSHamadanizadehKPublic health status of the civil population of Sardasht 15 years following large-scale war time exposure to sulfur mustard, 2003J Burns Surg Wound Care20032718
- KhateriSGhaneiMSoroushMHainesDIncidence of lung, eye and skin lesions as late complications in 34,000 Iranians with wartime exposure to mustard agentJ Occup Environ Med20034521136114314610394
- EmadARezaianGRThe diversity of the effects of sulfur mustard gas inhalation on respiratory system 10 years after a single, heavy exposureChest19971127347309315808
- Balali-MoodMHefaziMComparison of early and late toxic effects of sulfur mustard in Iranian veteransBasic Clin Pharmacol Toxicol20069927328217040211
- GhaneiMMokhtariMMohammadMMAslaniJBronchiolitis obliterans following exposure to sulfur mustard: chest high resolution computed tomographyEur J Radiol20045216416915489074
- GhaneiMTazelaarHDChilosiMAn international collaborative pathologic study of surgical lung biopsies from mustard gas exposed patientsRespir Med200810282583018339530
- HanSEspinozaLALiaoHBoularesAHSmulsonMEProtection by antioxidants against toxicity and apoptosis induced by the sulphur mustard analog 2-chloroethylethyl sulphide (CEES) in Jurkat T cells and normal human lymphocytesBr J Pharmacol200414179580214769780
- KorkmazAYarenYTopalTOterSMolecular targets against mustard toxicity: Implication of cell surface receptors, peroxynitrite production, and PARP activationArch Toxicol20068066267016552503
- ToewsGBImpact of bacterial infections on airway diseasesEur Respir Rev2005146268
- ShohratiMGhaneiMShamspourNGlutathione and malondialdehyde levels in late pulmonary complications of sulfur mustard intoxicationLung2010188778319862574
- EbrahimiMRoudkenarMHImani FooladiAADiscrepancy between mRNA and protein expression of neutrophil gelatinase-associated lipocalin in bronchial epithelium induced by sulfur mustardJ Biomed Biotechnol2010201082313120508729
- ThirumoorthyNManisenthil KumarKTSundarSAMetallothionein: An overviewWorld J Gastroenterol20071399399617373731
- LuHHuntDMGantiRMetallothionein protects human retinal pigment epithelial cells aganist apoptosis and oxidative stressExp Eye Res200274839211878821
- HaqFMahoneyMKoropatnickJSignaling events for metallothionein inductionMutat Res200353321122614643422
- DavisSRCousinsRJMetallothionein expression in animals: A physiological perspective on functionJ Nutr20001301085108810801901
- SatoMKondohMRecent studies on metallothionein: Protection against toxicity of heavy metals and oxygen free radicalsTohoku J Exp Med200219692212498322
- YanagisawaRTakanoHInoueKComplementary DNA microarray analysis in acute lung injury induced by lipopolysaccharide and diesel exhaust particlesExp Biol Med (Maywood)20042291081108715522845
- NouraniMROwadaYKitanakaNLocalization of epidermal-type fatty acid binding protein in macrophages in advanced atretic follicles of adult miceJ Mol Histol20053639140016400526
- GhaneiMAslaniJKhateriSHamadanizadehKPublic health status of the civil population of Sardasht 15 years following large-scale war time exposure to sulfur mustardJ Burns Wounds200327
- GreeneLAsthma, oxidant stress and dietNutrition19991589990710575668
- RahmanIAdcockIMOxidative stress and redox regulation of lung inflammation in COPDEur Respir J20062821924216816350
- GhaneiMShohratiMJafariMGhaderiSAlaeddiniFAslaniJN-acetylcysteine improves the clinical conditions of mustard gas-exposed patients with normal pulmonary function testsBasic Clin Pharmacol Toxicol20081042843218801028
- NaghiiMRSulfur mustard intoxication, oxidative stress, and antioxidantsMil Med200216757357512125850
- ThornalleyPJVasakMPossible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicalsBiochim Biophys Acta198582736442981555
- KumariMVHiramatsuMEbadiMFree radical scavenging actions of metallothionein isoforms I and IIFree Radic Res199829931019790511
- MinKSNishidaKOnosakaSProtective effect of metallothionein to ras DNA damage induced by hydrogen peroxide and ferric ionnitrilotriacetic acidChem Biol Interact199912213715210682935
- ZanggerKShenGOzGOtvosJDArmitageIMOxidative dimerization in metallothionein is a result of intermolecular disulphide bonds between cysteines in the alpha-domainBiochem J200135935336011583581
- PittBRSchwarzMWooESOverexpression of metallothionein decreases sensitivity of pulmonary endothelial cells to oxidant injuryAm J Physiol1997273L856L8659357862
- WesselkamperSCMcDowellSAMedvedovicMThe role of metallothionein in the pathogenesis of acute lung injuryAm J Respir Cell Mol Biol200634738216166738
- HelalGKHelalOKMetallothionein attenuates carmustine-induced oxidative stress and protects against pulmonary fibrosis in ratsArch Toxicol200983879418528683
- DeSKMcMasterMTAndrewsGKEndotoxin induction of murine metallothionein gene expressionJ Biol Chem199026515267152742203773
- SatoMSasakiMHojoHTissue specific induction of metallothionein synthesis by tumor necrosis factor-alphaRes Commun Chem Pathol Pharmacol1992751591721570401
- WaelputWBroekaertDVandekerckhoveJBrouckaertPTavernierJLibertCA mediator role for metallothionein in tumor necrosis factor-induced lethal shockJ Exp Med20011941617162411733576
- InoueKTakanoHShimadaASatohMRole of metallothionein in inflammatory lung diseasesCurr Respir Med Rev20095611
- EmadAEmadYCD4/CD8 ratio and cytokine levels of the BAL fluid in patients with bronchiectasis caused by sulfur mustard gas inhalationJ Inflamm (Lond)20074217224076
- TakanoHInoueKYanagisawaRProtective role of metallothionein in acute lung injury induced by bacterial endotoxinThorax2004591057106215563705
- InoueKTakanoHKaewamatawongTRole of metallothionein in lung inflammation induced by ozone exposure in miceFree Radic Biol Med2008451714172218929643
- CourtadeMCarreraGPaternainJLMetallothionein expression in human lung and its varying levels after lung transplantationChest19981133713789498954
- DekhuijzenPNRAntioxidant properties of N-acetylcysteine: Their relevance in relation to chronic obstructive pulmonary diseaseEur Respir J20042362963615083766
- NouraniMRYazdaniSRoudkenarMHHO1 mRNA and protein do not change in parallel in bronchial biopsies of patients after long term exposure to sulfur mustard, gene regulation and systems biologyGene Regul Syst Bio200948390
- BartelDPMicroRNAs: Target recognition and regulatory functionsCell200913621523319167326