Abstract
Purpose
Pharyngitis is commonly caused by a self-limiting upper respiratory tract infection (URTI) and symptoms typically include sore throat. Antibiotics are often inappropriately used for the treatment of pharyngitis, which can contribute to antimicrobial resistance, therefore non-antibiotic treatments which have broad antiseptic effects may be more appropriate. Amylmetacresol (AMC) and 2,4-dichlorobenzyl alcohol (DCBA) are present in some antiseptic lozenges and have established benefits in providing symptomatic relief and some in vitro antiviral action.
Methods
Seven bacterial species associated with pharyngitis, namely Streptococcus pyogenes, Fusobacterium necrophorum, Streptococcus dysgalactiae subspecies equisimilis, Moraxella catarrhalis, Haemophilus influenza, Arcanobacterium haemolyticum and Staphylococcus aureus, were exposed to an AMC/DCBA lozenge dissolved in artificial saliva. In vitro bactericidal activity was measured as a log reduction in colony-forming units (CFUs).
Results
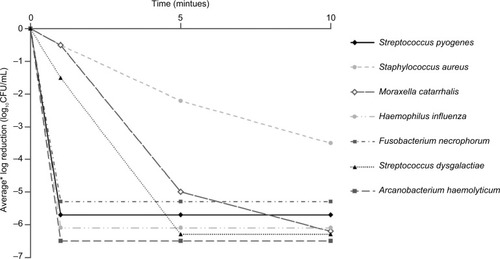
Bactericidal activity was recorded against all organisms after 1 minute. Greater than 3 log10 reductions in CFUs were observed at 1 minute for S. pyogenes (log10 reduction CFU/mL ± SD, 5.7±0.1), H. influenza (6.1±0.1), A. haemolyticum (6.5±0.0) and F. necrophorum (6.5±0.0), at 5 minutes for S. dysgalactiae (6.3±0.0) and M. catarrhalis (5.0±0.9) and at 10 minutes for S. aureus (3.5±0.1).
Conclusion
An AMC/DCBA lozenge demonstrated a greater than 99.9% reduction in CFUs against all tested species within 10 minutes, which is consistent with the time a lozenge remains in the mouth. Patients with uncomplicated bacterial pharyngitis may benefit from the antibacterial action of antiseptic AMC/DCBA lozenges. Furthermore, AMC/DCBA lozenges may be more relevant and appropriate than antibiotics for pharyngitis associated with a self-limiting viral URTI.
Plain language summary
Pharyngitis is a common condition. It can last several days and is usually the result of self-limiting viral infections, such as the common cold, although occasionally, pharyngitis can be caused by a bacterial infection. The most commonly reported symptom is sore throat. Antibiotics do not work against the viruses that in most cases cause pharyngitis but are often prescribed anyway. This contributes to antimicrobial resistance, where bacteria become immune to antibiotics and treatment for infections becomes difficult. Alternative treatments could help reduce inappropriate prescriptions of antibiotics for pharyngitis, and previous studies have demonstrated the antiviral and pain-relieving qualities of some antiseptic lozenges. The authors conducted a laboratory- based study to assess the ability of antiseptic lozenges to kill a broad range of bacteria known to cause pharyngitis. They found that, when lozenges containing two antiseptic ingredients were dissolved in a solution similar to human saliva, the mixture killed 99.9% of all pharyngitis-associated bacteria that were tested within 10 minutes. These results suggest that patients with uncomplicated bacterial pharyngitis may benefit from the antibacterial and pain-relieving action of antiseptic lozenges, including those taking antibiotics. Additionally, antiseptic lozenges may be more relevant and appropriate than antibiotics for pharyngitis of a viral origin.
Table 1 Control and test counts of challenge organisms
Introduction
Pharyngitis is associated with inflammation of the pharynxCitation1 and is one of the most common reasons patients seek health care professional advice.Citation2 Acute pharyngitis is predominantly caused by a viral upper respiratory tract infection such as the common coldCitation3,Citation4 and is usually self-limiting with symptoms, such as sore throat, lasting ~3–7 days.Citation5 Despite this, antibiotics are still frequently inappropriately used for the treatment of pharyngitis even though patients consulting their doctor are often primarily seeking reassurance and symptomatic relief.Citation6,Citation7 Antibiotics are ineffective against the viruses that causê90% of cases, do not offer symptomatic relief and inappropriate antibiotic prescription can contribute to antimicrobial resistance, which is a serious threat to global public health.Citation8 Consequently, there is a need for non-antibiotic treatments,Citation9,Citation10 which have broad anti-infective effects while meeting patient needs for relief of symptoms.
Antiseptics are a class of antimicrobial agent which kill via a physical action on the bacteria.Citation11 In addition to bactericidal activity, some antiseptics – such as amylmetacresol (AMC) and 2,4-dichlorobenzyl alcohol (DCBA) – have been shown to have antiviral effects in vitroCitation12,Citation13 and anesthetic-like effectsCitation14 with established benefits in providing symptomatic relief of pain.Citation15,Citation16
Bacterial infections contribute to 5%–15% of pharyngitis cases in adults.Citation4,Citation17,Citation18 The most common bacterial cause of acute pharyngitis, and the reason for legitimate antibiotic prescribing to prevent complications, is group A β-hemolytic Streptococcus (GABHS or Streptococcus pyogenes).Citation3,Citation19 It is responsible for ~30% of cases in childrenCitation20 and is less frequent in adults at ~10% of cases,Citation17 but rarely results in complications.Citation3 A number of other bacteria have also been implicated in infections of the throat, which may present with a more complicated pathology or represent either opportunistic infection or an underlying medical condition.
Less common species recovered from patients presenting with symptoms of pharyngitis or with a clinical diagnosis of pharyngitis include Fusobacterium necrophorum,Citation21 described in a recent study as a true pathogen rather than a colonizer of the oropharynx,Citation22 and the Streptococcus dysgalactiae subspecies equisimilis, which can cause severe or recurrent pharyngitis,Citation3,Citation17,Citation23 although there is insufficient evidence of a role for S. dysgalactiae in other adverse outcomes.Citation3 Moraxella catarrhalis has been frequently isolated from patients with pharyngitis in combination with S. pyogenes,Citation24 which may be significant considering that separate studies have demonstrated that M. catarrhalis potentiates the adhesion of S. pyogenes to the nasopharyngeal epithelium.Citation25,Citation26 Other bacteria cultured from patients with pharyngitis include Haemophilus influenza,Citation27 Arcanobacterium haemolyticumCitation28 and the opportunistic pathogen, Staphylococcus aureus, although the clinical significance of S. aureus association is not known.Citation19
In patients diagnosed with tonsillitis, F. necrophorum, appears to be a clinically important species, with a prevalence significantly higher in subjects with clinical tonsillitis compared to subjects without tonsillitis.Citation29 S. aureus has also been identified as a common cause of tonsillitisCitation30,Citation31 and was the most common pathogen isolated from patients undergoing tonsillectomy due to recurrent tonsillitis.Citation30 H. influenza has similarly been recovered from patients with tonsillitis, although the clinical significance is currently unknown.Citation27
Non-antibiotic antimicrobial treatments could potentially benefit patients with bacterial pharyngitis by offering not only antimicrobial activity but also symptomatic relief. The in vitro activity of 10 lozenge formulations has previously been investigated against S. pyogenes and S. aureus.Citation32 In this study, the in vitro bactericidal activity of AMC/DCBA lozenges against a broader range of potentially pathogenic oropharyngeal bacteria was assessed to evaluate the potential in vivo action of these lozenges against organisms associated with pharyngitis.
Methods and materials
Test samples
For the bactericidal assay, AMC 0.6 mg, DCBA 1.2 mg lozenges (Strepsils Honey and Lemon, Reckitt Benckiser Healthcare Ltd, Slough, UK) were dissolved into 5 mL of artificial saliva medium (0.1% meat extract [VWR International, Lutterworth, UK], 0.2% yeast extract [VWR International], 0.5% protease peptone [Oxoid, Basingstoke, UK], 0.02% potassium chloride [Fisher Scientific, Loughborough, UK], 0.02% sodium chloride [Fisher Scientific], 0.03% calcium carbonate [Fisher Scientific], 0.2% glucose [VWR International], 0.2% mucin from porcine stomach Type II [Sigma Aldrich, Gillingham, Dorset, UK], pH 6.7±0.3) at 44°C±1°C.
Test organisms and incubations
S. aureus (NCTC7445, Public Health England, Salisbury, UK) were cultured on tryptone soya agar (SGL, Corby, UK) at 32°C±2°C; S. pyogenes (NCTC12696, Public Health England) were cultured on Columbia blood agar with 5% defibrinated sheep blood (SGL) at 36°C±2°C; M. catarrhalis (NCTC3622, Public Health England) were cultured on Columbia blood agar (SGL) at 32°C±2°C; H. influenza (NCTC4842, Public Health England) were cultured on chocolate blood agar (SGL) at 32°C±2°C; F. necrophorum (NCTC12238, Public Health England) were cultured on anaerobic blood agar (FAA) with 5% horse blood (SGL) at 37°C±2°C anaerobically; A. haemolyticum (NCIMB702294, NCIMB, Aberdeen, UK) were cultured on Columbia blood agar with 5% defibrinated sheep blood at 36°C±2°C; S. dysgalactiae (ATCC12388, LGC, Teddington, UK) were cultured on Columbia blood agar with 5% defibrinated sheep blood at 36°C±2°C.
Bactericidal assay
The bactericidal assay was performed following a protocol similar to the Clinical and Laboratory Standards Institute approved guideline.Citation33 Specifically, inoculum cultures were prepared for each challenge organism to give an approximate population of 108 colony-forming unit (CFU)/mL in saline (0.9% sodium chloride [Fisher Scientific]). One inoculum suspension was prepared for each replicate tested. Test sample (4.9 mL) was prepared as above and inoculated with 0.1 mL of the inoculum suspension. The solution was vortexed thoroughly to mix and then tested after 1-, 5- and 10-minute contact times, consistent with the time a lozenge takes to dissolve in the mouth,Citation16 by removing 1 mL of sample/inocula mixture and transferring into 9 mL of neutralizing diluent (0.1% peptone water [VWR International], 0.9% sodium chloride [Fisher Scientific], 0.3% lecithin [MP Biomedicals, Illkirch-Graffenstaden, France], 1% polysorbate 80 [Univar, Bradford, UK], pH 6.6±0.2). Neutralization validation was carried out against all test organisms. Solutions were serially diluted to 10−5, plated onto the appropriate agar medium and incubated for a minimum of 3 days. A positive control sample of 4.9 mL artificial saliva medium and 0.1 mL of the test inoculum for each organism was also prepared without exposure to test samples and assayed at a 30-minute time point. Test control counts were performed to confirm the total population of the culture suspensions used for each test replicate. The test controls were used to calculate the log reduction on exposure to test samples. Mean log reduction in CFUs per milliliter was calculated from three test replicates.
Results
In vitro bactericidal activity of AMC/DCBA lozenges
For all test organisms, evidence of bactericidal activity was recorded at the 1-minute time point (, ), and test control counts demonstrated that the test method and media did not affect the survival of the organisms. For S. pyogenes, H. influenza, A. haemolyticum and F. necrophorum, the decrease in CFU/mL at 1 minute exceeded 3 log10 (99.9% decrease), whereas greater than 3 log10 reductions were recorded at 5 minutes for S. dysgalactiae and M. catarrhalis and at 10 minutes for S. aureus. Additionally, at all time points, the SD () of the replicates was small (≤0.9 log10 CFU/mL), indicating consistent and reproducible observations.
Figure 1 Bactericidal activity of an AMC/DCBA lozenge.
Abbreviations: AMC, amylmetacresol; CFUs, colony-forming units; DCBA, 2,4-dichlorobenzyl alcohol.
Discussion
This study examined the bactericidal action of an antiseptic lozenge containing AMC and DCBA. The organisms tested included gram-positive cocci (S. pyogenes, S. aureus, S. dysgalactiae) and bacilli (A. haemolyticum), as well as gram-negative cocci (M. catarrhalis) and bacilli (H. influenza, F. necrophorum), representing a broad range of bacterial cell structures and sensitivities.
The results demonstrated that the AMC/DCBA lozenge exhibits broad bactericidal activity against a range of organisms implicated in pharyngitis and the rapid activity observed is consistent with the time taken for a lozenge to dissolve in the mouth.Citation16
For all test organisms, evidence of bactericidal activity for the AMC/DCBA lozenge was recorded at the 1-minute time point. Of particular interest is the robust bactericidal activity against S. pyogenes, the most frequent cause of bacterial pharyngitis.Citation4 Reductions exceeding 99.9% were achieved by 1 minute for S. pyogenes, H. influenza, A. haemolyticum and F. necrophorum, by 5 minutes for S. dysgalactiae and M. catarrhalis and by 10 minutes for S. aureus. The bactericidal activity of an AMC/DCBA lozenge within a 10-minute period is important as it is consistent with the duration that a lozenge remains in the mouth; furthermore, the active ingredients were also tested at the expected concentration achieved when a lozenge is dissolved in the mouth, assuming a volume of 5 mL of saliva.
A previous in vitro evaluation of the bactericidal activity of antiseptic lozenges ([DCBA 1.2 mg, menthol 8 mg, AMC 0.6 mg] and [DCBA 1.2 mg, AMC 0.6 mg]) against S. pyogenes and S. aureus demonstrated antibacterial effectiveness.Citation32 Both AMC and DCBA formulations were highly active against the bacteria tested within 5 minutes of exposure, in contrast to the slow and weak action of the local antibiotic tyrothricin.Citation33 The data generated in this study support and expand upon these previously published observations, providing further evidence of effectiveness against a broader range of bacterial species under in vitro conditions, including those where knowledge of their clinical pathology in pharyngitis is continuing to evolve or those that represent either an opportunistic infection or an underlying medical condition. These data likewise complement recent studies showing the in vitro viricidal effects of lozenges containing AMC/DCBA (and the active ingredients as free substances) against parainfluenza virus type 3, cytomegalovirus, respiratory syncytial virus, influenza A and severe acute respiratory syndrome coronavirus.Citation12,Citation13 In addition to antimicrobial activity, AMC and DCBA are proven to provide relief from the symptoms of pharyngitis, particularly sore throat, likely through their demonstrated local anesthetic-like action against voltage-gated neuronal sodium channels,Citation14,Citation34 and therefore may benefit patients presenting with either bacterial or viral pharyngitis. Furthermore, by relieving symptoms and managing patient expectations, the number of instances of inappropriate antibiotic prescribing for viral pharyngitis may be reduced.
A limitation of this study is that these observations were performed in vitro and therefore do not fully reflect the environment of the throat. For example, the throat may contain multiple microorganisms whereas this study tested the bactericidal activity against organisms in isolation. The role of the patient’s immune system and swallowing action on the antimicrobial activity of the lozenge or active ingredients can also not be determined using in vitro methodology. However, the incidence of these bacteria is relevantly low in the general population; therefore, studying the bactericidal activity of AMC/DCBA in vivo can be challenging. Consequently, an in vitro approach is advantageous allowing the rapid generation of robust data, for multiple organisms simultaneously, that can be used to evaluate the potential of AMC/DCBA for efficacy in vivo.
Conclusion
These data show that an AMC/DCBA lozenge demonstrates bactericidal activity against all test organisms, representing a broad range of bacterial cell structures, from 1 minute and achieves greater than 99.9% kill for all test organisms within 10 minutes, which is consistent with the duration that a lozenge remains in the mouth.
Therefore, patients with uncomplicated bacterial pharyngitis, including those taking antibiotics, from low-risk populations and without additional risk factors, may benefit from the antiseptic action of AMC/DCBA against a range of bacterial species associated with pharyngitis. Most cases of pharyngitis should not require antibiotics as they are typically self-limiting and often viral in origin. Therefore, over-the-counter antiseptics like AMC/DCBA may be more appropriate, unless the condition deteriorates or a streptococcal infection is diagnosed.
Data sharing statement
All data generated or analyzed during this study are included in this manuscript.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Aisat Fatade Ogunpola (a former employee of Reckitt Benckiser Healthcare Ltd, UK) for laboratory support. Medical writing assistance was provided by Daniel East at Elements Communications Ltd, Westerham, UK and was funded by Reckitt Benckiser Healthcare Ltd, UK. This work was supported by Reckitt Benckiser Healthcare Ltd, UK.
Disclosure
Derek Matthews, Robert Atkinson and Adrian Shephard are employees of Reckitt Benckiser Healthcare Ltd, UK. The authors report no other conflicts of interest in this work.
References
- RennerBMuellerCAShephardAEnvironmental and non-infectious factors in the aetiology of pharyngitis (sore throat)Inflamm Res201261101041105222890476
- VincentMTCelestinNHussainANPharyngitisAm Fam Physician20046961465147015053411
- ESCMID Sore Throat Guideline GroupPelucchiCGrigoryanLGuideline for the management of acute sore throatClin Microbiol Infect201218Suppl 1128
- WorrallGJAcute sore throatCan Fam Physician200753111961196218000276
- SpinksAGlasziouPPDel MarCBAntibiotics for sore throatCochrane Database Syst Rev201311CD000023
- van DrielMLDe SutterADeveugeleMAre sore throat patients who hope for antibiotics actually asking for pain relief?Ann Fam Med20064649449917148626
- LinderJASingerDEDesire for antibiotics and antibiotic prescribing for adults with upper respiratory tract infectionsJ Gen Intern Med2003181079580114521641
- World Health OrganizationAntimicrobial Resistance Fact Sheet Available from: http://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance. Published February 15, 2018Accessed July 07, 2018
- DekkerARVerheijTJvan der VeldenAWInappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patientsFam Pract201532440140725911505
- GullifordMCDreganAMooreMVContinued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practicesBMJ Open2014410e006245
- SheldonATJrAntiseptic “resistance”: real or perceived threat?Clin Infect Dis200540111650165615889364
- OxfordJSLambkinRGibbIBalasingamSChanCCatchpoleAA throat lozenge containing amyl meta cresol and dichlorobenzyl alcohol has a direct virucidal effect on respiratory syncytial virus, influenza A and SARS-CoVAntivir Chem Chemother200516212913415889535
- ShephardAZybeshariSVirucidal action of sore throat lozenges against respiratory viruses parainfluenza type 3 and cytomegalovirusAntiviral Res201512315816226408353
- FoadiNde OliveiraRCBuchholzVA combination of topical antiseptics for the treatment of sore throat blocks voltage-gated neuronal sodium channelsNaunyn Schmiedebergs Arch Pharmacol201438710991100025012093
- McNallyDSimpsonMMorrisCShephardAGoulderMRapid relief of acute sore throat with AMC/DCBA throat lozenges: randomised controlled trialInt J Clin Pract201064219420719849767
- WadeAGMorrisCShephardACrawfordGMGoulderMAA multicentre, randomised, double-blind, single-dose study assessing the efficacy of AMC/DCBA Warm lozenge or AMC/DCBA Cool lozenge in the relief of acute sore throatBMC Fam Pract2011121621332976
- ShephardASmithGAspleySSchachtelBPRandomised, double-blind, placebo-controlled studies on flurbiprofen 8.75 mg lozenges in patients with/without group A or C streptococcal throat infection, with an assessment of clinicians’ prediction of “strep throat”Int J Clin Pract2015691597125296661
- RadkovaEBurovaNBychkovaVDevitoREfficacy of flurbiprofen 8.75 mg delivered as a spray or lozenge in patients with sore throat due to upper respiratory tract infection: a randomized, non-inferiority trial in the Russian FederationJ Pain Res2017101591160028740426
- DagnelieCFTouw-OttenFWKuyvenhovenMMRozenberg-ArskaMRde MelkerRABacterial flora in patients presenting with sore throat in Dutch general practiceFam Pract19931043713778168671
- ShaikhNLeonardEMartinJMPrevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysisPediatrics20101263e557e56420696723
- BattyAWrenMWPrevalence of Fusobacterium necrophorum and other upper respiratory tract pathogens isolated from throat swabsBr J Biomed Sci2005622667015997879
- EatonCSwindellsJThe significance and epidemiology of Fusobacterium necrophorum in sore throatsJ Infect201469219419624642207
- HarringtonATClarridgeJE3rdImpact of identification of Streptococcus dysgalactiae subspecies equisimilis from throat cultures in an adult populationDiagn Microbiol Infect Dis2013761202323537788
- GergovaRTPetrovaGGergovSMinchevPMitovIStratevaTMicrobiological features of upper respiratory tract infections in Bulgarian children for the period 1998–2014Balkan Med J201633667568027994923
- LafontaineERWallDVanlerbergSLDonabedianHSledjeskiDDMoraxella catarrhalis coaggregates with Streptococcus pyogenes and modulates interactions of S. pyogenes with human epithelial cellsInfect Immun200472116689669315501804
- VerhaeghSJFloresARvan BelkumAMusserJMHaysJPDifferential virulence gene expression of group A Streptococcus serotype M3 in response to co-culture with Moraxella catarrhalisPLoS One201384e6254923626831
- MihanceaNFrequency and distribution per species, biotypes, resistance to antibiotics and beta-lactamase production of the hemophils isolated from patients with respiratory diseasesRoum Arch Microbiol Immunol199857212513711845430
- CarlsonPRenkonenOVKontiainenSArcanobacterium haemolyticum and streptococcal pharyngitisScand J Infect Dis19942632832877939427
- JensenAHansenTMBankSKristensenLHPragJFusobacterium necrophorum tonsillitis: an important cause of tonsillitis in adolescents and young adultsClin Microbiol Infect2015213266.e1e3
- KatkowskaMGarbaczKStromkowskiJStaphylococcus aureus isolated from tonsillectomized adult patients with recurrent tonsillitisAPMIS20171251465127862322
- ZautnerAEKrauseMStropahlGIntracellular persisting Staphylococcus aureus is the major pathogen in recurrent tonsillitisPLoS One201053e945220209109
- RichardsRMXingDKIn vitro evaluation of the antimicrobial activities of selected lozengesJ Pharm Sci19938212121812208308699
- CLSIMethods for Determining Bactericidal Activity of Antimicrobial Agents. Approved Guideline, CLSI Document M26-AWayne, PAClinical and Laboratory Standards Institute (CLSI)1999
- BuchholzVLeuwerMAhrensJFoadiNKrampflKHaeselerGTopical antiseptics for the treatment of sore throat block voltage-gated neuronal sodium channels in a local anaesthetic-like mannerNaunyn Schmiedebergs Arch Pharmacol2009380216116819367399
