Abstract
Cholangiocarcinoma (CCA) is a tumor with increasing prevalence around the world. The prevalence of CCA is highest in East Asia and most significantly in the countries through which the Mekong River flows, owing to the presence of liver flukes, which are consumed in raw fish dishes. Outside Asia, the causes of bile duct cancers for the most part are unknown. In this review, we assess the current state of knowledge in both fluke-associated and sporadic CCA, from etiological, diagnostic, and treatment perspectives.
Introduction
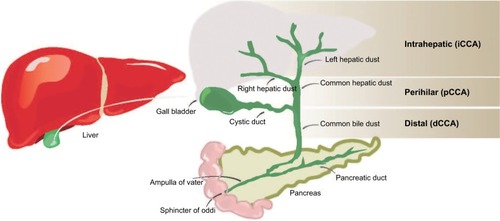
Cholangiocarcinoma (CCA), or bile duct carcinoma, is a malignancy that originates from the cholangiocytes lining the biliary tree. It is the second most common primary liver malignancy, following hepatocellular carcinoma (HCC), accounting for 10%–20% of all hepatic cancers.Citation1 Based on where the tumor arises in the biliary tree, CCA tumors are classified into intrahepatic (iCCA) or distal, while tumors developing in the bile duct bifurcation are classified as perihilar (pCCA; ). The majority of CCA tumors are in the perihilar and distal regions, and only 10% are intrahepatic.Citation2 The typical age at presentation is the seventh decade, with a slight male predominance.Citation3
Figure 1 Locations of CCA in the biliary tree.
Abbreviations: CCA, cholangiocarcinoma; iCCA, intrahepatic CCA.
Epidemiology
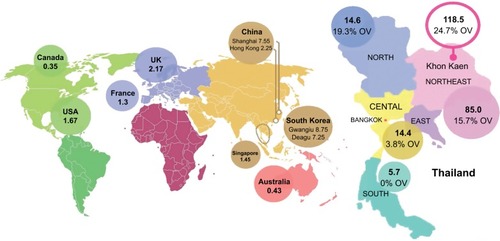
Recent studies have shown an unexplained global increase in CCA incidence and mortality. For instance, in England, the number of CCA deaths increased from 1,720 in 2010 to 2,161 in 2013.Citation4 A similar trend was found in the United States; the incidence rate of iCCA increased by 165% in 30 years, from the1970s to the 1990s, from 0.32 per 100,000 to 0.85 per 100,000.Citation1 The geographical distribution of CCA cases is uneven (), and it closely mirrors the prevalence of its predisposing risk factors. The vast majority of CCA cases in the Western developed nations have a sporadic nature. Primary sclerosing cholangitis (PSC), a chronic condition causing biliary obliterative fibrosis, is the commonest risk factor in Western countries, but it is associated with only 10% of cases, indicating the diversity of influencing factors on disease risk.Citation5 The highest reported CCA incidence internationally is in Northeast of Thailand, 118.5 per 100,000, in Khon Kaen, which is some 100 times higher than the global rate. The high CCA burden is associated with liver fluke infestations that is endemic to countries through which the Mekong River traverses on its route from Yunnan Province in China through to its delta in southern Vietnam.Citation6
Figure 2 Estimated global CCA incidence.
Abbreviation: CCA, cholangiocarcinoma.
Liver fluke infection is caused by water-borne parasites, Opisthorchis viverrini, Clonorchis sinensis, and Opisthorchis felineus, which are transmitted to humans by the consumption of raw, pickled, or undercooked infected fish. O. viverrini is common in the Mekong Basin region, including Thailand, Laos, central Vietnam, and Cambodia, whereas in Korea, China, and northern Vietnam, C. sinensis is more prevalent. O. felineus is reported to be endemic in parts of Russia, Kazakhstan, and Ukraine.Citation7
The distribution of biliary duct carcinoma in Thailand shows high variability (); regions with high O. viverrini endemicity show higher CCA burden. In Southern Thailand, where O. viverrini infection is sparse, the incidence is 5.7 per 100,000, compared with 14.6 per 100,000 and 85 per 100,000 in the North and Northeast regions, where O. viverrini infects 19.3% and 15.7% of the population, respectively.Citation8
Liver flukes
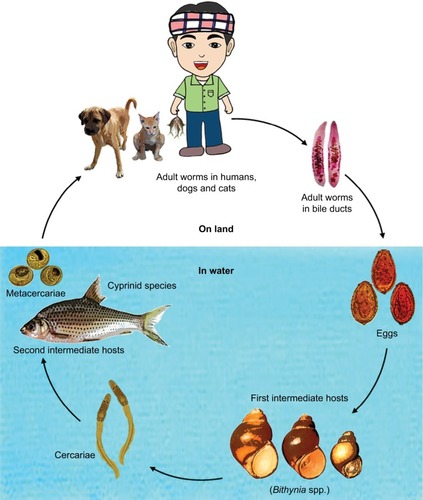
Leiper reported the first human cases of O. viverrini infection in the cadavers of Thai prisoners in the early part of the last century.Citation9 Eight decades later, in 1994, O. viverrini was classified, along with C. sinensis, as a group-1 carcinogen by the International Agency for Research on Cancer (IARC), based on their strong association with hepatobiliary carcinomas.Citation10 The majority of opisthorchiasis and clonorchiasis cases are found in the Mekong River basin villages where the flukes infect around 90%–95% of cyprinid fish (fish with scales), and eating freshly caught fish raw is a long-standing tradition enjoyed among the locals.Citation11 Koi pla (raw fish salad) and plasom (fermented fish) are a few of the most common infective dishes eaten in the region surrounding the 4,350-km long river, where fishing is one of the main sources of food and livelihood. Fish is the cheapest source of animal protein for the majority of the 67 million inhabitants of the Mekong Basin.Citation12
The people of this region are also among the poorest in the world with an average income of <1 US dollar per day.Citation13 Sithithaworn et al named the fluke infestation and its deadly consequences “the fatal disease of the poor”.Citation14 Mortality from liver and bile duct cancer is the leading cause of death in Thai males and third in females, causing a total of 28,000 deaths per year. The highest number of liver cancer mortality cases is reported in the Northeast of Thailand, comprising 70% of the cases.Citation15 O. viverrini is endemic in the wetland south of the Mekong region, affecting an estimated 8 million people in Thailand and 2 million in Laos. Until recently, the endemicity level in Cambodia was uncertain, but a large surveillance examining 16,082 fecal samples showed that the infection is highly endemic, reaching an alarming 65.1% in one of the villages in the Southern region. Eating raw fish in endemic areas was reported in 70%–100% of the locals.Citation16 An estimated 601 million people are currently at risk of infection with C. sinensis, 570 million are in China and Taiwan alone, and 67.3 million people are at risk of O. viverrini infection.Citation7
The fluke life cycle is complex, involving two intermediate hosts (snail to fish) and several morphological changes (). After the ingestion of fish containing viable metacercariae, infected humans excrete the eggs produced by the mature adult worms in their feces. If they reach fresh water, the eggs are ingested by freshwater snails and the larva (or miracidia) hatch in the digestive tract of these snails. The infected snails shed thousands of cercariae into the water, which penetrate the skin between the scales of freshwater fish, and encyst, forming metacercariae. In 21 days they become infective with metacercariae, and when ingested by humans, they excyst in the duodenum and ascend to the bile duct via the ampulla of Vater. Using their two suckers and body contraction, the metacercariae migrate further into the smaller, proximal bile ducts where they become mature worms within a month and are able to sexually reproduce.
Adult worms (measuring 10–25 mm long and 3–5 mm wide) are able to produce 1000–5000 eggs per day, and are estimated to survive up to 25 years in the biliary tree, causing only mild symptoms.Citation10,Citation17 Conventional diagnosis of liver flukes is based on microscopic examination of the feces for eggs. The infection is usually asymptomatic in mild cases (fewer than 100 flukes). Malaise, abdominal discomfort, and diarrhea are sometimes present in early infection.Citation18
Long-term complications associated with chronic infection include, hepatomegaly, cholecystitis, gallstones, and periportal fibrosis. The severity of such manifestations is linked to the intensity and duration of infection. Chronic inflammation is found to be a major etiological precursor of hepatobiliary malignancy, predominantly of CCA. Carcinomas typically present 30–40 years after infection, prognosis is generally poor, and death tends to occur within 3–6 months after diagnosis.Citation8 Praziquantel, an anthelmintic drug, is used to eliminate the worms. Although it is an effective therapy, praziquantel does not reverse periductal fibrosis and inflammation in all patients; subsequently, it may not prevent the progression to CCA.Citation19 In addition, the cycle of infection, dosing with praziquantel, and reinfection is recognized as a major challenge.Citation20 Repeated infection cycles, typically by regular self-dosing with praziquantel after the initial infection to eliminate the worms, can potentially increase the susceptibility to cholangiocarcinogenesis due to oxidative stress and inflammatory responses.Citation20
Chronic host inflammatory responses may persist even after the eradication of the liver flukes through the eggs they produce, which can remain entrapped in the periductal tissue causing granulomatous inflammation.Citation8,Citation20 Therefore, comprehensive assessment of reinfection rate and eradication potential is difficult to establish.
The association between the biliary parasitosis and CCA is well established, and extensively reviewed in the last two decades.Citation8,Citation18 CCA genesis is hypothesized to be initially induced via multifactorial mechanistic pathways: 1) mechanical damage caused by the fluke suckers, 2) fluke toxic secretary products, and 3) immunopathological host response. All of these factors result in chronic inflammation and hepatobiliary abnormalities, which trigger a proliferative response and formation of precursor lesions (eg, epithelial and adenomatous hyperplasia, and goblet cell metaplasia).Citation8 Gene–environment interactions play an important role in the individual’s susceptibility to carcinogenesis. As an example, genetic polymorphism of glutathione-S-transferase, a detoxifying enzyme, is found to be associated with seropositivity for opisthorchiasis, and may in turn alter the individual’s risk to CCA.Citation21
Nitrosamines, known carcinogens found in foods such as fermented food and commonly used fish paste in Northeastern Thailand and Laos, “plara”, add an additional risk factor.Citation8 Some authors hypothesized that nitrosamine compounds are a primary carcinogen leading to hepatobiliarytumors.Citation18
Primary sclerosing cholangitis
PSC, a progressive cholestatic biliary disease with unknown etiology, is characterized by chronic inflammation that leads to destruction of the intra- and extrahepatic bile ducts.Citation22 PSC incidence ranges from 0 to1.3 per 100,000 people/year in Western countries.Citation23 The disease is asymptomatic in the majority of the patients, and is often diagnosed incidentally through routine check-up (eg, elevated liver enzymes). Fatigue is the most common symptom, whereas pruritus and jaundice occur in advanced stages as a result of cholestasis. Inflammatory bowel disease is highly common in PSC patients, up to 80%, and often diagnosed prior to hepatobiliary disease.Citation24,Citation25
The mean age at diagnosis with PSC is 40 years, with a median life expectancy of 9–12 years from diagnosis. PSC is associated with portal hypertension, cirrhosis, and hepatobiliary and colorectal cancer. Progression to CCA is unrelated to the duration of the inflammation.
It tends to be diagnosed in 6%–36% of PSC patients within a few years after diagnosis, making CCA from PSC onset at a younger age (∼34 years old), compared with the general population where biliary carcinoma commonly presents in the seventh decade of life.Citation25 The only curative treatment to date for PSC is liver transplantation.Citation24
Biliary stones
Stones formed anywhere in the biliary tree are generally associated with increased CCA risk, substantially when the stones arise in the intrahepatic bile ducts, or hepatolithiasis, where it is estimated that 10% of the patients progress to develop iCCA. Hepatolithiasis rarely predisposes to CCA in Western patients, but it is a relatively common risk factor in several Asian countries, where the incidence ranges from 2% to 25%.Citation26 The presence of intrahepatic biliary stones was found in nearly 70% of CCA patients undergoing surgical resection in Taiwan.Citation27
Hepatolithiasis is typically concomitant with biliary stasis, cholangitis, strictures, and bacterial infections, subsequently, leading to prolonged inflammatory status and biliary injury, which in turn triggers malignant cholangiocyte growth, in some cases.Citation26 Cholecystolithiasis (gallbladder stones) and choledocholithiasis (stones in the common bile ducts) were found to be associated with increased intrahepatic cholangiocarcinogenesis with an odds ratio of 4.0 and 23.97, respectively, in a large-scale Danish case-control study (n=764 iCCA cases and n=3,056 population controls).Citation28
Choledochal cysts
Choledochal cysts are a rare congenital dilatation of the biliary tree (incidence of 1 per 100,000 people). The cysts can be single or multiple, and arise in the intra- or extrahepatic bile ducts. The disease is idiopathic, and is mostly common in Asian females. Several hepatobiliary complications can present with choledochal cysts including, cholelithiasis, cholecystitis, cholangitis, cirrhosis, portal hypertension, pancreatitis, pancreatic duct obstruction, and CCA. The risk of CCA in patients with choledochal cyst is 10%–30%, and even though surgical resection of cysts reduces the risk to 0.7%–6%, malignant transformation can still occur, and life-long follow-up is recommended for the majority of patients.Citation25,Citation29 Moreover, the coincidence of abnormal pancreaticobiliary duct junctions increases the possibility of cholangiocarcinogenesis. Pancreatic enzymes reflux, cholestasis, and elevated bile acid concentrations can further induce malignant proliferation in patients with choledochal cysts.Citation29
Chemical carcinogens
Exposure to Thorotrast, a radiographic contrast agent banned in the1960s, is reported to be associated with a 300-fold increased risk of CCA, occurring 16–45 years after exposure.Citation30 Thorotrast is primarily deposited in the liver (∼60%), and continues a lifelong emission of internal radioactive particles, as it has a biological half-life of 400 years.Citation31 Pathogenesis from Thorotrast exposure to carcinogenesis has not been fully proven. Radiation-induced liver insult has been shown to promote hepatocyte remodeling, genomic instability, DNA methylation, and disrupt the liver architecture. Few genetic mutations have been reported to be associated with Thorotrast-induced hepatobiliary malignancy, such as K-ras-2 and TP53.Citation32
In addition, occupational exposure to a halogenated hydrocarbon solvent, 1,2-dichloropropane, was found to be associated with CCA among printing factory workers in Japan, where the substance is used in ink removal. Long-term exposure (via inhalation and dermal contact) was linked to CCA induction among the young factory employees. The substance was categorized as class-1 carcinogen in 2014 by the IARC.Citation33
Other risk factors
Other hepatic diseases associated with CCA include alcoholic liver disease, cirrhosis, and cholangitis with odds ratios of 19.22, 75.9, and 6.3, respectively, reported in a Danish cohort.Citation28 CCA risk in viral hepatitis was recently investigated in a Korean study with n=276 CCA cases, hepatitis B virus significantly increasing iCCA risk with an odds ratio of 4.1.
The risk is greater in the presence of diabetes (odds ratio=12.2), and diabetes independently was associated with both intra- and extrahepatic biliary carcinoma.Citation34 Obesity was unrelated to CCA risk in both the Korean and Danish cohorts.
Inflammatory bowel disease is also implicated in cholangiocarcinogenesis. This, however, might be confounded by the presence of PSC which is often not controlled for in epidemiological studies. Data on alcohol intake and smoking in association to CCA risk are inconsistent, but they are both recognized as potential risk factors.Citation25 Several genetic polymorphisms related to cellular DNA repair, protection against toxins, or immunological surveillance have been implicated in biliary tract carcinogenesis.Citation2
Tumor phenotypes
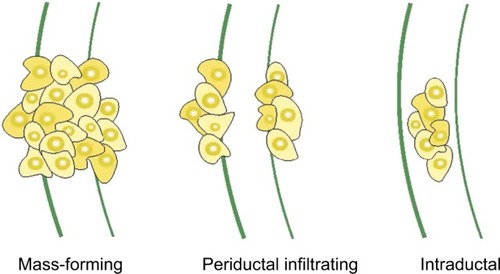
CCA is an extremely heterogeneous group of malignancies in its genomic, histological, morphological, biological, and clinical features. Apart from the anatomical classification of tumors (ie, intrahepatic, perihilar, or distal), they are further divided into mass-forming, periductal, and intra-ductal, based on their gross appearance, as illustrated in . CCAs can present as a single or multiple growth type, such as periductal infiltrating plus mass-forming. The most common growth type is mass-forming, accounting for 40%–42% of tumors.Citation35
Figure 4 Morphologic classification of CCA.
Abbreviation: CCA, cholangiocarcinoma.
Histologically, >90% of CCA are adenocarcinomas. Other microscopic types include adenosquamous and squamous carcinoma, and rare subtypes.Citation36 The tumors show various grades of differentiation: poor, moderate, and well. Those with low-grade or well-differentiated types have better prognosis and lower incidence of distal metastases than with high-grade carcinomas.Citation35 Combined hepatocellular–CCAs can present within the same liver. It is a rare type of primary liver cancer that shows histopathological features of both HCC and CCA, and accounts for 1%–15% of all CCAs.Citation36
Various risk factors of CCA and associated pathologies, diverse histological features, and tumoral genomic heterogeneity cast doubt on the cellular origin. Additionally, due to the fact that CCA can arise as a result of hepatocyte injury, such as viral hepatitis and alcoholic liver disease, it is probable that CCA can arise from hepatocytes, and not invariably from the cholangiocytes lining the biliary tree.Citation37
CCA carcinogenesis from hepatocytes was observed incidentally via the activation of the oncogenes, NOTCH and AKT, in vivo. Evidence from hepatocyte fate-tracing experiments, which enables tracking of the fate of cells in living mice, showed that activated NOTCH signaling, in combina tion with AKT overexpression, promotes tumorigenesis in the early stages of intrahepatic bile duct carcinomas.Citation38 NOTCH signaling is also known to play an important role during the embryonic development of liver architecture, including cholangiocyte differentiation and biliary duct development.
Genome-wide analysis studies revealed the global gene expression patterns and transcription mutations in CCA. Intrahepatic tumors showed greater genomic heterogeneity than tumors arising outside the liver (2,354 genes with altered expression vs 545).Citation39 To further capture CCA molecular profile, somatic mutational screening studies identified several mutated genes. KRAS mutations and loss-of-function mutations of TP53 are the most commonly altered genes in CCA, reported in around 10%–45% and 21% of cases, respectively. Multiple gene alterations were identified by various molecular profiling techniques and described molecular heterogeneity of CCA tumors. Different molecular expression profiles were associated with the tumor anatomical location, and several mutations were shown to be significantly associated with poor prognosis. The molecular mutations that characterize CCAs include IDH1, IDH2, ARID1A, PIK3CA, BAP1, and NRAS.Citation40
Furthermore, exome sequencing has identified characteristic mutational patterns in liver fluke-related bile duct cancers, distinct from non-infection-related bile duct cancers.Citation41 In addition, it is of interest to note that infection with O. felineus induces intraepithelial neoplasia of the biliary tract in a rodent model, further garnering evidence for the direct link between liver-fluke infections and CCA.Citation42
Overall, tumoral phenotypic characterization (genomic, epigenetic, and molecular) is associated with tumor aggressiveness, prognosis, and response to therapy, thus potentially indicating the best therapeutic approach to tailor personalized treatment for each patient. Therefore, it should be a key strand of future research to identify new potential therapeutic targets for treating CCAs, based on their phenotypic signature, in order to enable patient stratification.Citation3
Diagnosis
CCA diagnosis is often complex and requires the use of multiple diagnostic modalities to 1) establish strictures anatomical location; 2) distinguish between benign and malignant strictures; 3) differentiate CCA from gallbladder, other primary liver tumors, and combined hepatocellular–CCAs; 4) stage and grade the tumors; and 5) plan treatment approach. This is often difficult owing to the tumor anatomical location, desmoplastic nature, and lack of definitive diagnostic tests.Citation36,Citation43
Clinical presentation
Clinical symptoms and signs of CCA are often dependent on where the lesions originate in the biliary tree and are typically absent in early stage tumors. Malignant strictures originating within the right or left intrahepatic bile ducts tend to cause nonspecific symptoms (such as weight loss, abdominal discomfort, and malaise). Jaundice, pruritus, and pale stool are associated with lesions originating from the hilar and distal biliary ducts when the tumor is large enough to obstruct the biliary flow.Citation36
Blood biomarkers
To date, there is no specific blood test available to diagnose CCA. Conventional liver function markers such as serum bilirubin, alkaline phosphatase, and aminotransferase enzymes are elevated in the presence of biliary obstruction, and are not indicative of malignancy per se.Citation43
Serum tumor markers, carbohydrate antigen (CA)19–9, CA-125, and carcinoembryonic antigen (CEA) are the most widely used markers for suspected CCA. They are clinically used in conjunction with other diagnostic tools, and should not be used alone due to their poor diagnostic performance and inherent limitations.Citation36
For example, CA19–9, the most used marker, is nonspecific to CCA and is elevated in pancreatic, colorectal, and gastric cancers, and also in nonmalignant conditions, such as PSC and obstructive jaundice. In a follow-up study, 37% of PSC patients with elevated CA19–9 were negative for CCA.Citation44 Patients lacking Lewis antigen (10% of the general population) cannot produce CA19–9 and will not benefit from CA19–9 testing. The sensitivity and specificity of CA19–9 in CCA patients range from 40% to 70% and 50% to 80%, respectively, with a positive predictive value of 16%–40%.Citation36
CEA is raised in 30% of patients with CCA, whereas CA-125 is elevated in around 40%–50%. Both markers are nonspecific and insufficient for accurate diagnosis.Citation36 Several other potential serum tumor markers have been linked to CCA (eg, CYFRA21–1, CA-195, CA-242, DU-PAN-2, IL-6, and trypsinogen-2), but their clinical utility is still unclear, and they are not in routine use.Citation36,Citation45
Imaging and other techniques
In suspected CCA, ultrasonography (US) is initially used to exclude gallstones, assess biliary dilatation, and localize the obstruction site. Yet, US alone is insufficient to confirm diagnosis; it is operator-dependent, cannot accurately define tumor extent, and may miss small tumors. High-resolution imaging, computed tomography, and magnetic resonance imaging (MRI) provide greater sensitivity for CCA detection than US, but may not be readily accessible in Thailand and resources-limited settings. MRI with cholangiopancreatography is the technique of choice to visualize the ductal and vascular structure, define tumor extent, and detect distant metastases. The advantages of the procedure is its high sensitivity and specificity, noninvasiveness, and the fact that there is no risk of radiation.Citation43
Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography are more invasive, but they offer the possibility of therapeutic intervention, as well as diagnostic assessment. They allow sampling of strictures for brush cytology or tissue biopsy, and stent insertion to relieve biliary obstruction. These sampling methods are highly specific (100%), but they have a low sensitivity(46%–73%). Cytology is positive in <50% of CCA cases. Thus, negative results do not exclude malignancy. Biopsy, in addition to cytology, can enhance positive findings to 40%–70%, but tissue is not always easy to obtain, owing to the scirrhous nature of desmoplastic tumors and risk of tumor seeding.Citation36 Once CCA is confirmed, comprehensive staging must be carried out to screen for metastatic disease. The above diagnostic techniques are complementary and selection is dependent on the clinical needs of the individual. Multiple imaging techniques might be necessary for surgical assessment of resectability.
Treatment and prognosis
Surgical resection is the only curative therapy for CCA. Patients with intrahepatic tumors are candidates for partial hepatectomy, whereas pancreatoduodenectomy is most suitable for distal tumors. For pCCA, the extent of surgical intervention required is based on Bismuth–Corlette classification, and it is generally associated with the worst resectability rate, compared with distal tumors (56% vs 91%).Citation46
The majority of CCA cases present too late for surgical intervention. The latest treatment guidelines recommend a combination of Gemcitabine and Cisplatin chemotherapy for inoperable cases. This approach was adapted following encouraging results from two large randomized studies, ABC-02 in the UK and BT22 in Japan. The regimen is an acceptable standard first-line therapy for patients with advanced biliary tract cancer, where in some cases it was successful to downstage tumors to operable state and improve survival.Citation36,Citation47
Currently, there is no evidence to support the routine use of radiotherapy postoperatively or for unresectable disease.Citation3,Citation36
Srinagarind Hospital, the leading center in CCA treatment in the Northeast of Thailand, published a prospective study on Thai CCA patient outcome. Out of 163 patients with biliary carcinoma, only 10 cases (6.1%) were eligible for curative resection. The vast majority presented too late for any intervention, stage IV, and were referred to palliative care from the first visit. The overall median survival was 4 months, but patients who received surgical resection with negative tumor margins had 100% 4-year survival rates.Citation48
The prognosis for inoperable Thai patients with bile duct cancer receiving palliative chemotherapy is poor. Gemcitabine chemotherapy has a median survival of 10 months.Citation49 Moreover, resection margins are important prognostic factors, and complete surgical resection at an early stage is the only treatment that significantly improves patient survival.
Long-term survival and recurrence rate post hepatic resection were examined in a Korean retrospective study: iCCA recurred in 60% of cases, either in the remnant liver and in lymph nodes or as distant metastasis, commonly in the lung. Tumor size, lymph node metastasis, and multiple tumors were significant predictors of recurrence.Citation50
Molecular therapies for solid biliary tumors are generally complex to develop, owing to their therapeutic resistance, and anatomical, histological, and phenotypic heterogeneity.Citation2 Elucidation of the molecular mechanisms and pathways underlying cholangiocarcinogenesis may expedite development of personalized therapy for patients based on their particular tumor phenotype.
Screening strategies
Screening strategies for CCA vary according to the disease etiology, where it moves from small-scale, high-risk patient surveillance, such as for PSC patients, to large, community-based screening programs as in the O. viverrini prevention campaign in Thailand.
Cholangiocarcinoma screening in Western countries
Established risk factors account for less than a third of CCA cases in the West.Citation36 Due to the disease rarity and the ambiguous definition of asymptomatic at-risk population, surveillance guidelines of sporadic CCA are yet to be established. Patients with predisposing hepatobiliary disease undergo series of annual screening tests. PSC patients are typically screened mainly for CCA, but also for HCC and colorectal cancer. If biliary dominant strictures are present, regular ERCP with brush cytology is recommended. This is not ideal for surveillance; it is invasive, costly, and has low diagnostic accuracy.Citation51 Solely screening for CCA might not be feasible. To overcome this limitation, it is proposed to design a multicancer detection panel that combines CCA markers with other markers of uncommon respiratory and digestive tract cancers or aerodigestive cancers. This screening modality may justify and permit CCA screening in countries with low incidence.Citation51
Cholangiocarcinoma screening and control in Thailand
Approximately, 36% of the Thai population (23 million) live around the Mekong river wetlands, and are at potential risk of opisthorchiasis.Citation52 Primary prevention of CCA through opisthorchiasis control began in Thailand in 1950 as local treatment units in few high-endemic provinces. When praziquantel was introduced in the early1980s, the Thai Ministry of Health started four opisthorchiasis treatment units, which examined over half a million people and treated 400,452 cases.Citation53
In 1987, a national-scale opisthorchiasis screening program began as a part of the country’s national health plan. Over 5 million stool samples were examined, and 1.8 million positive cases were identified and treated in the North-Eastern region. The program expanded to cover provinces in the North and Central Thailand in 1992, resulting in a fall in the national opisthorchiasis prevalence, from 63.6% to 9.6% in 2001.Citation53
Despite the intensive health campaigns targeting O. viverrini prevention, the prevalence remains remarkably high in some parts in Thailand, and subsequently, CCA burden remains alarmingly high. Opisthorchiasis prevalence reached 42.2% in the latest epidemiological surveillance, named the Cholangiocarcinoma Screening and Care Program (CASCAP). CASCAP was developed by the Medical Faculty of Khon Kaen University in cooperation with the Cholangiocarcinoma Foundation, as a community-level screening program that aimed to document the current status of opisthorchiasis-related CCA in the region.Citation54
Repeated infection is likely to occur after the administration of praziquantel and might explain the persistent high infection rate. In an animal model, repeated infection of hamsters with O. viverrini exhibited an inflammatory response and showed higher levels of parasite-specific antibody, which positively correlated with a greater degree of fibrosis.Citation55 About 89% of the CASCAP cohort had previously eaten raw or fermented fish,Citation54 indicating a poor response to the educational campaigns against raw fish consumption among rural communities. Moreover, the vast majority are farmers (79.9%), and raw fish based-diet is embedded in their cultural identity. The magnitude of the problem is greater than telling people to “stop eating raw fish”; it has social, political, and environmental implications.
Additionally, the perceived political tension between the rural Northeast and the Bangkok government may have created resistance in accepting the health promotion messages communicated from the government to villages.Citation56 Reinfection cycles are also influenced by poor sanitation practice and lack of sewerage infrastructure. Commonly, during important growing/harvest cycles in the rice-fish Mekong Basin cultures, temporary homes are used which are typically huts with no latrines.Citation53
Currently, the screening modality used in Thailand is US imaging-based, which has several limitations. Experienced radiologists are required, and the effectiveness of the diagnosis depends on the operator skills. US is time-consuming, and not suitable for rapid screening of at-risk people in endemic areas. The rate of early tumor detection using US is unknown, and repeated US procedure is required for at-risk patients every 6–12 months; therefore, storage of the digital information require reliable server and network infrastructure. On the other hand, US is noninvasive, and with extensive training, optimizing sonographic equipment, and procedure standardization, US was shown to have early CCA detection potential by the detection of periductal fibrosis.Citation57
The absence of prognostic techniques limits therapeutic options. Therefore, there is an urgent unmet demand for the development of biomarkers that hold clinical potential for timely diagnosis of the CCA in Thailand and neighboring countries. CCA incidence in Thailand appears to be increasing, not decreasing, and even if the worm is eradicated, it will take 20–30 years to achieve CCA-free population. Hence, those with history of parasitosis are still at risk of developing malignant biliary tumors.
Future directions
The mortality from CCA remains equal to the disease incidence. New biomarkers for screening, diagnosis, staging, and prognosis would be crucial in the management of CCA, as current methods are ineffective in detecting small tumors. To date, several serum markers, including the clinically available iCA19–9 and CEA, and potential markers such as CA242, mucin glycoproteins, and several cytokines have been reported to be adequate diagnostic and prognostic markers for CCA.Citation58 Yet, the diagnostic accuracy of such markers is not sufficient for a timely and reliable diagnosis of CCA.
A high-throughput “omics” approach and the development of multi-marker panel would be of future benefit to explore potential diagnostic targets in disease screening applications. At the individual level, metabolic profiling provides the possibility of personalized health care, which aims to deliver targeted therapies and predict individual’s response to treatment.Citation59
Such methodology may have the potential to provide a new diagnostic window to CCA. Furthermore, metabolic phenotyping of CCA may shed light on understanding environmental interactions underpinning CCA genesis and may lead to the discovery of diagnostic markers in biofluids.
Acknowledgments
The authors were funded by grants from the Welcome Trust ISSF Fund at Imperial College, London, and AMMF – the Cholangiocarcinoma Charity (Stansted, Essex, UK). All authors acknowledge the support of the United Kingdom National Institute for Health Research Biomedical Research Centre at Imperial College, London, for infrastructure support.
Disclosure
MA was funded by the StratiGrad PhD programme at Imperial College, London. The authors report no other conflicts of interest in this work.
References
- ShaibYEl-SeragHBThe epidemiology of cholangiocarcinomaSemin Liver Dis200424211512515192785
- RazumilavaNGoresGCholangiocarcinomaThe Lancet2014383993521682179
- BanalesJMCardinaleVCarpinoGExpert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA)Nat Rev Gastroenterol Hepatol201613526128027095655
- NCIN2015National Cancer Intelligence Network: rare and less common cancers, incidence and mortality in England, 2010 to 2013 Technical report, Public Health England
- ZabronAEdwardsRJKhanSAThe challenge of cholangiocarcinoma: dissecting the molecular mechanisms of an insidious cancerDis Model Mech20136228129223520144
- BragazziMCardinaleVCarpinoGCholangiocarcinoma: epidemiology and risk factorsTransl Gastrointest Cancer2012112132
- KeiserJUtzingerJEmerging foodborne trematodiasisEmerg Infect Dis200511101507151416318688
- SripaBKaewkesSSithithawornPLiver fluke induces cholangiocarcinomaPLoS Med200747e20117622191
- LeiperRNotes of the occurrence of parasites presumably rare in manJ R Army Med Corps1915246569575
- IARC1994Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis.) Technical report, Public Health England
- VichasriSViyanantVUpathamESOpisthorchis viverrini: intensity and rates of infection in cyprinoid fish from an endemic focus in Northeast ThailandSoutheast Asian J Trop Med Public Health19821311381417112213
- ZieglerADPetneyTNGrundy-WarrCDams and disease triggers on the lower Mekong riverPLoS Negl Trop Dis201376e216623853695
- CogelsO2004Water resources and poverty alleviation in the lower Mekong BasinPresented at: IUCN World Conservation CongressNovember 18, 2004Bangkok, Thailand
- SithithawornPAndrewsRHNguyenVDThe current status of opisthorchiasis and clonorchiasis in the Mekong BasinParasitol Int2012611101621893213
- SripaBPairojkulCCholangiocarcinoma: lessons from ThailandCurr Opin Gastroenterol200824334935618408464
- MiyamotoKKirinokiMMatsudaHField survey focused on Opisthorchis viverrini infection in five provinces of CambodiaParasitol Int201463236637324342554
- IARC2012Monographs on the evaluation of carcinogenic risks to humans (Opisthorchis viverrini and Clonorchis sinensis). Technical report, Public Health England
- KaewpitoonNKaewpitoonSJPengsaaPSripaBOpisthorchis viverrini: the carcinogenic human liver flukeWorld J Gastroenterol200814566667418205254
- MairiangEHaswell-ElkinsMRMairiangPSithithawornPElkinsDBReversal of biliary tract abnormalities associated with Opisthorchis viverrini infection following praziquantel treatmentTrans R Soc Trop Med Hyg19938721941978337727
- SaengsawangPPromthetSBradshawPReinfection by Opisthorchis viverrini after treatment with praziquantelAsian Pac J Cancer Prev201617285786226925692
- HonjoSSrivatanakulPSriplungHGenetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast ThailandInt J Cancer2005117585486015957169
- KarlsenTHBobergKMUpdate on primary sclerosing cholangitisJ Hepatol201359357158223603668
- BoonstraKBeuersUPonsioenCYEpidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic reviewJ Hepatol20125651181118822245904
- LindorKDKowdleyKVHarrisonMEAmerican College of GastroenterologyACG clinical guideline: primary sclerosing cholangitisAm J Gastroenterol2015110564665925869391
- TysonGLEl-SeragHBRisk factors for cholangiocarcinomaHepatology201154117318421488076
- KimHJKimJSJooMKHepatolithiasis and intrahepatic cholangiocarcinoma: a reviewWorld J Gastroenterol20152148134181343126730152
- ChenMFPeripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatmentJ Gastroenterol Hepatol199914121144114910634149
- WelzelTMMellemkjaerLGloriaGRisk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control studyInt J Cancer2007120363864117109384
- SöreideKKörnerHHavnenJSöreideJABile duct cysts in adultsBr J Surg200491121538154815549778
- KatoIKidoCIncreased risk of death in Thorotrast-exposed patients during the late follow-up periodJpn J Cancer Res19877811118711922826375
- KaulAMuthHThorotrast kinetics and radiation doseRadiat Environ Biophys1978153241259746119
- WangLLiuDShimizuTFukumotoMMechanisms of liver carcinogenesis by chronic exposure to alpha-particles form internally deposited ThorotrastInt Congr Ser20051276192194
- KumagaiSKurumataniNArimotoAIchiharaGCholangiocarcinoma among offset colour proof-printing workers exposed to 1,2-dichloropropane and/or dichloromethaneOccup Environ Med201370750851023493378
- LeeBSParkECParkSWNamCMRohJHepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in KoreaWorld J Gastroenterol201521250251025593465
- LeongTY-MWannakrairotPLeeESLeongAS-YPathology of cholangiocarcinomaCurr Diagn Pathol20071315464
- KhanSADavidsonBRGoldinRDGuidelines for the diagnosis and treatment of cholangiocarcinoma: an updateGut201261121657166922895392
- SeehawerMHeinzmannFD’ArtistaLNecroptosis micro-environment directs lineage commitment in liver cancerNature20185627725697530209397
- FanBMalatoYCalvisiDFCholangiocarcinomas can originate from hepatocytes in miceJ Clin Invest201212282911291522797301
- MillerGSocciNDDhallDGenome wide analysis and clinical correlation of chromosomal and transcriptional mutations in cancers of the biliary tractJ Exp Clin Cancer Res2009286219435499
- RuzzenenteAFassanMConciSCholangiocarcinoma heterogeneity revealed by multigene mutational profiling: clinical and prognostic relevance in surgically resected patientsAnn Surg Oncol20162351699170726717940
- Chan-OnWNairismägiMLOngCKExome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancersNat Genet201345121474147824185513
- GouveiaMJPakharukovaMYLahaTInfection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent modelCarcinogenesis201738992993728910999
- van BeersBEDiagnosis of cholangiocarcinomaHPB (Oxford)2008102879318773062
- SinakosESaengerAKKeachJKimWRLindorKDMany patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinomaClin Gastroenterol Hepatol20119543443921334457
- BridgewaterJGallePRKhanSAGuidelines for the diagnosis and management of intrahepatic cholangiocarcinomaJ Hepatol20146061268128924681130
- NakeebAPittHASohnTACholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumorsAnn Surg199622444634758857851
- FuruseJOkusakaTBridgewaterJLessons from the comparison of two randomized clinical trials using gemcitabine and cisplatin for advanced biliary tract cancerCrit Rev Oncol Hematol2011801313921094052
- LuviraVNilpraphaKBhudhisawasdiVPugkhemAChamadolNKamsa-ArdSCholangiocarcinoma patient outcome in northeastern Thailand: single-center prospective studyAsian Pac J Cancer Prev201617140140626838246
- ButthongkomvongKSirachainanEJhankumphaSKumdangSSukhontharotOUTreatment outcome of palliative chemotherapy in inoperable cholangiocarcinoma in ThailandAsian Pac J Cancer Prev20131463565356823886146
- GilEJohJ-WParkHCYuJIJungSHKimJMPredictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: a retrospective studyWorld J Surg Oncol2015131125622525
- LozadaMEChaiteerakijRRobertsLRScreening for hepatocellular carcinoma and cholangiocarcinoma: can biomarkers replace imaging?Curr Hepatol Rep201514212813826328266
- MahasarakarmO2007An introduction to the Mekong fisheries of Thailand Technical Report 5Mekong River CommissionVientiane, Lao PDR
- JongsuksuntigulPImsomboonTOpisthorchiasis control in ThailandActa Trop200388322923214611877
- KhuntikeoNChamadolNYongvanitPCohort profile: cholangiocarcinoma screening and care program (CASCAP)BMC Cancer20151545926054405
- PinlaorSSripaBSithithawornPYongvanitPHepatobiliary changes, antibody response, and alteration of liver enzymes in hamsters reinfected with Opisthorchis viverriniExp Parasitol20041081–2323915491546
- AsavarutPNorsworthyPJCookJTaylor-RobinsonSDHarrisonRVDiet and disease: transgressing boundaries between science and society—understanding neglected diseases through the lens of cultural studies and anthropologyMed Humanit201642318118327279641
- ChamadolNPairojkulCKhuntikeoNHistological confirmation of periductal fibrosis from ultrasound diagnosis in cholangiocarcinoma patientsJ Hepatobiliary Pancreat Sci201421531632224420706
- WongkhamSSilsirivanitAState of serum markers for detection of cholangiocarcinomaAsian Pac J Cancer Prev201213Suppl172723480761
- WishartDSEmerging applications of metabolomics in drug discovery and precision medicineNat Rev Drug Discov201615747348426965202