Abstract
Purpose
We collected data from an online survey of 200 of our patients, which evaluated the efficacy of dapsone (diaminodiphenyl sulfone, ie, DDS) combined with other antibiotics and agents that disrupt biofilms for the treatment of chronic Lyme disease/post-treatment Lyme disease syndrome (PTLDS). We also collected aggregate data from direct retrospective chart review, including laboratory testing for Lyme, other infections, and associated tick-borne coinfections. This helped us to determine the frequency of exposure to other infections/coinfections among a cohort of chronically ill Lyme patients, evaluate the efficacy of newer “persister” drug regimens like DDS, and determine how other infections and tick-borne coinfections may be contributing to the burden of chronic illness leading to resistant symptomatology.
Patients and methods
A total of 200 adult patients recruited from a specialized Lyme disease medical practice had been ill for at least 1 year. We regularly monitored laboratory values and participants’ symptom severity, and the patients completed the online symptom questionnaire both before beginning treatment and after 6 months on DDS combination therapy (DDS CT). Paired-samples t-tests and Wilcoxon signed-rank nonparametric test were performed on each of eight major Lyme symptoms, both before DDS CT and after 6 months of therapy.
Results
DDS CT statistically improved the eight major Lyme symptoms. We found multiple species of intracellular bacteria including rickettsia, Bartonella, Mycoplasma, Chlamydia, Tularemia, and Brucella contributing to the burden of illness and a high prevalence of Babesia complicating management with probable geographic spread of Babesia WA1/duncani to the Northeast. Borrelia, Bartonella, and Mycoplasma species, as well as Babesia microti had variable manifestations and diverse seroreactivity, with evidence of persistence despite commonly prescribed courses of anti-infective therapies. Occasional reactivation of viral infections including human herpes virus 6 was also seen in immunocompromised individuals.
Conclusion
DDS CT decreased eight major Lyme symptoms severity and improved treatment outcomes among patients with chronic Lyme disease/PTLDS and associated coinfections.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Lyme disease is the number one vector-borne illness in the United States. There has been a 320% increase in the number of US counties affected within the past 20 years,Citation1 as well as an alarming threefold increase in the number of vector-borne disease cases reported to public health authorities from 2004 to 2016.Citation2 The May 1, 2018 edition of the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report found that of the almost 650,000 reported cases during a 12-year period, over 491,000 were tick-borne and over 150,000 were mosquito-borne.Citation3 Recently there was another 22% increase in tick-borne infections where “[c]ases of Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis (including Rocky Mountain spotted fever [RMSF]), babesiosis, tularemia, and Powassan virus disease all increased – from 48,610 cases in 2016 to 59,349 cases in 2017.”Citation4
These numbers may not however adequately represent the true public health burden. The underreporting of tick-borne diseases is a well-established healthcare phenomenon, and some states like New York who “estimate” cases are not counted in the federal numbers.Citation5 Those patients with symptoms of chronic Lyme disease, both diagnosed and undiagnosed, are also significantly contributing to the healthcare burden. They are known to be as functionally disabled as patients with chronic congestive heart failure according to prior NIH studies,Citation6 with 42% of affected individuals reporting that they stopped working.Citation7
The establishment of a new paradigm to account for why patients struggle with disabling symptoms after commonly prescribed treatments for chronic Lyme/post-treatment Lyme disease syndrome (PTLDS) is of vital importance based on the significant numbers of individuals contracting vector-borne diseases, including multiple Borrelia sensu lato species, relapsing fever borrelia, and other tick-borne infections. A chart review of a large cohort of chronically ill Lyme patients was therefore undertaken to better define the role of multiple variables on the 16-point Multiple Systemic Infectious Disease Syndrome (MSIDS) map and the role of overlapping infections/tick-borne coinfections in those suffering from resistant symptoms of chronic Lyme disease/PTLDS.
In part I of this observational study, we collected data from an online survey of 200 of our patients. They volunteered to participate in a study that evaluated the efficacy of diaminodiphenyl sulfone, that is, dapsone (DDS) combined with other antibiotics and agents that disrupt biofilms for the treatment of chronic Lyme disease/PTLDS. DDS combination therapy (DDS CT) had previously been published to be effective as a novel “persister” drug regimen for the treatment of chronic Lyme disease/PTLDSCitation8 in 100 patients who had previously failed commonly prescribed antibiotic therapies. We also collected aggregate data from direct retrospective chart review, which included laboratory testing for Lyme, other infections, and associated tick-borne coinfections. This helped us to determine the frequency of exposure to other infections/coinfections among a cohort of chronically ill Lyme patients, evaluate the efficacy of newer “persister” drug regimens like DDS, and determine how other infections and tick-borne coinfections may be contributing to the burden of chronic illness leading to resistant symptomatology.
Table 1 Percent severity ratings before dapsone and with dapsone combination therapy
Table 2 Frequency and percentage of positive CDC IgG and IgM Western blots with positive bands 31 and 34 kDa and a negative ELISA or negative C6 ELISA
Table 3 Babesia subtypes by demographic region in 200 Lyme disease patients
Patients and methods
Participants
Participants included in this retrospective chart review (N=200) were adults recruited from a specialized Lyme disease medical practice using email and telephone contacts. Although situated in the Northeastern United States, the medical practice attracts patients from all over the world. Of 200 participants, 67 (33.5%) were male, 133 (66.5%) were female. Age ranged from 18 to 84 years (M=52.04, SD =16.66). Out of 200 participants, four (2%) were Asian (Non-Hispanic), while the rest were White (non-Hispanic). Participants were mostly from the United States (N=193), which was divided into demographic regions: West Coast (N=1), Midwest (N=16), East Coast (North) (N=155), East Coast (South) (N=20), and Other (Hawaii) (N=1).
The majority of patients enrolled in the study had been ill for at least 1 year and had been treated by multiple health-care providers failing traditional antibiotic therapy for Lyme disease, which included but was not limited to tetracyclines, macrolides, penicillins, and cephalosporins. Potential participants were sent an email invitation containing a link to the online survey. After clicking the link, the participants viewed a page that explained the purpose of the survey and provided information necessary for them to make an informed decision about whether to participate or not. All survey participants were identifiable in order to compare their survey responses against their clinical record.
Methodology
Institutional Review Board approval was not required for this research since this was a retrospective review of patients’ charts who were previously undergoing treatment for tick-borne illness under the care of the first author. After signing informed consent forms that outlined the proposed benefits and potential risks of our study, patients volunteered to enroll in a preliminary DDS trial at our medical center based on the drug’s action on “persister” bacteria.Citation9–Citation12 There were two treatment arms: those patients on a tetracycline, rifampin, and DDS and those who were also placed on a cephalosporin (cefuroxime axetil or cefdinir) or a macrolide (clarithromycin or azithromycin). The choice of treatment was based on their prior clinical response to these medications (cephalosporins and or macrolides) and/or their coinfection status. All patients were placed on at least three different probiotics for gastrointestinal (GI) support, with regular laboratory monitoring of complete blood counts (CBCs), comprehensive metabolic profiles (CMPs), and methemoglobin levels before, during, and after DDS therapy.
Blood testing for Lyme disease and coinfection status were conducted using several Clinical Laboratory Improvement Amendments-certified national reference laboratories (Quest Diagnostics, Secaucus, NJ, USA; LabCorp, Burlington, NC, USA; BioReference, Elmwood Park, NJ, USA; and AccuReference Medical Lab, Wappingers Falls, NY, USA), local state laboratories (ie, Sunrise Medical Laboratories, Hicksville, NY, USA; NorDx, Scarborough, ME, USA; and Affiliated Laboratory Inc., Rutland, VT, USA), several hospital-based laboratories, and specialty laboratories for tick-borne diseases (Imugen, Norwood, MA, USA; IgeneX, Palo Alto, CA, USA; MDL Laboratory, Hamilton Township, NJ, USA; The State University at New York at Stony Brook Lyme Disease Laboratory, Stony Brook, NY, USA; Milford Molecular Diagnostics, Milford, CT, USA; Galaxy Diagnostics, Morrisville, NC, USA; and Immunosciences Lab Inc, Los Angeles, CA, USA). More than one laboratory was used for each patient depending on the laboratory capability, patient insurance, and availability in their home state (Galaxy Diagnostics was not available in NY).
We monitored participants’ symptoms at each consultation (6–8 weeks), and the patients completed the online symptom questionnaire both before beginning treatment and after 6 months on DDS CT. The length of the trial varied, but those patients who continued to show clinical improvement remained on DDS CT after the 6-month period until they reached a plateau with no further improvement in symptoms. Paired-samples t-tests were performed on each of eight major Lyme symptoms with pre-DDS and DDS conditions, along with a Wilcoxon signed-rank nonparametric test to evaluate the small range of severity ratings.
Rigorous patient safeguards were in place, and each participant received detailed instructions that outlined the need for blood test every 3 weeks, dietary guidelines, and the name and phone number of the medical center’s head nurse if anyone had questions or medical issues. Email address of a medical staff as well as an emergency beeper number were also provided. Medication refills were only given if blood testing was up to date, and patients were contacted by the nursing staff if any abnormal laboratory results required a change in medication or for an updated appointment.
Surveys were conducted in office, online, and via telephone to gather patient information. The symptom questionnaire that the patients have completed is derived from a larger validated Lyme questionnaireCitation13 and the work of Shadick et al.Citation14 Symptom severity of eight symptoms was gathered both before beginning DDS CT and after 6 months of treatment, following a protocol from our previous study by Horowitz and Freeman on DDS.Citation8
Symptoms measured were:
Fatigue and/or tiredness
Muscle and/or joint pain
Headache
Tingling and/or numbness and/or burning of extremities
Sleep problems
Forgetfulness and/or brain fog
Difficulty with speech and/or writing
Day sweats and/or night sweats and/or flushing
Participants rated the severity of these symptoms both before DDS CT and after 6 months, to examine whether DDS CT decreased the severity of these symptoms. Data were gathered using a 5-point severity scale wherein patients indicated the severity of each symptom, where 1 represented no symptom, and 5 represented the most severe.
Inclusion criteria
All 200 patients in our retrospective chart review met the criteria for a clinical diagnosis of Lyme disease supported by a physician documented erythema migrans (EM) rash and/or positive laboratory testing, including a positive ELISA/enzyme immunoassay, and/or C6 ELISA, immunofluorescent antibody (IFA), Centers for Disease Control and Prevention (CDC) positive IgM and/or IgG Western blot (WB), PCR, Borrelia-specific bands (23, 31, 34, 39, 83/93) on a WB,Citation15 and/or positive ELISpot (lymphocyte transformation test [LTT]). These patients had either failed or had an inadequate response to prior antibiotic therapy and/or had relapsed with persistent symptoms after stopping anti-infective therapy.
Exclusion criteria
Patients under the age of 18 years, having a known allergy to DDS or any medication used in the trial, and/or having significant laboratory abnormalities including a pre-trial anemia were excluded from our study.
Surveys included
Surveys with questions regarding DDS status (currently or previously taking the drug), DDS dosage, coinfections, other drugs, side effects, and symptom severity for common Lyme symptoms before and after at least 6 months of DDS treatment (the full survey is available on request from the authors) were included.
Results
DDS status consisted of 53.70% currently taking DDS for at least 6 months, and 46.30% no longer taking DDS. Dosages included 25 mg (12%), 50 mg (23.5%), 75 mg (12%), and 100 mg (51.5%). DDS treatment is occasionally pulsed – instead of taking the medication daily, it can be taken 3 days a week, or every other day, and so on, depending on the severity of Herxheimer reactions with regular use. About 20.5% of participants indicated that their DDS treatment was pulsed. Reasons for stopping DDS treatment were “My symptoms improved” (N=22), “I had an adverse reaction” (N=46), and “I switched to a different protocol” (N=22).
The percent severity rating for each symptom in pre-dapsone (pre-DDS) and DDS-treated patients are available in . Thirty-five cases were missing both pre-DDS and DDS severity data or either one. These cases were removed from this table and from t-test analyses leaving symptom severity data for 165 patients.
In order to analyze this, paired-samples t-tests were performed on each symptom with pre-DDS and DDS conditions of: fatigue and/or tiredness: t(164)=10.69, P<0.001, muscle and/or joint pain: t(164)=8.13, P<0.001, headache: t(164)=5.35, P<0.001, tingling and/or numbness and/or burning of extremities: t(164)=6.71, P<0.001, sleep problems: t(164)=6.17, P<0.001, forgetfulness and/or brain fog: t(164)=9.84, P<0.001, difficulty with speech and/or writing: t(164)=8.70, P<0.001, and day sweats and/or night sweats and/or flushing: t(164)=8.36, P<0.001.
A Wilcoxon signed-rank nonparametric test (which was run due to the small range of severity ratings) showed a statistically significant change in severity ratings of the same eight symptoms for pre-DDS and DDS conditions. Results indicated that for fatigue and/or tiredness: Z=−8.624, P<0.001, muscle and/or joint pain: Z=−7.295, P<0.001, headache: Z=−5.587, P<0.001, tingling and/or numbness and/or burning of extremities: Z=–7.302, P<0.001, sleep problems: Z=−6.363, P<0.001, forgetfulness and/or brain fog: Z=–8.169, P<0.001, difficulty with speech and/or writing: Z=−7.873, P<0.001, day sweats and/or night sweats and/or flushing: Z=–8.081, P<0.001.
These results further confirm that patients had a significant change in all eight chronic Lyme symptoms ().
We used two antibiotic combinations (these were the only combinations with similar group sizes, an assumption which must be met to do statistical analyses) to evaluate which combinations were the most effective:
DDS and a tetracycline with rifampin
DDS and a tetracycline with rifampin and a cephalosporin or a macrolide
(Most people were on rifampin, having one group with rifampin and another without would have given us grossly disproportionate group sizes).
There was no significant difference between the two groups, and both groups were effective in decreasing symptomatology. Further detailed data analysis will be necessary to determine if the addition of a cephalosporin and/or a macrolide temporarily improved symptoms (we often rotate antibiotics during the clinical course based on a patient’s response). Unpublished scientific research done by Dr Eva Sapi’s team at the University of New Haven evaluated DDS CTs in culture, and their effect on lowering Borrelia biofilm mass. These results were presented as a poster presentation at the ILADS 16th International Lyme Conference in 2016 in BostonCitation16 and showed that DDS combined with doxycycline, rifampin and stevia was the most effective three drug/herbal biofilm combination therapy, confirmed in our clinical study. Further translational clinical research, moving from the laboratory to the bedside will be necessary however to evaluate the efficacy of one regimen over another, especially in patients with associated coinfections. We were also not able to differentiate changes in symptom outcome based on different DDS doses, due to inadequate numbers of individuals in each dosage group.
Before rating the severity of their symptoms via questionnaire, patients were asked for a pre-DDS and DDS “Percent Normal” (on a 100-point scale) apart from evaluating their eight hallmark symptoms of Lyme disease. These data come from symptom ratings collected via an online Surveymonkey. com questionnaire. (https://www.surveymonkey.com/). Success in the DDS trial was operationally defined as improvement in percent of normal after 6 months on DDS. Failure was operationally defined as remaining the same or worsening of percentage of normal after at least 6 months of DDS CT. A paired-samples t-test demonstrates a significant difference from pre-DDS to DDS conditions: t(180)=12.83, P<0.001 (pre-M=51.34%, DDS M=65.85%). Of 181 participants who gave both pre-DDS and DDS percentage scores, 14 participants reported feeling worse currently than they did before the DDS, 22 participants reported no difference, while all other participants (145) currently reported a higher percentage of normal. Of the 145 participants who reported higher percentage of normal for current symptoms (some participants did not answer this question), difference in percentage ratings ranged from 2% to 75% (M=16.09, SD =13.52).
Side effects of DDS
There are four common side effects of DDS, which need to be monitored to minimize reactions and “do no H.A.R.M.” (ie, Herxheimer reactions, Anemia, Rashes, Methemoglobinemia). Herxheimer reactions represent inflammatory cytokine release (ie, tumor necrosis factor-alpha, interleukin-6 [IL-6], and IL-8) associated with bacterial killing.Citation17,Citation18 Anemia is secondary to the drugs’ effect on folic acid metabolism, but could potentially be due to hemolytic anemia if there was glucose-6-phosphate dehydrogenaseCitation19 deficiency (which was ruled out in our patients). Rashes may be due to sulfa sensitivity (although many patients allergic to sulfamethoxazole/trimethoprim tolerate DDS), and methemoglobinemiaCitation20,Citation21 is due to increased oxidative stress affecting the ability of hemoglobin to efficiently carry oxygen. Elevated methemoglobin levels can result in increased fatigue, headaches, blue hands, and lips as well as shortness of breath. About 66.70% of participants experienced Herxheimer reactions, 48.30% anemia, 7% rashes (which were mild), and 18.4% elevated methemoglobin levels with 3.5% experiencing blue hands, 3% blue lips, and 21% shortness of breath. Shortness of breath may have also been due to active and inadequately treated babesiosis, which can cause cough/and shortness of breath with respiratory distress. Side effects resolved quickly once stopping the drug and using higher doses of folic acid to reverse anemia, and glutathione with N-acetyl-cysteine to lower elevated methemoglobin levels,Citation22 based on the published scientific literature and the first author’s clinical experience. Methylene blue can also lower methemoglobin levels.Citation23 Minor side effects of DDS included temporary elevations in bilirubin,Citation24 especially in those with Gilbert’s syndrome.Citation25
No patients suffered any long-term effects or developed Clostridium difficile during the months of DDS CT. All patients were instructed to take a minimum of three different probiotics with a total of over 100 billion live organisms/day, which contained mixtures of prebiotics (lecithin and oleic acid), lactobacilli (L. rhamnosis, L. acidophilus, L. paracasei), bifidobacterium (B. lactis, strains BL-04 and Bi-07), and Saccharomyces boulardii. It has been published in the scientific literature that these strains significantly decrease the incidence of antibiotic-associated diarrhea and support a healthy intestinal environment and immune health, although only S. boulardii has been shown to be effective for the prevention of C. difficile diarrhea.Citation26–Citation29
Lyme disease testing
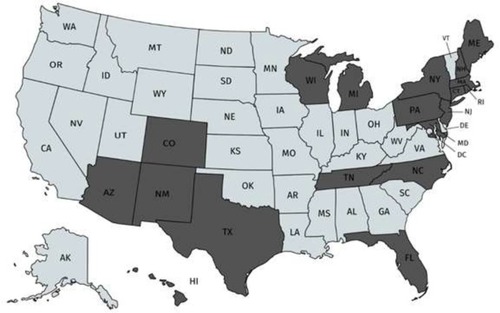
Our study showed that the most common IgM and IgG Borrelia-specific bands were 23, 31, 34, 39, 83/93 kDa for patients during their treatment in our medical facility, which occasionally spanned years ( and ). A chart review revealed that these bands changed and/or expanded over time.
Figure 1 Percentage of 200 patients with IgM Western blot band(s) over the course of treatment.
Abbreviation: IgM, immunoglobulin M.
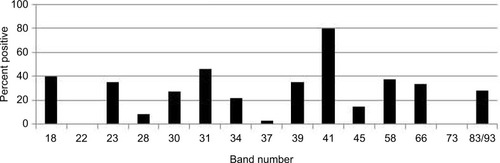
Figure 2 Percentage of 200 patients with IgG Western blot band(s) over the course of treatment.
Abbreviation: IgG, immunoglobulin G.
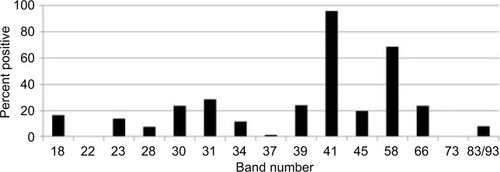
Indeterminate WB bands (weak positives) were not included in our study to eliminate false positives and increase sensitivity. Most patients studied did however have new indeterminate Borrelia-specific WB bands (23, 31, 34, 39, 83/93 kDa) appear and change over time during their illness. compares the number of individuals with EM rashes, positive CDC criteria WBs (IgM and IgG), and positive Lyme testing by IFA, ELISA, C6 ELISA, and ELISpot (LTT). Only a small number of patients were tested using LTT, which were done by other medical providers prior to enrolling in the study. Positive CDC WBs were defined as at least 2/3 reactive IgM WB bands (23, 39, 41 kDa) and a positive CDC IgG WB was defined as having at least 5/10 IgG WB bands (18, 23 [OspC], 28, 30, 39 [BmpA], 41 [Fla], 45, 58, 66, and 83/93 kDa; ).
Figure 3 Percentage of patients with EM rashes and positive Lyme testing.
Abbreviations: CDC, Centers for Disease Control and Prevention; EM, erythema migrans; IFA, immunofluorescent antibody; IgM, immunoglobulin M; LTT, lymphocyte transformation test.
Repeat WB testing during treatment showed that the 31 kDa band was positive on the IgM WB 46.2% of the time (), and positive on the IgG WB 28.4% of the time (). For the IgM WB, the 34 kDa band appeared 21.5% of the time and was positive on the IgG WB 11.7% of the time. shows the frequencies of negative ELISA and C6 ELISA with the presence of bands 31 and 34 kDa and negative/positive CDC IgG and IgM WBs ().
Co-infections
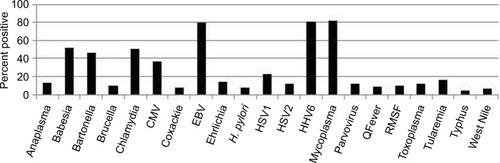
Many Lyme patients in our study struggled with other tick-borne illnesses. For the current work, we included information on the following tick transmissible infections as well as other infectious agents that participants may have been exposed to during their lifetime (unrelated to tick transmission). These were collected directly from the patient charts using indirect testing, that is, antibody titers (which indicate prior exposure, but not necessarily active infection) and direct testing, that is, PCR, fluorescent in situ hybridization (FISH) (which are indicative of active infection): Anaplasma, (N=27, 13.5%), Babesia (Babesia microti and Babesia duncani [N=104, 52%]), Bartonella (B. henselae and B. quintana [N=93, positive by titer, PCR, FISH, and/or VEGF, 46.5%]), Brucella (N=20, 10%), Chlamydia pneumoniae (N=102, 51%), cytomegalovirus (N=74, 37%), Coxsackie, (N=15, 7.5%) Epstein–Barr virus (EBV) (N=160, 80%), Ehrlichia (N=29, 14.5%), Helicobacter pylori (N=15, 7.5%), Herpes simplex virus (HSV1) (N=46, 23%), HSV2 (N=23, 11.5%), human herpes virus 6 (HHV6) (N=162, 81%), Mycoplasma (M. pneumonia, M. fermentans, and M. penetrans [N=164, 82%]), Parvovirus (N=23, 11.5%), Q-Fever (Coxiella burnetti [N=17, 8.5%]), RMSF (Rickettsia [N=20, 10%]), Toxoplasmosis (N=23, 11.5%), tularemia (N=33, 16.5%), typhus (N=10,5%), and West Nile Virus (N=13, 6.5%). Participants tested positive for exposure to between 0 and 16 infections (M=5.87, SD=2.29). In addition to their Lyme disease, of 200 participants, 0.5% had no evidence of other infections/coinfections, 26% had evidence of prior exposure to 2–4 infections/coinfections, 64% had evidence of prior exposure to 5–8 infections/coinfections, 8% had evidence of prior exposure to 9–12 infections/coinfections, and 1.5% had evidence of exposure to >12 infections/co-infections. illustrates the high frequency of tick transmissible infections and other infectious agents in our 200 patients, most notably for Babesia, Bartonella, C. pneumoniae, EBV, HHV6, and mycoplasma.
Figure 4 Percent positive testing for coinfections over the course of treatment among 200 Lyme disease patients. These results demonstrate a high percentage of co-infections.
New findings: co-infections are the rule and several tick species have spread
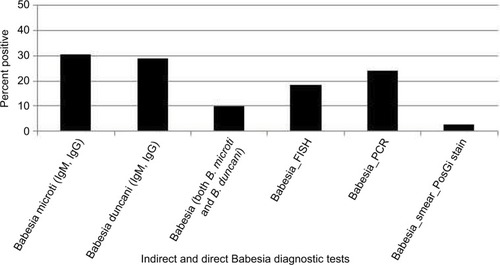
New findings include evidence of more than one co-infection in the majority of the 200 patients () and serological evidence of not only B. microti (N=51, 25.5%) but also B. duncani (N=56, 28%). Some patients had evidence of antibody titers for both the species (N=20, 10%; ). Out of 32 patients who were seronegative for B. microti and/or B. duncani, 37.5% were positive by direct testing (PCR, FISH). One was PCR positive (3%) and 11 were FISH positive (34.5%), illustrating the need for a broad screening approach to detect babesiosis.
Figure 5 Indirect and direct Babesia diagnostic testing among 200 patients with Lyme disease.
Abbreviations: FISH, fluorescent in situ hybridization; PosGi, positive Giemsa.
B. duncani babesiosis has been reported to exist primarily in the Western United States, although the spread of WA-1 babesiosis has occasionally been found in diverse geographic areas of the United States.Citation30 Based on positive antibody titers for B. duncani from patients living in the Northeast, Upper Midwest, and Southwestern United States (; ), which were reported from several national and specialty laboratories (Quest Diagnostics, LabCorp, BioReference Laboratory, Medical Diagnostics Laboratory, and IgeneX), this finding implies that there has been a spread of B. duncani across the United States, and especially in the Northeastern United States. illustrates antibody-positive B. duncani cases by home state in 200 patients.
Discussion
The significant increase in Lyme disease cases during the past 20 yearsCitation12 highlights the imperative of performing and patients receiving an accurate diagnostic evaluation. To diagnose Lyme disease, physicians often rely on clinical presentation (the appearance of an EM rash) and/or two-tiered testing when a patient presents with a chronic fatiguing, musculoskeletal illness with cognitive difficulties. Schutzer et al recently reported limitations of serodiagnostic testing which have a number of shortcomings, including the inability to distinguish active infection, past infection, or reinfection.Citation31
In this retrospective chart review/observational study of 200 patients with tick-borne disorders, only 19.5% had evidence of an EM rash. Although prior studies indicate that EM occurs in 70%–80% of cases,Citation32 multiple variables including rash appearance and gender may influence accurate reportingCitation33,Citation34 and an updated study on the diagnosis and treatment of Lyme disease found it to be present in only a minority of cases (as low as 9%).Citation35 Some patients might not notice an EM rash. Our patient population also had a low incidence of positive IFAs (10 %), ELISAs (20.5%), and C6 ELISAs (10%). These are first-line tests that some clinicians use to determine if a WB is needed, regardless of CDC recommendations that surveillance case definitions should not be relied upon to make a clinical diagnosis.Citation36 Despite a low incidence of positive IFAs, ELISAs, and C6 ELISAs, we found that 11.5% had CDC-positive IgG WBs and 45% of patients had CDC-positive IgM WBs. Some clinicians choose to ignore the significance of a positive IgM WB in a patient with chronic Lyme symptoms, attributing it to a false-positive test, although the medical literature has shown that positive IgM blots can be found in late Lyme.Citation37 That was the case in our patient population who responded positively to DDS CT. A sizeable number of these patients with a chronic fatiguing, musculoskeletal, neurocognitive illness with a negative ELISA and/or C6 ELISA also had a significant percentage of Borrelia-specific bands (23, 31, 34, 39, 83/93 kDa) on their WB, pointing to probable exposure with a Borrelia species (B. sensu lato). A recent 2017 study by Embers et al using non-human primates showed that variable manifestations of Lyme disease can exist with diverse seroreactivity. After antibiotic treatment for disseminated infection, posttreatment persistence with intact, metabolically-active B. burgdorferi was found and may not be reflected by maintenance of specific antibody production by the host.Citation38
Diagnostic testing for Lyme is known to lack adequate sensitivity, specificity, and reliability,Citation39–Citation41 and false seronegativity has been extensively reported in the peer review medical literature.Citation42–Citation52 Many B. sensu lato species and relapsing fever borrelia (ie, Borrelia miyamotoi), as well as some species of Babesia, Bartonella, and Mycoplasma may also not be found on commonly used antibody testing.Citation53 For example, patients were oftentimes positive for one species of Babesia by antibody testing, but negative for another, or only were positive on PCR and/or FISH testing. In 32 of our patients who were antibody negative for either B. microti and/or B. duncani, 37% were positive by direct testing (one was PCR positive and eleven were FISH positive). One patient with Chronic Variable Immune Deficiency (CVID) with negative Lyme antibody testing (IFA, C6 ELISA), but with Borrelia-specific bands on a WB, was positive for antibodies against the relapsing fever borrelia, B. miyamotoi (GlpQ+). This presents a clinical dilemma for both healthcare providers and patients who are searching for answers for their chronic illness.
A recently validated symptom questionnaire for Lyme disease, the Horowitz Multiple Systemic Infectious Disease Syndrome Questionnaire (HMQ) may be useful to evaluate the pretest probability of a tick-borne disorder.Citation13 The HMQ was shown to demonstrate satisfactory psychometric properties, including construct validity, divergent validity, and predictive validity. Scores for the HMQ in the “likely” or “highly likely” categories should lead providers to do a broad panel of tick-borne testing. Testing should include not only an ELISA, which has limited sensitivityCitation41 but also a C6 ELISACitation54 and IgM/IgG WBs, focusing on the presence of Borrelia-specific bandsCitation15 to capture the varied species of Borrelia. B. sensu latoCitation55 and relapsing fever BorreliaCitation56 which are now known to also cause chronic illness.
The presence of Borrelia-specific bands helped to confirm the clinical diagnosis of Lyme disease after other diseases had been ruled out.Citation15,Citation57 These bands include the 23 kDa, Osp C (21–24 kDa), 31 kDa (OspA), 34 kDa (OspB), 39 kDa and 83/93 kDa bands. The 41 kDa and Borrelia proteins of the 60–75 kDa range (especially 66 kDa) are non-specific and can cross-react with non-borrelial antigens.Citation58,Citation59 New Borrelia-specific bands appearing over time in individuals with ongoing symptoms (especially migratory pain) was suggestive of an active infection in the proper clinical setting, and several patients were also PCR-positive despite seemingly “adequate” antibiotic therapy for months or years prior to DDS therapy (N=29, 14.5%). Some patients (16.8%) also had evidence of exposure to Borrelia hermsii, a relapsing fever borrelia, although false seropositivity due to an infection with Borrelia burgdorferi could not be ruled out.Citation59 B. hermsii and B. burgdorferi infections produce antibodies that also cross-react with B. miyamotoi antigens, making the serodiagnosis difficult.Citation60
A 2018 publication by Strobino et alCitation61 indicated that for convalescent EM, combinations of the C6 ELISA with a second-tier ELISA or line blot may provide useful alternatives to WB-based testing algorithms, but this needs to be confirmed in larger clinical studies. For those without an EM rash (and not meeting the definition of PTLDS), the two-tiered testing strategy for diagnosing B. burgdorferi misses a large proportion of cases,Citation62 and this approach cannot diagnose new species of Borrelia, including B. miyamotoi and B. burgdorferi sensu lato, which are also known to cause chronic illness.Citation63,Citation64 These infections are spreading across the United States and Europe. Recently, B. miyamotoi was found to have a higher prevalence of infection in Ixodes pacificus, western black-legged ticks compared to its eastern counterpart, and other tick species not currently recognized as competent vectors (Ambylomma americanum and Arum maculatum) were also observed to have B. miyamotoi infections.Citation65 Although there can be a great deal of heterogeneity in the protein patterns among B. sensu lato species, proteins OspA (31 kDa) and OspB (34 kDa) have been reported (plus OspA at 33 kDa),Citation66 as well as OspC (23 kDa), 39 kDa, and 83 kDa.Citation67 These are the same proteins we frequently found in Lyme-MSIDS patients in our study ( and ). Non-specific antibody can be present in sera and bind to a protein that co-migrates to the same position on a WB as OspA.Citation68 Although this protein can be found in certain viral infections like EBV, Lyme immunoblots with recombinant OspA may be a solution to improve testing specificity.Citation69
Osp A is not considered as a major antigen produced in mammals (early inhibition of OspA and OspB is needed for vertebrate infection, since B. burgdorferi mutants that express OspA and Osp B are rapidly killed by host immune responses), but the protein appears to be produced in some patients with chronic Lyme symptoms.Citation70 These proteins have been linked to more severe arthritic and neurological diseases (encephalopathy, myelopathy, and peripheral neuropathy) with autoimmune manifestations.Citation71–Citation73 Repeat IgM and IgG WB testing during treatment showed that the 31 kDa band was positive 46.2% and 28.4% of the time, respectively. For the IgM WB, the 34 kDa band appeared 21.5% of the time and was positive on the IgG WB 11.7% of the time. Unfortunately, not all laboratories report the full spectrum of bands since OspA and OspB are not part of the CDC surveillance criteria for either a positive IgM or IgG WB. These bands can be specific for exposure to multiple Borrelia species assuming there has been no recent viral infection or Lyme vaccination. The serological findings of patients in our study showed a considerable number with negative ELISA and C6 ELISA test results (), but were positive on OspA (31 kDa) and OspB (34 kDa) bands on a WB, as shown in and . We therefore suggest that these bands be reported and followed in all WB specimen results to inform the healthcare provider as to the probability of exposure and active infection in chronic disease. This is especially important based on the spread of new Borrelia species in the United States and Europe, where the ability to cause EM differs between B. burgdorferi sensu lato isolates.Citation74
Most of our 200 patients (N=165) statistically improved their eight major Lyme symptoms on DDS CT (P<0.001) despite failing or relapsing on prior therapy. Patients served as their own controls. Resistant fatigue, myalgias, joint and nerve pain, headaches, sleep and cognitive disorders all improved, as well as malarial-like symptoms (day and night sweats, chills, flushing). The majority also had evidence of exposure to multiple infections/coinfections. These included Anaplasma (13.5%), Ehrlichia (14.5%), Babesia (52%), Bartonella (46.5%), Mycoplasma (82%), RMSF (10%), Q-Fever (8.5%), typhus (5%), tularemia (16.5%), Brucella (10%), C. pneumoniae (N=102, 51%), and H. pylori (7.5%). Viral infections included HSV1 (23%), HSV2 (11.5%), HHV6 (81%), parvovirus (11.5%), and West Nile virus (6.5%). Parasites found were toxoplasmosis, as well as B. microti and B. duncani. Inherent limitations of our testing strategies (which were done primarily using antibody and PCR technology) interfered however with our ability to detect multiple species of coinfections that could be causing symptoms yet would not be found on commonly used testing. For example, some patients with Babesia were seronegative and only positive with RNA testing, patients were not routinely tested for all species of Mycoplasma (including M. fermentans and M. penetrans), and although a considerable percentage of our patients tested positive for Bartonella henselae, there are at least 38 different species and subspecies of Bartonella (and proposed Candidatus species), some of which have been associated with human disease.Citation75 We did not have access in our NY clinic to specialty laboratories that are able to evaluate a broader range of bartonella infections unless done in other states. As per the Report of the Other Tick-Borne Diseases and Coinfections Subcommittee to the Tick-Borne Disease Working Group posted on the Health and Human Services (HHS) website on May 4, 2018,Citation76 Bartonella species can also be extremely difficult to diagnose by clinicians, as there is evidence that they hide in the intracellular compartment. Without broad-based sensitive serology, testing can be negative. The sensitivity of confirming a diagnosis by direct detection also continues to be an area that needs improvement in other tick-borne diseases and co-infections. Therefore, the true number of individuals with Babesia and Bartonella infections and less frequently diagnosed tick-borne infections/coinfections like RMSF, Q-fever, tularemia, and Brucella needs to be confirmed in future studies on PTLDS/chronic Lyme disease. Low positive titers for these infections could be the result of cross reactivity with other rickettsial and intracellular infections, although some individuals reactivated their infections over time with fourfold increases in titers, or positive agglutination testing that is, Brucella. We found a 10% incidence of positive titers to RMSF (N=20), and as per the HHS report, “about 10% of people in the United States (and worldwide) have pre-existing spotted fever group rickettsiae (SFGR)-specific antibodies. Thus, a healthcare provider might treat a patient with a positive reaction to a Rocky Mountain spotted fever (RMSF) serological test for RMSF when they should be looking at another cause of the patients’ disease… This illustrates the power and limitations of serology and the need for paired tests, which cannot definitively confirm or rule out the presence of infection.”Citation76
The considerable number of B. duncani antibody tests (N=175, 28%) discovered in residents living in the Northeast was unexpected. False-positive serology to B. duncani is possible, which can be associated with acute EBV infection, but all the patients in our study with EBV had prior exposure, without evidence of an active infection (by PCR). Only one patient was EBV PCR positive in 2003, who was also HHV-6 PCR positive and B. microti antibody negative at that time, whose B. duncani/WA-1 titer turned positive 10 years later in 2013. At that point in time, she was EBV and HHV-6 PCR negative with decreasing EBV capsid IgG antibody titers. It is therefore not likely that B. duncani/WA-1 antibody reactivity in any of our patients was attributable to polyclonal B-cell activation from viral infections. Similarly, although travel to the West/Pacific Coast cannot be ruled out as a potential cause of exposure of B. duncani, two recent publications in 2018 reported range expansion of human babesiosis in the northeastern United States,Citation77 as well as widespread distribution of B. duncani across Canada.Citation78 A considerable proportion of those cases (67.7%) reported by Scott et al in 2018 were found in Quebec, New Brunswick, Newfoundland, and Nova Scotia, on the eastern Canadian seaboard close to Maine. Seven percent of the 200 patients in our study lived in Maine and tested positive for B. duncani. This implies spread of the babesia parasite by migratory songbirds,Citation79,Citation80 although it is also possible that the original discoverers of Babesia WA-1 (later termed B. duncani) failed to recognize its presence in other geographic areas. Other tick-borne infections are known however to be spread through migratory birdsCitation81–Citation83 such as B. sensu lato spp. in northwestern California,Citation84,Citation85 and rickettsia fever was spotted throughout Europe.Citation82
New ticks and tick-borne infections can appear in areas where they were not previously recognized. That was the case for the Asian longhorn tick (Haemaphysalis longicornis), foreign to the United States, which was recently found in New Jersey,Citation86 Virginia, West Virginia, Arkansas, and North CarolinaCitation87 as well as Connecticut and New York.Citation88 This tick is known to be a competent vector for a phlebovirus causing severe fever with thrombocytopenia syndrome, as well as being able to transmit the alpha gal allergy in other parts of the world.Citation89 Although these infections and tick-borne disorders have not yet been associated with H. longicornis in the United States (the primary cause of alpha gal allergy in the United States is the lone star tick, A. americanum),Citation90 enhanced screening and prevention practices are necessary. One of the patients we evaluated from the Southern United States had a borderline positive IgE antibody against galactose-a-1, 3-galactose (alpha-gal) with symptoms consistent with alpha gal meat allergy (delayed urticarial, GI disorder symptoms, and/or airway angioedema minutes to hours post ingestion of meat or gelatin capsules containing BSA).
Tick species are expanding in areas not previously seen (lone star ticks have expanded from the Southeastern States to Long Island, NY, USA leading to increasing cases of alpha gal allergy), and in 2018 seroprevalence to other flaviviruses like the Powassan virus was shown to be increasing in Lyme endemic areas across the United States.Citation91 Thirty three viruses, including 24 putative novel viral species, were also recently discovered in Ixodes scapularis, A. americanum, and Dermacentor variabilis ticks collected in New York, Connecticut, and Virginia in 2018.Citation92 Although the role of these viruses has not yet been determined, ticks containing multiple tick-borne infections including bacteria, viruses, and parasites (Babesia spp.) are increasing and require ongoing surveillance, especially since multiple tick-borne infections like Babesia also pose a risk for the blood supplyCitation93 along with the possibility of maternal–fetal transmission.Citation94
Increasing co-infection of blacklegged ticks with Babesia spp. and B. burgdorferi is expected in the United States as reported by researchers at the Cary Institute of Ecosystems Studies in New York.Citation95 Based on our study, B. duncani may have spread to areas not previously recognized, just as exposure to the Powassan virus has been shown to be increasing. Since “there have only been 13 B. duncani (WA-1 babesiosis) cases for which incontrovertible evidence of the disease etiology (direct detection of the agent by blood smear or PCR; hamster inoculation; in vitro culture) has been reported and accepted by the Centers for Disease Control and Prevention…,”Citation76 our finding needs to be confirmed in larger clinical studies, using well-validated samples. Many patients who were B. microti negative and B. duncani positive with malarial like symptoms (fevers, sweats, chills, flushing, “air hunger” with an unexplained cough) did improve with Babesia therapy (atovaquone, atovaquone/proguanil, azithromycin, and clindamycin). B. duncani testing should therefore be considered in any patient with an unexplained fatiguing, febrile illness and suggestive lab testing (anemia, thrombocytopenia, and transaminitis) living in the northeastern United States, who becomes ill after a tick bite or blood transfusion. Increased morbidity and mortality from babesiosis can be seen in the very young,Citation96 elderly, immunosuppressed,Citation97 (and asplenic) as well as in those with associated comorbidities.Citation98
Other testing to consider
A CBC and CMP to look for the presence of leukopenia, thrombocytopenia, and transaminitis should be performed in patients with suspected tick-borne infections, as these can occur with Ehrlichia, Anaplasma, rickettsial infections,Citation99 as well as Borrelia species including B. miyamotoi.Citation100 Other tick-borne infections like babesiosis should also be considered in severely ill patientsCitation101 since it is frequently transmitted at the same time as borreliosis and can cause similar hematological abnormalities (leucopenia, thrombocytopenia, and transaminitis) as well as hemolytic anemia (Babesia divergens increases the risk).Citation102 Recent findings in the mouse model suggest that B. burgdorferi coinfection attenuates parasite growth while B. microti presence exacerbates Lyme disease-like symptoms.Citation103 Any patient who complains of unexplained fevers, day and night sweats, chills, flushing, an unexplained cough, and shortness of breath (air hunger), which are questions number 1 and number 22 (Section 1 of the HMQ), may have a concomitant infection with babesiosis.Citation102,Citation104,Citation105 Malarial-like symptoms can however be present with or without associated laboratory abnormalities, including leucopenia, thrombocytopenia, transaminitis, and hemolytic anemia, and patients who are co-infected and/or with low levels of parasitemia may not present with the classical symptoms of babesiosis.Citation53 Hematological abnormalities including transaminitis usually resolved with anti-infective therapy unless there were other underlying etiologies (one patient was found to have alpha-1-antitrypsin deficiency, and another was positive for hemochromatosis). A Babesia panel approach with a Giemsa stain, Babesia titers (IFA) for multiple species of Babesia (B. microti, B. duncani [WA-1], B. divergens, and Babesia sp. EU1), with PCRCitation106 and FISH testingCitation107 may help to establish the diagnosis in the United States and Europe,Citation108 while ruling out other causes of these symptoms. The Babesia PCR screen used in our study detected Babesia-specific DNA for B. microti and/or B. duncani. Several of our patients were FISH (RNA) positive (34.5%) while being antibody/PCR negative, highlighting the importance of a broad screening approach to detect babesiosis.
Other potential tick-borne transmitted infections, including BartonellaCitation109 and tularemia,Citation110 should also be considered in the differential diagnosis in those experiencing an unexplained chronic fatiguing, musculoskeletal illness, as well as brucellosis, which has been found in a small percentage of individuals from the Midwest United States diagnosed with chronic fatigue syndrome (CFS).Citation111 Approximately 46% of our patients tested positive for Bartonella species and 16.5% had positive antibody titers for tularemia. One patient in our study with an unexplained autoimmune disease (Behcet’s syndrome) who had failed almost 20 years of various disease modifying anti-rheumatic drug therapies initially had negative Bartonella titers, a low positive tularemia titer, and a negative VEGF, along with low positive HHV6 titers. After using DDS combination intracellular therapy, her Bartonella titer and VEGF turned positive for the first time in years, tularemia titers increased fourfold (1:320+) and HHV-6 titers similarly increased to 1:1,280, suggesting that this protocol unmasked occult seronegative intracellular infections.Citation112
The discovery of chronic HHV6, Bartonella, and Mycoplasma was confirmed in other patients in our study with Lyme disease. One patient with Lyme and B. henselae (IgG 1:64+) had a positive Bartonella PCR and two positive Lyme PCRs several years apart although they had been on commonly used antibiotic therapy for both infections. Another patient with Lyme disease, Anaplasmosis, and babesiosis (positive B. microti [IgM 1:16+], B. duncani [1:256+], Babesia PCR and FISH+) had three positive HHV6 PCRs, positive repeat Babesia FISH testing (despite atovaquone and azithromycin), as well as positive PCR testing for B. henselae and Mycoplasma species despite several years of combination intracellular antibiotic therapy for the above infections.
Intracellular co-infections like Mycoplasma have been found in patients with CFS, who were subsequently diagnosed with B. burgdorferi.Citation113 Most of the patients in our study showed exposure to Babesia, Bartonella, and Mycoplasma, with smaller numbers showing exposure to Anaplasma, Ehrlichia, Rocky Mountain Spotted Fever, Q-fever, tularemia, and brucella. In prior clinical studies, ~70% of chronic Lyme patients showed exposure to systemic Mycoplasma species infections, and the species predominantly found in Lyme patients was M. fermentans. Citation53M. fermentans has been found in ticks in Northern California, New York, and New Jersey,Citation114 and evidence for disseminated Mycoplasma fermentans in New Jersey residents with antecedent tick attachment and subsequent musculoskeletal symptoms has been published in the scientific literature,Citation114 although the role of Mycoplasma species and proof of vector competency of ticks still needs to be confirmed in larger clinical studies.
Once the diagnosis has been established, the advantage of using DDS in the setting of individuals exposed to Lyme and Babesia, other intracellular coinfections, with/or without associated autoimmune phenomenon, is that DDS has been shown to be effective for autoimmune illnesses like Behcets syndromeCitation10 while also being efficacious against borrelial and malarial type parasitic infections like Babesia.Citation8 Combining it with doxycycline and rifampin helps to broaden the intracellular coverage. These clinical findings (exposure to Borrelia, Babesia, and intracellular infections along with autoimmune phenomenon) were often present in chronically ill individuals suffering with tick-borne disease in our study. Well controlled studies are needed in the laboratory and clinical setting to establish the role of DDS CT, biofilms, and persisters in tick-borne illness.
We also did not control for a placebo effect. The three hallmarks of a placebo effect that influence treatment outcomes are the roles of conditioning, expectancy, and doctor–patient relationship.Citation115 Patients who had come to us from other medical practices would have been exposed to these same factors, yet their symptoms did not improve or only had short lived benefit to their treatment. Placebo effects are also typically short-lived phenomenon especially in pain,Citation116 and our patients had sustained musculoskeletal and neuropathic pain relief with DDS CT. Since more than 66% of our patients had Herxheimer reactions (and oftentimes improved afterward), a “nocebo” effect is also possible and cannot be ruled out. It is less likely however in the setting of chronic Lyme/PTLDS, since Herxheimer reactions are a well-established phenomenon secondary to antibiotics causing cytokine release.
One of the clinical challenges for healthcare providers and patients that emerged from our study is that Borrelia, Bartonella, and Mycoplasma species, as well as B. microti, were all shown to persist despite commonly prescribed courses of antibiotics or antimalarial/Babesia therapy. This has been confirmed in other scientific research studies.Citation64,Citation97,Citation111,Citation117–Citation128 One of the patients in our study even had evidence of persistent Babesia by both DNA (PCR) and RNA (FISH) analyses despite using clindamycin and quinine, as well as atovaquone and azithromycin. Although previously reported as far back as 1999 by Horowitz,Citation129 some of the immune and genetic etiologies responsible for persistence of the Babesia parasite were not determined until 2010 by WormserCitation130 as well as Lemieux et al in 2016Citation131 when mutations in the cytb and rpl4 genes of B. microti were discovered in patients with resistant infections. Research into more effective treatments for babesiosis are urgently needed.
Tick-borne bacterial infections including Lyme disease, bartonella species, rickettsia (Q-fever, Coxiella burnetti), tularemia, and mycoplasma species (M. fermentans) can exist as chronic infections located in the intracellular compartment, subsequently increasing inflammation. In our clinical study, the majority of patients showed exposure to M. pneumonia,Citation132 and five patients (2.5%) tested positive by PCR for M. fermentans, while two patients tested positive (1%) by PCR for M. penetrans (not all patients were evaluated for these subspecies). One of the patients in our study was PCR positive for M. fermentans three times consecutively over a 15-month period. In a prior retrospective study done in 2003, persistence of Mycoplasma species was seen for up to almost 1 year with single drug intracellular therapy,Citation128 which included the use of tetracyclines, macrolides, or quinolones. Another patient was Bartonella FISH (RNA) positive despite prolonged antibiotic therapy. Doxycycline, rifampin, and DDS CT along with Plaquenil (which alkalanizes the intracellular compartment and has an effect on round body forms)Citation133,Citation134 and grapefruit seed extractCitation135 (which also addresses cyst forms/cell wall deficient forms) combined with agents that disrupt biofilms (Stevia, oregano oil)Citation136 have the ability to affect different forms of multiple bacteria and parasites simultaneously, even if seronegativity exists. This triple intracellular antibiotic regimen produced statistical improvement in our group of patients with Lyme and associated coinfections, despite prior use of one or two drug regimens. Whether that combination is effective against the broad range of intracellular co-infections we discovered in our patients still needs to be confirmed in larger controlled studies.
When Lyme is combined with coinfections like Anaplasma, Babesia, and Bartonella, it is especially important to address factors interfering with normal immunity. Although most of our patients had comorbidities (ie, multiple coinfections, sleep disorders, nutritional deficiencies, and other abnormalities on the MSIDS map affecting inflammation), many of our patients also had evidence of immune deficiency. Thirty six of 175 patients tested had low IgG levels (20.6%), including 14 who had CVID; 45 patients had low IgG subclass 1 (N=163, 27.6%), 30 patients had low IgG subclass 2 (N=164, 18.3%), 51 patients had low IgG subclass 3 (N=164, 31.1%), and 14 patients had low IgG subclass 4 (N=164, 8.5%) with several patients having a poor response to a pneumococcal challenge (N=4, 7.8%). These immune deficiencies could impact both the production of positive antibodies and associated morbidity from babesiosis, and other infections like Anaplasma. Anaplasma can have adverse effects on the immune system, and 13.5% of our patients had positive antibodies against A. phagocytophilum. A. phagocytophilum uses multiple evasion strategies to inhibit neutrophil antimicrobial functions. One mechanism is its ability to inhibit the fusion of the lysosomes with the cytoplasmic vacuoles while arresting or inhibiting signaling pathways. Leukopenia and impaired function of neutrophils in patients with HGA can promote susceptibility to secondary and opportunistic infections. There is some evidence of immunosuppression in patients with HGA, and small numbers of our patients showed exposure to Anaplasmosis. During infection, A. phagocytophilum also upregulates the production of chemokine IL-8 as well as pro-inflammatory cytokines, contributing to its pathophysiological effects. We see similar increases in symptomatology when Lyme patients are exposed to Babesia and Bartonella, and other sources of inflammation found on the 16-point MSIDS map. These may include genetic causes of autoimmunity, environmental toxins, nutritional deficiencies, food allergies/sensitivities, imbalances in the microbiome with or without leaky gut,Citation137 and/or sleep disorders. These may all contribute to free radical/oxidative stress, further increasing inflammation and symptomatology with downstream effects.Citation38–Citation149
Chronic symptoms could therefore possibly overlap multifactorial causes on the MSIDS model. Fatigue for example could be due to infections, inflammation, autoimmunity, neuropsychiatric and/or sleep disorders, environmental toxins, mitochondrial dysfunction, nutritional deficiencies, food allergies/sensitivities, GI disorders and leaky gut, Hypothalamic-Pituitary-Adrenal (HPA) axis, autonomic nervous system dysfunction, as well as deconditioning; however these potential factors were often simultaneously or previously treated, and treating infections had significant positive effects. These factors will be discussed in detail in Precision Medicine: The Role of the MSIDS Model in Defining, Diagnosing and Treating Chronic Lyme Disease/PTLDS and Other Chronic Illness: Part 2.Citation150
Conclusion
Many of our patients infected with Lyme disease and associated coinfections had severe symptoms, often relapsed with commonly used therapies, and did not present with an EM rash nor meet the CDC two-tiered surveillance criteria. Almost two-thirds of patients had been exposed to between five and eight infections/coinfections and 14.5% of patients were PCR positive for B. burgdorferi despite seemingly “adequate” antibiotic therapy for months or years prior to DDS therapy (N=29, 14.5%). Evidence of persistent infection with HHV6, Bartonella, and/or Mycoplasma was also confirmed by PCR in several patients, although many in our study had evidence of other medical problems accounting for ongoing symptoms. These included associated immune dysfunction/immune deficiency, inflammation, environmental toxins with detoxification problems, GI problems, allergies, nutritional deficiencies, hormone, and autonomic nervous system dysregulation as well as sleep and psychiatric disorders in those suffering with post treatment Lyme symptoms.Citation151 None of these factors had been addressed in the three prior NIH randomized controlled Lyme trialsCitation6,Citation152,Citation153 nor the European PLEASE trialCitation154 and could explain in part why patients remained ill. Many patients with late Lyme disease in those trials failed conventional beta lactam, tetracycline, macrolide, or other antibiotic therapies even if given for 4–6 weeks. These included those patients with evidence of carditis, arthritis, and neuroborreliosis, including but not limited to symptoms of diplopia (3rd and 4th cranial nerves [CN]), Bell’s palsy (7th CN), deafness (8th CN), disequilibrium with ataxia (8th CN), tongue palsy (12th CN), dropped shoulder (11th CN), and foot drop or leg paralysis (spinal cord involvement). Part II of this retrospective chart review will therefore address in detail the abnormalities found on the MSIDS map in those suffering with chronic Lyme disease/PTLDS, which could account for resistant, chronic symptomatology. MSIDS is a precision medical model that considers genetic, environmental, and lifestyle factorsCitation155 as well as individual differences in infectious burden, customizing and selecting appropriate and optimal therapies, tailoring and targeting treatments for individual differences. The goal of precision medicine is to address the individual needs of patients, improve clinical outcomes, and minimize side effects.
The rising numbers of individuals suffering with Lyme and other long-term disabling illnesses alert us to a necessary shift in the paradigm for the diagnosis and treatment of chronic diseaseCitation149 and consideration of this precision medical approach. The MSIDS model and the use of persister drugs like DDS and pyrazinamide traditionally used for slow growing mycobacterial infectionsCitation9 represent new effective therapeutic options in tick-borne disorders. Multi-drug therapies for extended duration have been used for successful eradication of Q-fever,Citation156 mycobacterial diseases (ie, DDS with antituberculous drugs have been used effectively in leprosy regimens for half a century), as well as in other illnesses involving intracellular microorganisms like Brucella.Citation157 DDS CT along with several agents that disrupt biofilms decreased the severity of eight major Lyme symptoms in our study in those with PTLDS/chronic Lyme disease, along with diagnosing and adequately treating multiple species of intracellular bacteria. Bartonella, Mycoplasma, rickettsia, tularemia, and Brucella along with parasitic infections like Babesia and viral infections including HHV-6 were all likely sources contributing to the burden of illness. In summary, DDS CT, along with identifying and treating tick-borne co-infections, and abnormalities on the MSIDS map all affected chronic symptomatology and provides an expanded medical resource for those failing traditional therapy.
Acknowledgments
We thank our Hudson Valley Healing Arts Center research team: Haley Moss Dillon, Sonja Siderias, Heather Orza, and Renee Nelson for their assistance, as well as Aron G Wiegand, Egamaria Alacam, and Connor Duncan who assisted us with data input. We acknowledge with thanks the Bay Area Lyme (BAL) Foundation and the MSIDS Research Foundation (MRF) for providing us research grants for the data mining portion of this study. Dr Richard I Horowitz would also like to express his appreciation to his colleagues and subcommittee members on the HHS Tick-Borne Disease Working Group for their dedication and expertise in the diagnosis and treatment of tick-borne disorders. The views expressed are those of Dr Richard I Horowitz and do not represent the views of the HHS Tick-Borne Disease Working Group. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript; and in the decision to publish the results.
Disclosure
The authors report no conflicts of interest in this work.
References
- KugelerKJFarleyGMForresterJDMeadPSGeographic distribution and expansion of human Lyme disease, United StatesEmerg Infect Dis20152181455145726196670
- How many people get Lyme disease?Lyme diseaseCDC Available from: https://www.cdc.gov/lyme/stats/humancases.htmlAccessed February 13, 2018
- RosenbergRLindseyNPFischerMVital signs: trends in reported vectorborne disease cases — United States and Territories, 2004–2016MMWR Morb Mortal Wkly Rep2018671749650129723166
- Record number of tickborne diseases reported in U.S. in 2017CDC online newsroomCDC Available from: https://www.cdc.gov/media/releases/2018/s1114-record-number-tickborne-diseases.htmlAccessed December 19, 2018
- National Lyme STATs underestimate local cases Available from: https://uw-media.poughkeepsiejournal.com/video/embed/94-817864?sitelabel=reimagine&continuousplay=true&placement=uw-smallarticleattophtml5&keywords=pou-lyme-disease%2Cticks%2Ccenters-for-disease-control-and-prevention%2Clyme-disease%2Cepidemiology%2Chealth&simpleTarget=&simpleExclusion=disasters&pagetype=storyAccessed May 21, 2018
- KlempnerMSHuLTEvansJTwo controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme diseaseN Engl J Med20013452859211450676
- JohnsonLWilcoxSMankoffJStrickerRBSeverity of chronic Lyme disease compared to other chronic conditions: a quality of life surveyPeer J20142e32224749006
- I HorowitzRIHFreemanPRThe use of dapsone as a novel “persister” drug in the treatment of chronic Lyme disease/post treatment Lyme disease syndromeJ Clin Exp Dermatol Res20160703345
- ZhangYYewWWBarerMRTargeting persisters for tuberculosis controlAntimicrob Agents Chemother20125652223223022391538
- BarrJA short history of dapsone, or an alternative model of drug developmentJ Hist Med Allied Sci201166442546720966036
- HorowitzRIFreemanPThe use of dapsone as a novel “persister” drug in the treatment of chronic Lyme disease/post treatment Lyme disease syndromeJ Clin Exp Dermatol Res20160703345
- WHO Model prescribing information: drugs used in leprosy Available from: http://apps.who.int/medicinedocs/en/d/Jh2988e/Accessed May 27, 2018
- CiteraMFreemanPRHorowitzRIEmpirical validation of the Horowitz multiple systemic infectious disease syndrome questionnaire for suspected Lyme diseaseInt J Gen Med20171024927328919803
- ShadickNAPhillipsCBSanghaOMusculoskeletal and neurologic outcomes in patients with previously treated Lyme diseaseAnn Intern Med19991311291992610610642
- MaBChristenBLeungDVigo-PelfreyCSerodiagnosis of Lyme borreliosis by western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferiJ Clin Microbiol19923023703761537905
- FearnleyAGuptaKFreemanPRHorowitzRIEffect of Dapsone and its Antimicrobial Combinations on Borrelia burgdorferi BiofilmsPresented at: the ILADS 16th Annual Meeting2016Boston, MA
- ButlerTThe Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesisAm J Trop Med Hyg2017961465228077740
- KaplanskiGGranelBVazTDurandJMJarisch-Herxheimer reaction complicating the treatment of chronic Q fever endocarditis: elevated TNFalpha and IL-6 serum levelsJ Infect199837183849733392
- WajcmanHGalactérosFLe déficit en glucose-6 phosphate dés-hydrogénase: protection contre le paludisme et risque d’accidents hémolytiquesComptes Rendus Biologies2004327871172015506519
- JoplingWHSide-effects of antileprosy drugs in common useLepr Rev19835442612706199637
- GuragainSUpadhayayNBhattaraiBMAdverse reactions in leprosy patients who underwent dapsone multidrug therapy: a retrospective studyClin Pharmacol20179737828721106
- MorrisonDBWilliamsEFMethemoglobin reduction by glutathione or cysteineScience1938872245151617816389
- BurkePJahangirKMrKKolberMRDapsone-induced methemoglobinemiaCan Fam Physician201359995896124029511
- EastJBlantonLSSymptomatic hyperbilirubinemia secondary to dapsone-induced hemolysis and atazanavir therapyAntimicrob Agents Chemother20125621081108322123706
- Gilbert’s syndrome - Symptoms and causes - Mayo Clinic Available from: https://www.mayoclinic.org/diseases-conditions/gilberts-syndrome/symptoms-causes/syc-20372811Accessed July 11, 2018
- NaXKellyCProbiotics in Clostridium difficile infectionJ Clin Gastroenterol201145SupplS154S15821992956
- McFarlandLVMeta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile diseaseAm J Gastroenterol2006101481282216635227
- LawleyTDClareSWalkerAWTargeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in micePLoS Pathog2012810e100299523133377
- CastagliuoloIRieglerMFValenickLLamontJTPothoulakisCSaccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosaInfect Immun19996713023079864230
- PrinceHELapé-NixonMPatelHYehCComparison of the Babesia duncani (WA1) IgG detection rates among clinical sera submitted to a reference laboratory for WA1 IgG testing and blood donor specimens from diverse geographic areas of the United StatesClin Vaccine Immunol201017111729173320861326
- Direct diagnostic tests for Lyme diseaseClinical infectious diseasesOxford Academic Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciy614/5126199Accessed December 19, 2018
- The presenting manifestations of Lyme disease and the outcomes of treatmentNEJM Available from: https://www.nejm.org/doi/full/10.1056/NEJM200306123482423Accessed July 25, 2018
- TibblesCDEdlowJADoes this patient have erythema migrans?JAMA2007297232617262717579230
- StrleFWormserGPMeadPGender disparity between cutaneous and non-cutaneous manifestations of Lyme borreliosisPLoS One201385e6411023737968
- StonehouseAStuddifordJSHenryCAAn update on the diagnosis and treatment of early Lyme disease: “focusing on the bull’s eye, you may miss the mark”J Emerg Med2010395e147e15117945460
- Surveillance case definitions for current and historical conditionsNNDSS Available from: https://wwwn.cdc.gov/nndss/conditions/Accessed August 3, 2018
- CraftJEFischerDKShimamotoGTSteereACAntigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illnessJ Clin Invest19867849349393531237
- EmbersMEHasenkampfNRJacobsMBVariable manifestations, diverse seroreactivity and post-treatment persistence in nonhuman primates exposed to Borrelia burgdorferi by tick feedingPLoS One20171212e018907129236732
- ElsnerRAHasteyCJOlsenKJBaumgarthNSuppression of long-lived humoral immunity following Borrelia burgdorferi infectionPLoS Pathog2015117e100497626136236
- MarangoniASparacinoMMondardiniVComparative evaluation of two enzyme linked immunosorbent assay methods and three Western blot methods for the diagnosis of culture-confirmed early lyme borreliosis in ItalyNew Microbiol2005281374315782625
- AngCWNotermansDWHommesMSimoons-SmitAMHerremansTLarge differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblotsEur J Clin Microbiol Infect Dis20113081027103221271270
- SteereACDiseaseSLSeronegative Lyme diseaseJAMA1993270111369b
- KaiserRFalse-negative serology in patients with neuroborreliosis and the value of employing of different borrelial strains in serological assaysJ Med Microbiol2000491091191511023188
- BrunnerMNew method for detection of Borrelia burgdorferi antigen complexed to antibody in seronegative Lyme diseaseJ Immunol Methods20012491–218519011226475
- DejmkováHHulínskaDTegzováDPavelkaKGatterováJVavríkPSeronegative Lyme arthritis caused by Borrelia gariniiClin Rheumatol200221433033412189466
- BreierFKhanakahGStanekGIsolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicusBr J Dermatol2001144238739211251580
- SchutzerSECoylePKBelmanALGolightlyMGDrulleJSequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme diseaseThe Lancet19903358685312315
- CookMPuriBCommercial test kits for detection of Lyme borreliosis: a meta-analysis of test accuracyInt J Gen Med2016942744027920571
- CookMPuriBApplication of Bayesian decision-making to laboratory testing for Lyme disease and comparison with testing for HIVInt J Gen Med20171011312328435311
- DattwylerRJVolkmanDJLuftBJDissociation of specific T-and B-lymphocyte responses to Borrelia burgdorferiN Engl J Med198831922144114463054554
- PikeljFStrleFMozinaMSeronegative Lyme disease and transitory atrioventricular blockAnn Intern Med1989111190
- LeeflangMMGAngCWBerkhoutJThe diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysisBMC Infectious Diseases201616114027013465
- Office of HIV/AIDS and Infectious Disease Policy AS for H (ASH)Report of Other TBDS and Co-Infections Subcommittee592018HHS.gov Available from: https://www.hhs.gov/ash/advisory-committees/tickbornedisease/reports/other-tbds-2018-5-9/index.htmlAccessed May 21, 2018
- BrandaJALinskeyKKimYASteereACFerraroMJTwo-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassayClin Infect Dis201153654154721865190
- RudenkoNGolovchenkoMGrubhofferLOliverJHUpdates on Borrelia burgdorferi sensu lato complex with respect to public healthTicks Tick Borne Dis20112312312821890064
- RoscoeCEpperlyTTick-borne relapsing feverAm Fam Physician200572102039204416342834
- PorwancherRA reanalysis of IgM Western blot criteria for the diagnosis of early Lyme diseaseJ Infect Dis199917941021102410068602
- ArnaboldiPMDattwylerRJCross-reactive epitopes in Borrelia burgdorferi p66Clin Vaccine Immunol201522784084325972406
- BruckbauerHRPreac-MursicVFuchsRWilskeBCross-reactive proteins of Borrelia burgdorferiEur J Clin Microbiol Infect Dis19921132242321597198
- KrausePJCarrollMFedorovaNHuman Borrelia miyamotoi infection in California: serodiagnosis is complicated by multiple endemic Borrelia speciesPLoS One2018132e019172529420552
- StrobinoBSteinhagenKMeyerWA community study of Borrelia burgdorferi antibodies among individuals with prior Lyme disease in endemic areasHealthcare20186269
- HorowitzRIApproach to diagnosing Lyme disease misses a large proportion of casesBMJ2016352i11326762319
- BrandaJARosenbergESBorrelia miyamotoi: a lesson in disease discoveryAnn Intern Med201315916123817704
- RudenkoNGolovchenkoMVancovaMClarkKGrubhofferLOliverJHIsolation of live Borrelia burgdorferi sensu lato spirochaetes from patients with undefined disorders and symptoms not typical for Lyme borreliosisClin Microbiol Infect2016223267:267.e9
- NietoNCPorterWTWacharaJCUsing citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United StatesPLoS One2018137e019964430001350
- BarantonGPosticDSaint GironsIGironsSIDelineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosisInt J Syst Bacteriol19924233783831380285
- HauserULehnertGLobentanzerRWilskeBInterpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu latoJ Clin Microbiol1997356143314449163458
- Improved Sensitivity of Lyme disease Western Blots Prepared with a Mixture of Borrelia Burgdorferi Strains 297 and B31 Available from: http://austinpublishinggroup.com/chronic-diseases/fulltext/chronicdiseases-v1-id1009.phpAccessed December 19, 2018
- LiuSCruzIRamosCTaleonPRamasamyRShahJPilot study of immunoblots with recombinant Borrelia burgdorferi antigens for laboratory diagnosis of Lyme diseaseHealthcare20186399
- WoodmanMECooleyAEStevensonBProduction of outer surface protein A by Borrelia burgdorferi during transmission from infected mammals to feeding ticks is insufficient to trigger OspA seroconversionFEMS Immunol Med Microbiol200854227728218793197
- StrotherKOHodzicEBartholdSWde SilvaAMInfection of mice with Lyme disease spirochetes constitutively producing outer surface proteins A and BInfect Immun20077562786279417371860
- AkinEMchughGLFlavellRAFikrigESteereACThe immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritisInfect Immun19996711731819864212
- AlaediniALatovNAntibodies against OspA epitopes of Borrelia burgdorferi cross-react with neural tissueJ Neuroimmunol20051591–219219515652419
- Tijsse-KlasenEPandakNHengeveldPTakumiKKoopmansMPGSprongHAbility to cause erythema migrans differs between Borrelia burgdorferi sensu lato isolatesParasit Vectors2013612323339549
- MozayeniBRMaggiRGBradleyJMBreitschwerdtEBRheumato-logical presentation of Bartonella koehlerae and Bartonella henselae bacteremiasMedicine20189717e046529703000
- Office of HIV/AIDS and Infectious Disease Policy AS for H (ASH)Report of Other TBDS and Co-Infections Subcommittee592018 Available from: https://www.hhs.gov/ash/advisory-committees/tickbornedisease/reports/other-tbds-2018-5-9/index.htmlAccessed May 21, 2018
- GoethertHKMolloyPBerardiVWeeksKTelfordSRSrtIZoonotic Babesia microti in the northeastern U.S.: evidence for the expansion of a specific parasite lineagePLoS One2018133e019383729565993
- ScottJScottCHuman babesiosis caused by Babesia duncani has widespread distribution across CanadaHealthcare20186249
- ScottJDLaDDurdenLAAmblyomma dissimile Koch (Acari: Ixodidae) parasitizes bird captured in CanadaSystematic and Applied Acarology2015208854860
- ScottJDFirst record of locally acquired human babesiosis in Canada caused by Babesia duncani: a case reportSAGE Open Med Case Rep20175 2050313X1772564
- HamerSAGoldbergTLKitronUDWild birds and urban ecology of ticks and tick-borne pathogens, Chicago, Illinois, USA, 2005–2010Emerg. Infect. Dis201218101589159523017244
- ElfvingKOlsenBBergstromSDissemination of spotted fever Rickettsia agents in Europe by migrating birds2018 Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0008572Accessed July 6
- MascarelliPEMcquillanMHarmsCHarmsRBreitschwerdtEBartonella henselae and B. koehlerae DNA in birdsEmerg Infect Dis201420491492
- NewmanEAEisenLEisenRJBorrelia burgdorferi sensu lato spirochetes in wild birds in northwestern California: associations with ecological factors, bird behavior and tick infestationPlos One2015102e011814625714376
- GirardYAFedorovaNLaneRSGenetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residentsJ Clin Microbiol201149394595421177909
- Asian ticks (mysteriously) turned up on a New Jersey sheepNPR.org Available from: https://www.npr.org/sections/goatsand-soda/2018/02/27/588408433/asian-ticks-mysteriously-turned-up-on-a-new-jersey-sheepAccessed July 6, 2018
- RosenbaumLThis invasive tick can clone itself and suck livestock dryScience News Available from: https://www.sciencenews.org/article/invasive-longhorned-tick-can-clone-itself-suck-livestock-dry. Published June 29, 2018Accessed July 6, 2018
- WCSU confirms first specimen of new tick in Connecticut – NewsTimes Available from: https://www.newstimes.com/local/article/WCSU-confirms-first-specimen-of-new-tick-in-13193573.phpAccessed September 3, 2018
- ChinukiYIshiwataKYamajiKTakahashiHMoritaEHaemaphysalis longicornis tick bites are a possible cause of red meat allergy in JapanAllergy201671342142526551325
- ComminsSPPlatts-MillsTATick bites and red meat allergyCurr Opin Allergy Clin Immunol201313435435923743512
- ThommAMSchotthoeferAMDupuisAPDevelopment and validation of a serologic test panel for detection of Powassan virus infection in U.S. patients residing in regions where Lyme disease is endemicmSphere201831e0046717
- TokarzRSameroffSTagliafierroTIdentification of Novel Viruses in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis TicksmSphere201832e006141729564401
- MoritzEDWintonCSTonnettiLScreening for Babesia microti in the U.S. Blood SupplyN Engl J Med2016375232236224527959685
- FederHMLawlorMKrausePJBabesiosis in pregnancyNew Engl J Med2003349219519612853599
- HershMHOstfeldRSMchenryDJCo-infection of Blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hostsPLoS ONE201496e9934824940999
- SaetreKGodhwaniNMariaMCongenital babesiosis after maternal infection with Borrelia burgdorferi and Babesia microtiJ Pediatric Infect Dis Soc201871e1e528992325
- KrausePJGewurzBEHillDPersistent and relapsing babesiosis in immunocompromised patientsClin Infect Dis200846337037618181735
- SethiSAlcidDKesarwalaHTolanRWProbable congenital babesiosis in infant, New Jersey, USAEmerg Infect Dis200915578879119402971
- AndersonABijlmerHFournierP-EDiagnosis and management of Q fever — United States2013 Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6203a1.htmAccessed May 21, 2018
- ChowdriHRGugliottaJLBerardiVPBorrelia miyamotoi Infection Presenting as Human Granulocytic AnaplasmosisAnn Intern Med20131591212723817701
- KrausePJTelfordSRSpielmanAConcurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illnessJAMA199627521165716608637139
- MylonakisEEleftheriosMWhen to suspect and how to monitor babesiosisAm Fam Physician200163101969197511388711
- Investigating disease severity in an animal model of concurrent babesiosis and Lyme disease Available from: https://www.ncbi.nlm.nih.gov/pubmed/30367867Accessed December 19, 2018
- HorowitzMLColettaFFeinAMDelayed onset adult respiratory distress syndrome in babesiosisChest19941064129913017924525
- KnappKLRiceNAHuman coinfection with Borrelia burgdorferi and Babesia microti in the United StatesJ Parasitol Res20152015111
- WilsonMGlaserKCAdams-FishDBoleyMMaydaMMolestinaREDevelopment of droplet digital PCR for the detection of Babesia microti and Babesia duncaniExp Parasitol2015149243125500215
- ShahJMarkOWeltmanHFluorescence in situ hybridization (fish) assays for diagnosing malaria in endemic areasPLoS One2015109
- LempereurLShielsBHeymanPA retrospective serological survey on human babesiosis in BelgiumClin Microbiol Infect201521196:96.e125636942
- EskowERaoRVMordechaiEConcurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae: evidence for a novel tick-borne disease complexArch Neurol20015891357136311559306
- Pérez-CastrillónJLBachiller-LuquePMartín-LuqueroMMena-MartínFJHerrerosVTularemia epidemic in northwestern Spain: Clinical description and therapeutic responseClin Infect Dis200133457357611462198
- NicolsonGLGanRHaierJMultiple co-infections (Mycoplasma, Chlamydia, human herpes virus-6) in blood of chronic fatigue syndrome patients: association with signs and symptomsAPMIS2003111555756612887507
- HorowitzRIFreemanPRAre Mycobacterium drugs effective for treatment resistant Lyme disease, Tick- borne co-infections, and autoimmune disease?ResearchGate Available from: https://www.researchgate.net/publication/314217187_Are_Mycobacterium_Drugs_Effective_for_Treatment_Resistant_Lyme_Disease_Tick-_Borne_Co-Infections_and_Autoimmune_DiseaseAccessed February 5, 2018
- NicolsonGLNicolsonNLHaierJChronic fatigue syndrome patients subsequently diagnosed with lyme disease Borrelia burgdorferi : evidence for Mycoplasma species coinfectionsJournal of Chronic Fatigue Syndrome2007144517
- EskowEAdelsonMERaoRVMordechaiEEvidence for disseminated Mycoplasma fermentans in New Jersey residents with antecedent tick attachment and subsequent musculoskeletal symptomsJ Clin Rheumatol200392778717041434
- BenedettiFPlacebo and the new physiology of the doctor-patient relationshipPhysiol Rev20139331207124623899563
- CastelnuovoGGiustiEMManzoniGMWhat is the role of the placebo effect for pain relief in neurorehabilitation? Clinical implications from the Italian consensus Conference on pain in neurorehabilitationFront Neurol2018931029867723
- MinnickMFBattistiJMPestilenceBJMPestilence, persistence and pathogenicity: infection strategies of BartonellaFuture Microbiol20094674375819659429
- MaggiRGMascarelliPEHavengaLNNaidooVBreitschwerdtEBCo-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarianParasit Vectors2013610323587235
- AllredDRBabesiosis: persistence in the face of adversityTrends Parasitol2003192515512586467
- KrausePJSpielmanATelfordSRPersistent parasitemia after acute babesiosisN Engl J Med199833931601659664092
- RudenkoNGolovchenkoMVancovaMClarkKGrubhofferLOliverJHIsolation of live Borrelia burgdorferi sensu lato spirochaetes from patients with undefined disorders and symptoms not typical for Lyme borreliosisClin Microbiol Infect2016223267:267.e9
- LeeSHVigliottiJSVigliottiVSJonesWMoorcroftTALantsmanKDNA sequencing diagnosis of off-season spirochetemia with low bacterial density in Borrelia burgdorferi and Borrelia miyamotoi infectionsInt J Mol Sci2014157113641138624968274
- LeeSHVigliottiJSVigliottiVSJonesWShearerDMDetection of borreliae in archived sera from patients with clinically suspect Lyme diseaseInt J Mol Sci20141534284429824619223
- EricsonMBalakrishnanNMozayeniBRBrMCulture, PCR, DNA sequencing, and second harmonic generation (SHG) visualization of Bartonella henselae from a surgically excised human femoral headClin Rheumatol20173671669167528028681
- HarmsADehioCIntruders below the radar: molecular pathogenesis of Bartonella sppClin Microbiol Rev2012251427822232371
- FinlayBBMcfaddenGAnti-Immunology: evasion of the host immune system by bacterial and viral pathogensCell2006124476778216497587
- NicolsonGLChronic bacterial and viral infections in neurodegenerative and neurobehavioral diseasesLab Med2008395291299
- HorowitzRIHorowitzRI2003Mycoplasma Infections in Chronic Lyme Disease: A Retrospective Analysis of Co-Infection and Persistence Demonstrated by PCR Analysis Despite Long Term Antibiotic Treatment [Abstract]Presented at: 16th International Scientific Conference on Lyme & Other Tick-Borne Disorders2003Hartford CT
- HorowitzRIChronic Persistent Babesiosis after Clindamycin and Quinine/Mepron and Zithromax. [Abstract]12th International Conference on Lyme Borreliosis. Presented at: 12th International Conference on Lyme BorreliosisApril 1999New York, NY
- WormserGPPrasadANeuhausEEmergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infectionClin Infect Dis201050338138620047477
- LemieuxJETranADFreimarkLA global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapseNat Microbiol2016171607927572973
- Sánchez-VargasFMGómez-DuarteOGMycoplasma pneumoniaean emerging extra-pulmonary pathogenClin Microbiol Infect200814210511517949442
- DontaSTMacrolide therapy of chronic Lyme diseaseMed Sci Monit2003911PI13614214586290
- BrorsonOBrorsonSHAn in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to hydroxychloroquineInt Microbiol200251253112102233
- GocARathMDontaSTThe anti-borreliae efficacy of phytochemicals and micronutrients: an updateTher Adv Infect Dis201633–4758227536352
- FengJZhangSShiWZubcevikNMiklossyJZhangYSelective essential oils from spice or culinary herbs have high activity against stationary phase and biofilm Borrelia burgdorferiFront Med20174169
- LernerAMatthiasTChanges in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune diseaseAutoimmun Rev201514647948925676324
- MoutaillerSValiente MoroCVaumourinECo-infection of ticks: the rule rather than the exceptionPLoS Negl Trop Dis2016103e000453926986203
- NelderMPRussellCBSheehanNJHuman pathogens associated with the blacklegged tick Ixodes scapularis: a systematic reviewParasit Vectors2016926527151067
- BreitschwerdtEBBartonellosis: one health perspectives for an emerging infectious diseaseIlar J2014551465824936029
- BreitschwerdtEBBartonellosis, one health and all creatures great and smallVet Dermatol201728196e2128133871
- NicolsonGHaierJRole of chronic bacterial and viral infections in neurodegenerative, neurobehavioural, psychiatric, autoimmune and fatiguing illnesses: Part 2Br J Med Pract201020103301310
- SimeckaJWRossSECassellGHDavisJKInteractions of mycoplasmas with B cells: antibody production and nonspecific effectsClin Infect Dis199317Suppl 1S176S1828399911
- FurrPMTaylor-RobinsonDWebsterADMycoplasmas and urea-plasmas in patients with hypogammaglobulinaemia and their role in arthritis: microbiological observations over twenty yearsAnn Rheum Dis19945331831878154936
- ChenWLiDPaulusBWilsonIChadwickVSHigh prevalence of Mycoplasma pneumoniae in intestinal mucosal biopsies from patients with inflammatory bowel disease and controlsDig Dis Sci200146112529253511713965
- MaedaYKurakawaTUmemotoEDysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestineArthritis Rheumatol201668112646266127333153
- ParksCGMillerFWPollardKMKmPExpert panel Workshop consensus statement on the role of the environment in the development of autoimmune diseaseInt J Mol Sci2014158142691429725196523
- JomovaKVondrakovaDLawsonMValkoMMetalsVMMetals, oxidative stress and neurodegenerative disordersMol Cell Biochem20103451–29110420730621
- HorowitzRIClinical roundup: selected treatment options for Lyme diseaseAltern Complement Ther2012184220225
- HorowitzRIFreemanPRPrecision medicine: the role of the MSIDS model in defining, diagnosing, and treating chronic Lyme Disease/Post treatment Lyme disease syndrome and other chronic illness: Part 2Healthcare201864129
- DoshiSKeilpJGStrobinoBMcelhineyMRabkinJFallonBADepressive symptoms and suicidal ideation among symptomatic patients with a history of Lyme disease vs two comparison groupsPsychosomatics201859548148929606281
- KruppLBHymanLGGrimsonRStudy and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trialNeurology200360121923193012821734
- FallonBAKeilpJGCorberaKMBaFKmCA randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathyNeurology20087013992100317928580
- BerendeATer HofstedeHJVosFJHofstede HjmTERRandomized trial of longer-term therapy for symptoms attributed to Lyme diseaseN Engl J Med2016374131209122027028911
- Larry JamesonJLongoDLMedicine—personalizedPProblematic, and promisingObstetrical & Gynecological Survey20157010612
- KershGJAntimicrobial therapies for Q feverExpert Rev Anti Infect Ther201311111207121424073941
- AlaviSMAlaviLTreatment of brucellosis: a systematic review of studies in recent twenty yearsCaspian J Intern Med20134263664124009951