Abstract
Thrombotic thrombocytopenic purpura (TTP) is a rare, life-threatening disorder. This paper describes the case of a 39-year-old Sudanese male who presented to the emergency room with fever, jaundice, decreased level of consciousness, and worsening kidney function for 7 days, a high lactate dehydrogenase level (1947), severe thrombocytopenia (platelets 8), and numerous schistocytes in the peripheral blood smear. The patient was admitted with a diagnosis of TTP for plasma exchange. Fourteen days later, his creatinine kinase (CK) level rose to >50,000 IU; rhabdomyolysis was suggested. Continuous venovenous hemodialysis (CVVHD) was started. The patient’s CK level remained high, despite CVVHD, until the 6th day, after which this parameter gradually started to decrease. This report highlights a resistant case of TTP that presented with concomitant severe rhabdomyolysis, which demanded aggressive, continuous intervention.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disorder classically presenting with fever, thrombocytopenia, microangiopathic hemolytic anemia, renal dysfunction, and neurological impairment as well as various multiorgan complications and a mortality rate reaching 95% for untreated cases.Citation1 The survival rate is 80%–90% with early diagnosis and treatment with plasma infusion and plasma exchange. This paper presents a unique case of resistant TTP with severe rhabdomyolysis.
Case report
A 39-year-old Sudanese male presented to the emergency room with fever, jaundice, and decreasing level of consciousness (inability to recognize relatives) for 7 days. There were no associated symptoms or past medical history. The following laboratory values were obtained: white blood cell (WBC) count, 8.6 × 109/L; hemoglobin (Hb), 67 g/L; platelet (PLT) count, 8 × 109/L; creatinine, 121 μmol/L; prothrombin time and partial thromboplastin time, normal; lactate dehydrogenase level (LDH), 1947 U/L; increased reticulocyte count; and blood smear showing schistocytes. A urine analysis showed the following: blood, +4; pH 5.5; protein, +2; red blood cell count, 15–20; WBC count, 1; and specific gravity, 1.0170. The presence of microangiopathic hemolytic anemia, thrombocytopenic purpura, neurologic abnormalities, fever, and renal disease confirmed the patient’s diagnosis.
The patient was admitted with a presumptive diagnosis of TTP for plasma exchange. He received a total of 28 sessions of plasmapheresis. His LDH level decreased to 534 U/L, Hb increased to 87 g/L, and PLT count increased to 36 × 109/L. Ten days later, his LDH rose to 1270 U/L, with Hb of 105 g/L and PLT count of 14 × 109/L. The patient was started on vincristine and rituximab with a high dose of dexamethasone. The patient was also started on cryoprecipitate supernatant because of the suboptimal response to plasmapheresis with fresh frozen plasma. This was thought to be a relapse of TTP; the condition was considered and managed as resistant TTP. Two days later, intravenous immunoglobulin (full dose) was started. Despite the treatment for resistant TTP, the patient worsened, requiring ICU (intensive care unit) admission because of the development of uncontrolled seizures, which required intubation and ventilation. The patient developed left-sided weakness and hypotension. He was started on vasopressors. Treatment with antibiotics and then antifungal therapy commenced; the case was labeled as refractory TTP. Repeated brain computed tomography scans were normal.
During this process, the creatinine kinase (CK) levels increased to >50,000 U/L, with worsening kidney function and decreased urine output. Acute renal failure in this patient was diagnosed according to the RIFLE (risk, injury, failure, loss, end stage) criteria: a rise in creatinine of more than three-fold, and urine output < 0.3 mL/kg/h over 24 hours. Urine alkalanization was started when creatine phosphokinase (CPK) started rising to a target urine pH of 8, with several fluid boluses targeting a urine output of >200 mL/h. Mannitol was used for 24 hours only. The serum calcium concentration was monitored on a daily basis throughout the patient’s stay in the ICU and after his discharge to a step-down unit. Continuous venovenous hemodialysis started, although the CPK levels did not decrease for 5 days. After maintaining the patient on dialysis for 2 weeks, the patient started to improve clinically and was more alert, with a CK level of 5360 U/L and PLT count of 95 × 109/L (see ).
Figure 1 Daily alteration of creatine, CPK, and Ca in association with CVVHD.
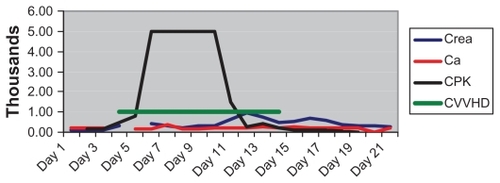
All possible differential diagnoses for rhabdomyolysis were investigated, including metabolism, viral serology, bacterial levels, toxins, and drugs. Compartment syndrome was ruled out because of the sustained CPK release.
The difficulty in weaning the patient from the ventilator stemmed from critical illness polyneuropathy and the consequences of the tracheostomy. The patient was eventually weaned from the ventilator. He was conscious and alert, with residual weakness/paresis of the lower limbs, which was managed with physiotherapy. The patient continued to require intermittent hemodialysis thrice every week.
Discussion
Rhabdomyolysis is most often caused by muscular trauma or crush injuries. Other types of causative injuries are nontraumatic and exertional or nontraumatic and nonexertional. Potential causes include malignant hyperthermia, neuroleptic malignant syndrome, near drowning/hypothermia, HIV (human immunodeficiency virus) infection, snake bites, heritable muscle enzyme deficiencies, metabolic and inflammatory myopathies, status epilepticus, status asthmaticus, eclampsia, prolonged labor, alcohol and cocaine abuse, copper sulfate, zinc phosphide, and other drugs. Traumatic and nontraumatic causes of rhabdomyolysis have been described in other reviews.Citation2,Citation3 Acute renal failure in rhabdomyolysis can be attributed to many mechanisms, such as precipitated myoglobin that blocks the renal tubules, oxygen free-radical injury, and renal arteriolar vasoconstriction induced by myoglobin. Acidic urine precipitates myoglobin into casts that can occlude the renal tubules, which blocks urine flow or output. The other mechanism of renal cellular injury is the peroxidation of lipids in the cellular membrane by the oxygen free radicals produced by iron-containing heme. Rhabdomyolysis releases platelet-activating factor, endothelin, and prostaglandins, which cause vasoconstriction and affect the glomerular filtration rate.Citation4
In this particular case, the cause of resistant, sustained rhabdomyolysis could be multifactorial, related most directly to the muscle necrosis caused by a microthrombotic process secondary to TTP. Rare reports have cited hematological factors in association with or as the cause of rhabdomyolysis.Citation5 In this patient, TTP was diagnosed based on the presence of thrombocytopenia, microangiopathic hemolytic anemia, and central neurological dysfunction. The patient developed resistant rhabdomyolysis, which caused renal failure. Notably, rhabdomyolysis is implicated as the cause of approximately 5%–25% of cases of acute renal failure.Citation6 There are two case reports in the literature worth mentioning. The first describes a patient with polymyositis who developed TTP, acute renal failure, and rhabdomyolysis after a flare-up of polymyositis following radiation therapy administered for uterine cervical cancer.Citation7 The second case was a patient with thrombotic microangiopathy who presented with fulminant rhabdomyolysis and multiorgan failure. Thrombotic microangiopathy typically presents as fulminating rhabdomyolysis with multiorgan dysfunction.Citation5
Although the patient developed multiorgan failure with TTP and severe sepsis, aggressive and continuous interventions including plasma exchange, chemotherapy, antibiotics, and continuous dialysis all contributed to the patient’s recovery, despite the lengthy stay in the ICU.
Conventional treatment for TTP, including plasma exchange, was not effective in isolation. When combined with chemotherapy and steroid therapy, this approach facilitated recovery.Citation8
In conclusion, early, proper treatment of TTP can reduce morbidity and mortality in such patients when administered in combination with the early introduction of interventions such as continuous dialysis, even after the normalization of kidney function. This very rare or less recognized presentation of a rare disease could contribute to a high mortality rate if not recognized and treated expediently. This case also underlines the importance of reevaluation or reassessment. Finally, it is important to note that the acute renal failure was persistent, possibly because of rhabdomyolysis and not because of TTP alone.
Disclosure
The author reports no conflicts of interest in this work.
References
- AmorosiElUltmannJEThrombocytopenic purpura: report of 16 cases and review of the literatureMedicine196645139159
- TalyABNairKPArunodayaGRNon-traumatic acute rhabdomyolysisNeurol India199947515410339709
- ChughKSSinghalPCNathIVAcute renal failure due to nontraumatic rhabdomyolysisPostgrad Med J197955386392482182
- SlaterMSMullinsRJRhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a reviewJ Am Coll Surg19981866937159632160
- IkhlaqueNChangJCThrombotic microangiopathy presenting as fulminating rhabdomyolysis with multiorgan dysfunction – a case reportHospital Physician2003395156
- RowlandLPMyoglobinuriaCan J Neurol Sci19841111136322948
- MakinoHNagakeYMoriwakiKThrombotic thrombocytopenic purpura and myoglobinuric acute renal failure following radiation therapy in a patient with polymyositis and cervical cancerIntern Med19953424277718974
- RockGAShumakKHBuskardNAComparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpuraN Engl J Med19913253933972062330