Abstract
Background:
Parkinson’s disease (PD) is a chronic neurodegenerative disorder. There is limited knowledge about the function of the hypothalamic-pituitary-adrenal axis in PD. The primary aim of this prospective study was to analyze diurnal salivary cortisol concentrations in patients with PD and correlate these with age, gender, body mass index (BMI), duration of PD, and pain. The secondary aim was to compare the results with a healthy reference group.
Methods:
Fifty-nine PD patients, 35 women and 24 men, aged 50–79 years, were recruited. The reference group comprised healthy individuals matched for age, gender, BMI, and time point for sampling. Salivary cortisol was collected at 8 am, 1 pm, and 8 pm, and 8 am the next day using cotton-based Salivette® tubes and analyzed using Spectria® Cortisol I125. A visual analog scale was used for estimation of pain.
Results:
The median cortisol concentration was 16.0 (5.8–30.2) nmol/L at 8 am, 5.8 (3.0–16.4) at 1 pm, 2.8 (1.6–8.0) at 8 pm, and 14.0 (7.5–28.7) at 8 am the next day. Total secretion and rate of cortisol secretion during the day (8 am–8 pm) and the concentration of cortisol on the next morning were lower (12.5 nmol/L) in the reference group. No significant correlations with age, gender, BMI, duration of PD, Hoehn and Yahr score, Unified Parkinson’s Disease Rating Scale III score, gait, pain, or cortisol concentrations were found.
Conclusion:
The neurodegenerative changes in PD does not seem to interfere with the hypothalamic-pituitary-adrenal axis. Salivary cortisol concentrations in PD patients were increased in the morning compared with the reference group, and were not influenced by motor dysfunction, duration of disease, or coexistence of chronic or acute pain.
Introduction
Parkinson’s disease (PD) is a chronic progressive neurodegenerative disorder accompanied by autonomic dysfunction and alterations in a number of regulatory mechanisms, including loss of circadian rhythms in dopaminergic systems and fluctuations in the kinetics of drugs used in treatment of the disease.Citation1
Pain in PD is common. Our own investigationsCitation2 reveal an incidence of pain of about 60%. Other studies in this area reveal incidence rates of 40%–80%.Citation3–Citation7 The pathogenesis of pain in PD is probably complex. Degeneration of descending dopaminergic pain-inhibiting fibers from the midbrain is probably one of the central pathways. Dystonia, a motor symptom of PD, is a common explanation for pain. Direct effects of levodopa on pain sensation support the hypothesis of an influence of the regulating mechanisms of autonomic and inhibitory modulation of pain input in dopamine-dependent centers.Citation8
Typically, signs of PD are hypokinesia, rigidity, and tremor, also referred to as the motor symptoms of the disease. Nonmotor signs, such as mood changes and pain, are frequent.Citation9 The pathogenesis of the nonmotor symptoms is not fully understood, and concomitant endocrine dysfunctions have been suggested. There have been few studies in PD on endocrinopathy, such as hypothyreosis and cortisol deficiency. Munhoz et al showed that the secretion of thyroid and cortisol hormones is changed in PD, possibly caused by disturbances in the hypothalamic-pituitary-adrenal axis.Citation10 A recent study by Aziz et al analyzed thyroid-stimulating hormone, free thyroxine, prolactin, and growth hormone, but not cortisol, in PD patients.Citation11
Influences of aging and stress on the glucocorticoid system may result in reduction of hormone production capacity, impaired neuronal adaptive responses to environmental challenges, and increased vulnerability to stress-induced loss of hippocampal neurons.Citation12 Aging is hypothesized to alter the function of the hypothalamic-pituitary-adrenal axis in both men and women. Increasing cortisol concentrations, especially nocturnal concentrations,Citation13,Citation14 have been described. Some studies have indicated that there are gender differences.Citation13–Citation16 One study showed significantly higher evening cortisol concentrations with increasing age, regardless of gender, while in the mornings this pattern was seen only in men. Diurnal cortisol variations were lower in older women but not in men.Citation17
Studies of plasma cortisol in PD have shown variable results. Higher cortisol concentrations have been reported in untreated PD patients without dementia compared with healthy controls,Citation18,Citation19 and also compared with age-matched and gender-matched patients with Alzheimer’s disease. However, another study found decreased plasma cortisol concentrations in untreated PD patients compared with healthy controls, suggesting that this may be a consequence of hypothalamic/hypophyseal disturbance. Further, a previous study showed higher plasma cortisol concentrations in the evening and during the night in PD patients.Citation18 One study reported higher concentrations of adrenocorticotrophic hormone in a subgroup of PD patients (both demented and nondemented) found to be dexamethasone nonsuppressors, suggesting higher concentrations of corticotrophin-releasing hormone.Citation20 This surprisingly high incidence of dexamethasone non-suppression in patients with PD could indicate a possible central pathologic disturbance of neurotransmitter function. However, levodopa medication and dopamine alone exert no influence on corticotrophin-releasing hormone, and the effects of other PD medications (such as dopamine agonists) on cortisol concentrations have not been studied.
In one study, acute levodopa intake has been shown to induce lower plasma cortisol levels in patients on long-term treatment for PD who were depleted of antiparkinsonian medication for 12 hours.Citation21 We believe that description of the diurnal salivary cortisol concentration curve in patients with chronic neurodegenerative diseases, such as PD, could be of great interest for future studies of pharmacological and nonpharmacological interventions, with the aim of reducing stress-related symptoms and lifelong suffering.
The primary aim of this study was to measure salivary cortisol concentrations in a well-defined group of PD patients, with and without chronic PD-related pain, with regard to age, duration of disease, body mass index (BMI), motor function (Unified Parkinson’s Disease Rating Scale [UPDRS] III, gait) and influence of concomitant pain. The secondary aim was to compare the salivary cortisol concentrations in these PD patients with those in a healthy reference group, matched by age, gender, and BMI.
Methods and materials
Patients
Patients with stable and well-defined PDCitation22 for more than two years, who were aged 40–80 years, with chronic pain (PD-P) or without chronic pain (PD no-P), were recruited from the outpatient departments of three medium-sized city hospitals in southern Sweden. The study was approved by the ethics committees at the University of Gothenburg and the University of Linkoping.
A period of two years since receiving the diagnosis of PD was chosen to decrease the risk of recruiting patients with an incorrect diagnosis, given that a number of other disorders can mimic PD. The individual course of PD is variable, and in our study the range of disease duration was 2–27 years (median 5–6 years). Stable PD was defined as lack of severe fluctuations of the disease (on-off symptomatology) in terms of need for frequent extra doses of antiparkinsonian medication and absence of dementia. Exclusion criteria were severe fluctuations in PD, concurrent epilepsy, active malignancy, polyneuropathy, or other serious disease of somatic or psychiatric origin that could interfere with the study. Patients with severe abnormalities of blood parameters, electrolytes, liver or renal parameters, including bilirubin >20 mmol/L, serum creatinine >130 mmol/L, SR >30 mm, fasting plasma glucose >6.7 mmol/L, were excluded. Patients on corticosteroids (oral, nasal, or inhalation), insulin, antiepileptic drugs, or medication for dementia, and those participating in other therapeutic or pharmacological studies were also excluded.
Chronic pain was defined as the occurrence of PD-related pain on at least three days per week during the three months prior to recruitment. A reference population consisting of healthy individuals, matched by gender, age, and BMI, were recruited from another project,Citation17 and consisted of 1700 healthy men and women aged 30–80 (mean 48) years. These individuals were recruited from the same catchment area as our patients, and their cortisol levels were analyzed using the same method and in the same laboratory. Demographic and clinical characteristics of the population with PD and the reference group are presented in .
Table 1 Characteristics of the PD population and the healthy reference group
Methods
Motor function was assessed by the UPDRS.Citation23 Duration and severity of pain was measured using a visual analog scale (VAS)Citation24 for five consecutive days before sampling. Maximal pain during each of these five days was registered in our protocol.
Collection of salivary cortisol samples for both groups was done in the community using a technique which has been well described elsewhere.Citation25–Citation29 To summarize, patients were instructed to have no intake of food within 30 minutes of sampling. Samples were taken at four time points, ie, at 8 am, 1 pm, and 8 pm, and then at 8 am the next morning. Cotton-based neutral Salivette® tubes (Landskrona, Sweden) were used. A swab was chewed for two minutes and then placed in a sterile plastic tube. This was then put in the patient’s refrigerator at home. The tubes for each patient were collected and sent by post to the laboratory within three days. The Salivette tubes were then centrifuged at 1711 G for 15 minutes at 20°C, and then frozen at −80°C until assayed. A commercial radioimmunoassay-based technique for measurement of salivary cortisol was used (Spectria™ Cortisol I125, Landskrona, Sweden). Total cortisol secretion during the day (8 am–8 pm) and during the night (8 pm–8 am) was calculated using the formula for area under the curve from the zero level (AUC0–AUCG), and the increase in cortisol secretion from the baseline level during the same time interval (AUCi) was calculated according to the method reported by Pruessner et alCitation30 and Fekedulegn et al.Citation31 The latter author has shown significant associations (r > 0.7; P = 0.001) between AUCG and cortisol concentrations. All analyses of saliva from the same person were performed at the same time to minimize interassay variance.
Statistical analysis
STATISTICA version 8.0 (Statsoft Inc, Tulsa, OK) and SPSS version 18.0 (SPSS Inc, Chicago, IL) were used for the statistical evaluations. Nonparametric, Mann–Whitney U, and Wilcoxon paired signed-rank tests were used. The Spearman’s rank correlation test was also used. The Wilcoxon signed-rank test for one sample was used when comparing median salivary cortisol levels in PD patients with the median in the reference population.
Results
Fifty-nine patients, consisting 24 men and 35 women aged 50–79 years of age (median 66.5/67.5 years for men/women) were recruited for this study. The PD-P group comprised 43 patients (16 men and 27 women aged 50–77 years [median 63/66 years for men/women]), and the PD no-P group comprised eight men and eight women (median 70/75 years for men/women). Age at onset of PD was 40–74 years (median 60.0) and duration of PD was 2–27 years, with a median of 5.0/6.0 years for men/women, respectively. Most patients (96%) were over 55 years of age at the start of the study. No patient was younger than 64 years in the PD no-P group compared with 18 of 43 (42%) patients in the PD-P group.
Time of awakening in the PD-P and PD no-P groups did not differ significantly, and varied from 4.35 am to 8.00 am on the two days when sampling took place. Time points for sampling varied in relation to specified time points, ie, 8 am (±30 minutes), 1 pm (−60/+30 minutes) and 8 pm (−95/+75 minutes). Time intervals between awakening and sampling in the morning were 0–220 minutes and 5–187 minutes in the PD-P group and PD no-P group, respectively, and in 10 patients was less than 45 minutes. A cortisol arousal reaction with an increase in salivary cortisol of 2.5 nmol/L was noted in only one patient.
Maximal pain in the PD patients during the five days prior to inclusion were calculated on the VAS scale as the median (10/90 percentiles), with a value of 6.2 (2.8/9.1) and minimal pain of 3.0 (0.0/6.5). Motor function as estimated by UPDRS III scores was 3–57, corresponding to mild to severe PD (see ).
Basal salivary cortisol
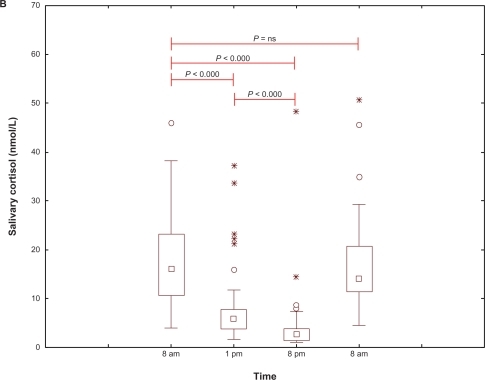
Salivary cortisol concentrations varied over a 24-hour period, as shown in and . Individual salivary cortisol values in the PD-P and PD no-P patients are shown in . There were no statistically significant differences in salivary cortisol concentrations between the two groups. One patient in the PD no-P group had extremely high values (174.6, 22.3, 14.6, and 110 nmol/L).
Figure 1A Individual diurnal salivary cortisol concentrations (nmol/L) in Parkinson’s disease with and without chronic pain.
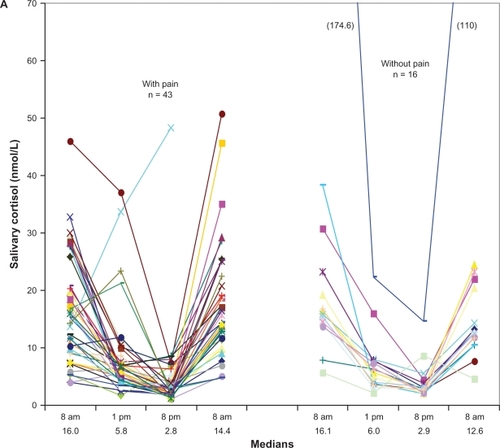
Figure 1B Diurnal salivary cortisol concentrations (nmol/L) in all 59 patients with Parkinson’s disease. Statistical analysis, between paired time points.
Abbreviation: ns, not significant.
Salivary cortisol concentrations at 8 am, 1 pm, 8 pm, and 8 am, along with delta values, are shown in and . Morning cortisol concentrations were higher in the PD group compared with the reference group, and the concentration was not dependent on time interval between awakening and time point of salivary sampling. There was also no difference in cortisol concentration between participants taking their levodopa medication within one hour either side of salivary sampling. The linear equation for the cortisol trend curve calculated to estimate AUC was y = 23.56–1.02x versus y = 17.84–0.69x for the PD and reference groups, respectively.
Table 2A Comparisons of salivary cortisol concentrations (nmol/L) in PD patients with (PD-P) and without chronic PD-related pain (PD no-P) and the reference group
Table 2B Differences in salivary cortisol concentrations between paired time points, delta cortisol (nmol/L)
Total cortisol secretion and secretion rate
Total cortisol secretion during the day (8 am–8 pm, AUC0–AUCG) was significantly increased in PD patients, at 112.8 nmolh versus 81.1 nmolh in the reference group (P < 0.001). The corresponding value for 8 pm–8 am in PD patients was 109.8 nmolh. The decrease in the salivary cortisol secretion rate during the day (8 am–8 pm, negative AUCi) in the PD and reference groups was −73.7 nmolh versus −49.9 nmolh. This difference was statistically significant (P = 0.001). The increase in salivary cortisol secretion rate during the night (8 pm–8 am, AUCi) was 72.6 nmolh, and similar to the day time value.
Somatic status and symptom correlations with cortisol
There was a highly significant correlation (r = 0.44; P < 0.01) between values at 8 am on day 1 and 8 am on day 2. There were also significant correlations between cortisol concentrations at 1 pm and for all other time points (r values 0.28–0.42; P 0.001–0.034). No significant correlations between BMI, motor dysfunction, measured as UPDRS III ≤ 20 compared with >20, gait (UPDRS III, item 30), acute pain (maximum VAS at screening), chronic pain, and cortisol concentrations were identified.
Discussion
In this study, we compared salivary cortisol concentrations in a group of patients with a diagnosis of PD for more than two years with those in a reference group of gender-, BMI-, and age-matched healthy individuals.Citation17 There were two groups of PD patients, ie, those with and those without PD-related pain.
The cortisol concentrations in this study were unrelated to age. This is in disagreement with the hypothesis that aging alters the function of the hypothalamic-pituitary-adrenal axis in both men and women. Increasing cortisol concentrations, especially at night, have been described previously.Citation13,Citation16 Even in our reference group, significantly higher evening cortisol concentrations were found with increasing age, regardless of gender, while in the mornings this pattern was seen only in men.Citation17
We found no gender differences in cortisol rhythm and/or amplitude in our PD patients. This is in contrast with findings in our reference group, where differences were found for cortisol concentrations in the morning, and the diurnal variations in cortisol were lower in older women but not in men. Other studies have also indicated gender differences.Citation13–Citation16
For estimation of total secretion and the secretion rate of cortisol, we analyzed AUC0–AUCG for cortisol during the daytime (8 am–8 pm) according to the recommendations of Fekedulegn et al.Citation31 The results were significantly higher in the PD group than in the reference group. The corresponding value for nocturnal AUCG (8 pm–8 am) was similar. The decreasing secretion rate (negative AUCi) was significantly higher in the PD group. Our results showing a significant increase in secretion rate and total cortisol secretion are potential evidence for a well functioning adrenal and hypothalamic-pituitary-adrenal axis, as reported by Fekedulegn et al.Citation31
The excellent correlation between salivary cortisol levels and plasma total and free biologically active cortisol levels in healthy men and women was reported as early as 1983 by Vining et al,Citation32 and this was confirmed in a subsequent study.Citation33 This method has been the “gold standard” for estimating stress in psycho-biologic-endocrine research for a number of years.Citation34 A recent report by Törnhage described the usefulness of salivary cortisol for assessing the hypothalamic-pituitary-adrenal axis.Citation27 These observations make it appropriate and convenient to use salivary cortisol sampling at home as a pain-free, simple, repeatable, and useful method for assessment of the hypothalamic-pituitary-adrenal axis.
Cortisol as a reflector of chronic stress in chronic disease has not undergone adequate investigation, although some studies have been performed. PD has several potentially stressful nonmotor symptoms, such as pain, mood change, and autonomic dysfunction, all of which could result in changes in cortisol secretion.
The time point for sampling in the evening differed between the PD and reference groups. Sampling occurred about two hours later in the reference group. We believe that the consequences of this are trivial, because we know that the negative slope is minimal between 8 pm and 10 pm.Citation35 We also estimated the linear equation for salivary cortisol in both groups. When we adjusted for the time difference, the salivary cortisol concentration at 8 pm in the reference group was 4.0 nmol/L compared with 3.2 nmol/L in the PD group, and this difference was not statistically significant.
The fact that the time point for awakening corresponds to sleep duration is important. A previous study found a positive correlation between sleep duration and cortisol concentration.Citation35 The time interval from awakening in the morning to the exact time point of sampling is also important because of the cortisol arousal reaction. In our study, we registered the exact time for sampling in all patients in order to control for both the cortisol arousal reaction and food intake. For most patients in our study, the exact time points for sampling were in accordance with those stipulated and stated for the reference group (±30 minutes). In 11 patients, the time interval was less than 60 minutes. In seven of these 11 patients, the interval was 30–45 minutes, with a high risk for an arousal effect. There was a cortisol arousal reaction in only one of 59 participants.
We found that patients in both PD groups generally showed a similar 24-hour rhythm of cortisol secretion. However, morning cortisol concentrations were higher in the PD group compared with those in the reference group. This is in disagreement with the findings of Hartmann et al, who reported normal morning plasma cortisol but increased concentrations in the evening and at night.Citation18
Our hypothesis that chronic neurodegenerative disease, using PD as an example, could change hypothalamic-pituitary-adrenal axis function was not confirmed. However, we found no correlation between duration of disease and cortisol concentrations. Some patients in our study showed very high morning salivary cortisol concentrations on both days. Function of the hypothalamic-pituitary-adrenal axis seems to be optimal, even at this age and after many years of disease. This, in turn, could indicate a normal-functioning hypothalamic-pituitary-adrenal axis in individuals with PD, thereby not supporting the hypothesis of earlier studies by Rabey et alCitation20 and Bellomo et alCitation36 concerning pathophysiological changes at the hypothalamic-hypophyseal-adrenal level.
There were marked differences in motor function of the patients according to UPDRS III scores. We predicted that motor dysfunction would be a severe stress factor resulting in increased cortisol concentration, but this was not confirmed. We found no correlation between gait problems, defined by UPDRS III item 30, and salivary cortisol concentration. This is in disagreement with Charlett et al who found a positive correlation between gait problems and plasma cortisol concentrations.Citation19 The reason for this difference is not obvious.
In this study, there was no correlation between BMI and cortisol concentrations in either the PD group or the reference group. In contrast, a study by Travison et al found a negative correlation between BMI and morning cortisol.Citation37
The hypothalamic-pituitary-adrenal axis seems to be preserved in patients with PD. Effects of acute or chronic pain were not seen in our study, in contrast with the findings by Heim et al.Citation38 The “glucocorticoid cascade hypothesis,” as described by McEwen,Citation12 ie, an acquired or primary decrease in hippocampal glucocorticoid receptor numbers leads to a reduction in central feedback sensitivity that results in basal glucocorticoid hypersecretion, is partially confirmed in our study. Possibly, the night-time lack of antiparkinsonian dopaminergic substitution contributes to the elevated cortisol concentration in the morning. The limitation of our study is the relatively small numbers of PD patients (n = 59), although this number compares well with those used in earlier studies in this field.Citation19,Citation20
Conclusion
PD patients with mild to severe PD have a normal diurnal cortisol rhythm, and higher morning cortisol concentrations and increased cortisol secretions during the day (8 am–8 pm) compared with healthy age-matched and gender-matched individuals. PD patients seem to have a normal cortisol arousal reaction and hypothalamic-pituitary-adrenal axis function, and their cortisol concentration is unrelated to age, duration of disease, gender, severity of motor dysfunction, and BMI, supporting the hypothesis that this neurodegenerative disorder itself does not interfere with hypothalamic-pituitary-adrenal axis function.
Acknowledgements
Financial support for this study was provided by the Research Fund at Skaraborg Hospital, the Research and Development Council of County Skaraborg, the Palle Ferb Foundation, the Academy for Healthcare, Jonkoping County Hospital, the Medical Research Council of Southeast Sweden, the Else Torgard Memorial Foundation, and the Skaraborg Institute of Research and Development. We thank Professor Bo Eriksson and Salmir Nasic for statistical support, and Astrid Borg for excellent secretarial and financial assistance and, last but not least, the study participants.
Disclosure
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported in this paper.
References
- BruguerolleBSimonNBiologic rhythms and Parkinson’s disease: A chronopharmacologic approach to considering fluctuations in functionClin Neuropharmacol200225419420112151906
- BorgATörnhageC-J“Parkitouch”-studien, Parkinsons sjukdom och effekten av beröringsmassage. [the study of “Parkitouch”, Parkinson’s disease and the effects of tactile touch]Parkinson Journalen200912224
- BeiskeAGLogeJHRonningenASvenssonEPain in Parkinson’s disease: Prevalence and characteristicsPain20091411–217317719100686
- Negre-PagesLRegraguiWRascolODoPaMiPSGChronic pain in Parkinson’s disease: The cross-sectional French DoPaMiP surveyMov Disord200823101361136918546344
- FordBPain in Parkinson’s diseaseClin Neurosci199852637210785830
- BuzasBMaxMBPain in Parkinson diseaseNeurology200462122156215715210873
- BorgmanParkinsonenkät-98Parkinsonjournalen2002
- SchestatskyPKumruHValls-SoleJNeurophysiologic study of central pain in patients with Parkinson diseaseNeurology200769232162216918056580
- SimuniTSethiKNonmotor manifestations of Parkinson’s diseaseAnn Neurol200864Suppl 2S65S8019127582
- MunhozRPTeiveHATroianoARParkinson’s disease and thyroid dysfunctionParkinsonism Relat Disord200410638138315261881
- AzizNAPijlHFrolichMRoelfsemaFRoosRADiurnal secretion profiles of growth hormone, thyrotropin and prolactin in Parkinson’s diseaseJ Neuroendocrinol201123651952421466597
- McEwenBSRe-examination of the glucocorticoid hypothesis of stress and agingProg Brain Res1992933653811480759
- Van CauterELeproultRKupferDJEffects of gender and age on the levels and circadian rhythmicity of plasma cortisolJ Clin Endocrinol Metab1996817246824738675562
- SeemanTESingerBMcEwenBGender differences in age-related changes in HPA axis reactivityPsychoneuroendocrinology200126322524011166486
- LaughlinGABarrett-ConnorESexual dimorphism in the influence of advanced aging on adrenal hormone levels: The Rancho Bernardo StudyJ Clin Endocrinol Metab200085103561356811061502
- KudielkaBMBuske-KirschbaumAKirschbaumCHPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and genderPsychoneuroendocrinology2004291839814575731
- LarssonCAGullbergBRastamLLindbladUSalivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: A cross-sectional studyBMC Endocr Disord200991619545400
- HartmannAVeldhuisJDDeuschleMStandhardtHHeuserITwenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: Ultradian secretory pulsatility and diurnal variationNeurobiol Aging19971832852899263193
- CharlettADobbsRJPurkissAGCortisol is higher in parkinsonism and associated with gait deficitActa Neurol Scand199897277859517856
- RabeyJMScharfMObermanZZoharMGraffECortisol, ACTH, and beta-endorphin after dexamethasone administration in Parkinson’s dementiaBiol Psychiatry19902765815912157505
- MullerTWelnicJMuhlackSAcute levodopa administration reduces cortisol release in patients with Parkinson’s diseaseJ Neural Transm2007114334735016932991
- CalneDBSnowBJLeeCCriteria for diagnosing Parkinson’s diseaseAnn Neurol199232SupplS125S1271510370
- RamakerCMarinusJStiggelboutAMVan HiltenBJSystematic evaluation of rating scales for impairment and disability in Parkinson’s diseaseMov Disord200217586787612360535
- ScottJHuskissonECGraphic representation of painPain1976221751841026900
- JonesMTGillhamBCampbellEAAl-TaherARChuangTTDi SciulloAPharmacology of neural pathways affecting CRH secretionAnn N Y Acad Sci19875121621752831772
- TornhageCJAlfvenGDiurnal salivary cortisol concentration in school-aged children: Increased morning cortisol concentration and total cortisol concentration negatively correlated to body mass index in children with recurrent abdominal pain of psychosomatic originJ Pediatr Endocrinol Metab200619684385416886592
- TornhageCJSalivary cortisol for assessment of hypothalamic-pituitary-adrenal axis functionNeuroimmunomodulation200916528428919571589
- ClementsADParkerCRThe relationship between salivary cortisol concentrations in frozen versus mailed samplesPsychoneuroendocrinology19982366136169802131
- TornhageCJReference values for morning salivary cortisol concentrations in healthy school-aged childrenJ Pediatr Endocrinol Metab200215219720411874185
- PruessnerJCKirschbaumCMeinlschmidGHellhammerDHTwo formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent changePsychoneuroendocrinology200328791693112892658
- FekedulegnDBAndrewMEBurchfielCMArea under the curve and other summary indicators of repeated waking cortisol measurementsPsychosom Med200769765165917766693
- ViningRFMcGinleyRAMaksvytisJJHoKYSalivary cortisol: A better measure of adrenal cortical function than serum cortisolAnn Clin Biochem198320Pt 63293356316831
- AardalEHolmACCortisol in saliva – reference ranges and relation to cortisol in serumEur J Clin Chem Clin Biochem199533129279328845424
- WeibelLMethodological guidelines for the use of salivary cortisol as biological marker of stressPresse Med20033218845851 French12870390
- LeproultRVan CauterERole of sleep and sleep loss in hormonal release and metabolismEndocr Dev201017112119955752
- BellomoGSantambrogioLFiacconiMScarponiAMCiuffettiGPlasma profiles of adrenocorticotropic hormone, cortisol, growth hormone and prolactin in patients with untreated Parkinson’s diseaseJ Neurol1991238119221851513
- TravisonTGO’DonnellABAraujoABMatsumotoAMMcKinlayJBCortisol levels and measures of body composition in middle-aged and older menClin Endocrinol (Oxf)2007671717717466009
- HeimCEhlertUHellhammerDHThe potential role of hypocortisolism in the pathophysiology of stress-related bodily disordersPsychoneuroendocrinology200025113510633533