Abstract
A 61-year-old woman with lung cancer developed delayed transfusion-related acute lung injury (TRALI) syndrome after transfusion of plasma- and leukoreduced red blood cells (RBCs) for gastrointestinal bleeding due to intestinal metastasis. Acute lung injury (ALI) recurred 31 days after the first ALI episode. Both ALI episodes occurred 48 hours after transfusion. Laboratory examinations revealed the presence of various antileukocyte antibodies including antiplatelet antibody in the recipient’s serum but not in the donors’ serum. The authors speculate that antiplatelet antibodies can have an inhibitory effect in the recipient, which can modulate the bona fide procedure of ALI and lead to a delay in the onset of ALI. This case illustrates the crucial role of a recipient’s platelets in the development of TRALI.
Introduction
Transfusion-related acute lung injury (TRALI) is a clinical syndrome associated with blood transfusions and is typically characterized by the sudden onset of dyspnea due to bilateral noncardiogenic pulmonary edema.Citation1 Although classic TRALI is defined as acute lung injury (ALI) that occurs within 6 hours following transfusion, late-onset ALI associated with blood transfusion has also been observed clinically. It is proposed that this condition is an independent disease entity termed “delayed TRALI syndrome.”Citation2 A recent laboratory investigation using the two-event animal model proposed that a TRALI event could be halted by the depletion of platelets from the recipient animal,Citation3 and in addition, there are a few reports on the participation of platelets in TRALI.Citation4,Citation5 However, the clinical evidence that supports this rationale is poorly understood. In this report, a patient who experienced a recurrence of delayed TRALI syndrome is presented. Antiplatelet antibodies were found in the recipient rather than in the donors.
Case report
The patient was a 61-year-old woman who was diagnosed with advanced nonsmall-cell lung cancer with multiple brain metastases. She received systemic chemotherapy (carboplatin + paclitaxel) for lung cancer. However, febrile neutropenia (38.5°C, neutrophil 753/μL, C-reactive protein 41.50 mg/dL) and rapidly progressing anemia (Hb 5.2 g/dL) due to intestinal tract bleeding developed after chemotherapy. She was immediately administered two bags (600 mL) of plasma- and leukoreduced red blood cells (RBCs) on hospital day 1. No apparent complications were observed within 6 hours after transfusion; however, 48 hours after the transfusion, sudden development of dyspnea and rapid progression of hypoxemia were observed. A chest radiograph showed bilateral diffuse pulmonary infiltrates without cardiomegaly (). B-type natriuretic peptide levels were within the normal limits. There was no evidence of microbial, Pneumocystis, tubercular, or fungal infection in the lungs. Methylprednisolone sodium succinate was administered for pulmonary edema, but pulmonary infiltrates and hypoxemia continued to worsen (). On day 23, chest radiography showed a significant improvement in pulmonary shadows (). However, bleeding from the intestinal tract continued, and two additional transfusions, each comprising two bags of RBCs, were required. No adverse events were associated with the two additional transfusions. On day 32, an additional transfusion of two bags of RBCs was performed and on day 34, there was a rapid deterioration of the respiratory condition again. Bilateral pulmonary infiltrates were again observed on a chest radiograph (). The patient died of multiple organ failure the next day. An autopsy was performed, and the pathological findings of the lungs indicated diffuse alveolar damage with hyaline membrane formation ().
Figure 1 Chest radiographs. (A) Onset of the first transfusion-related acute lung injury episode on hospital day 3. (B) At the initiation of mechanical ventilation on day 9. (C) A clear chest radiograph on day 23. (D) Onset of the second first transfusion-related acute lung injury episode on hospital day 34.
Figure 2 Pathological findings. (A and B) Findings of histological examination of the lung: (A) result of hematoxylin and eosin staining revealed diffuse alveolar damage with hyaline membrane formation (×200), (B) result of immunostaining for CD41 demonstrated the sequestration of platelets in the lung (×200). (C) Positive control of immunostaining for CD41 (normal bone marrow) (×200).
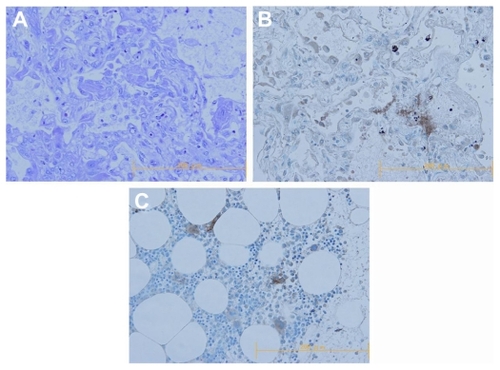
To elucidate the contribution of platelets to ALI, we tried an immunohistochemical staining for the platelet using anti- CD41 antibody against the pulmonary specimen obtained from the autopsy. The results clearly demonstrated the sequestration of platelets in the patient’s lung (). These findings strongly suggested the contribution of platelets to the pathogenesis of ALI in this case. Antileukocyte antibodies were not detected in the serum of donors from whom the blood products that led to both of the ALI episodes were obtained. However, antiplatelet Immunoglobulin M antibodies, in addition to the various antileukocyte antibodies (anti-human leukocyte antigen (HLA)-I, anti-HLA-II, antimonocyte, and unclassified antineutrophil antibody), were detected in the serum of the recipient.
Discussion
Classic TRALI is defined as an ALI that occurs within 6 hours after blood transfusion.Citation6 However, this diagnostic criterion for classic TRALI was not met in our case because the two ALI episodes did not occur within 6 hours after the corresponding transfusions. A recent report on the two-event animal model revealed that the environmental status of the recipient can affect the procedure in TRALI.Citation3 Looney et al described that platelets in the circulation of the recipient are essential for developing ALI and act as terminal effector cells.Citation3 The pathological findings also showed marked sequestration of platelets in the lung in which ALI developed. However, some antiplatelet antibodies have been shown to directly activate platelets.Citation7,Citation8
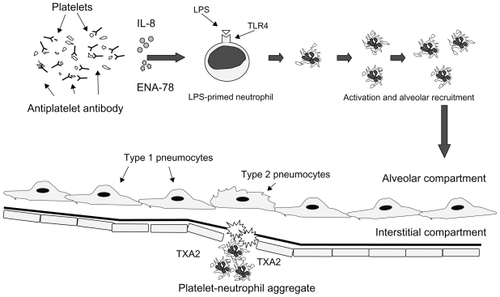
Platelets have been shown to contain members of the chemokine family of the C-C class, such as regulated on activation, normal T-cell expressed, and secreted and monocyte chemotactic peptide-3, as well as the CXC class, such as interleukin-8 and epithelial neutrophil-activating peptide-78.Citation9 Moreover, recent studies have shown that human platelets express Toll-like receptor 4Citation10 and can sequestrate in the lung in response to lipopolysaccharide (LPS) in a neutrophil-dependent manner.Citation11,Citation12 Taken together, these findings suggest that circulating platelets contribute to the development of TRALI throughout the whole process. In the case documented here, antileukocyte antibodies were not found in the donors, but antiplatelet antibodies were observed in serum from the recipient. It is hypothesized that severe bacterial infection associated with systemic chemotherapy plays a role in LPS-priming, and the transfusion of stored RBCs, which can contain any biological response modifier (eg, lysophosphatidylcholine, arachidonic acid, or hydroxyeicosatetraenoic acid), acts as a “second hit” initiating TRALI.Citation13 However, antiplatelet antibodies in the recipient might inhibit intra-alveolar platelet recruitment, resulting in a delay of platelet sequestration. Consequently, it is considered that this delay of platelet sequestration due to antiplatelet antibodies could cause a 48-hour eclipse time from the corresponding blood transfusion to the development of ALI. The present case report suggests an important role for the recipient’s platelets and antiplatelet autoantibodies in the development of TRALI (). These clinical findings could represent modulation of a TRALI event by environmental factors in the recipient, rather than in the donors.
Figure 3 Rationale of delayed transfusion-related acute lung injury. In delayed TRALI, antiplatelet autoantibodies interfere with the release of IL-8 and ENA-78, and plateletneutrophil aggregation and secretion of TXA2, resulting in a delay of onset of acute lung injury.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- PopovskyMAMooreSBDiagnostic and pathogenetic considerations in transfusion-related acute lung injuryTransfusion1985255735774071603
- MarikPECorwinHLAcute lung injury following blood transfusion: expanding the definitionCrit Care Med2008363080308418824899
- LooneyMRNguyenJXHuYVan ZiffleJALowellCAMatthayMAPlatelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injuryJ Clin Invest20091193450346119809160
- KhanSYKelherMRHealJMSoluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injuryBlood20061082455246216772606
- TuinmanPRGerardsMCJongsmaGViaarAPBoonLJuffermansNPLack of evidence of CD40 ligand involvement in transfusion-related acute lung injuryClin Exp Immunol201116527828421605114
- HolnessLKnippenMASimmonsLLachenbruchPAFatalities caused by TRALITransfus Med Rev20041818418815248168
- NomuraSYamaguchiKKidoHNew monoclonal anti-human Fc gamma receptor II antibodies induce platelet aggregationClin Exp Immunol1991861791841833099
- YanabuMNomuraSFukuroiTPlatelet activation induced by an antiplatelet autoantibody against CD9 antigen and its inhibition by another autoantibody in immune thrombocytopenic purpuraBr J Haematol1993846947018217830
- NomuraSInamiNRoles of platelet-derived chemokines in various clinical settingsGrinwaldLRChemokine Research TrendsNew YorkNova Science2007159169
- AndoneguiGKerfootSMMcNagnyKEbbertKVPatelKDKubesPPlatelets express functional Toll-like receptor-4Blood20051062417242315961512
- ZhangGHanJWelchEJLipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathwayJ Immunol20091827997800419494325
- NomuraSOzakiYIkedaYFunction and role of microparticles in various clinical settingsThromb Res200812382318667228
- SillimanCCMooreEEKelherMRIdentification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injuryTransfusion2011 [Epub ahead of print.]