Abstract
Chromobacterium violaceum is a common environmental bacterium that rarely causes disease in humans but has a high fatality rate if it does. Due to the rarity of the cases, clinicians are often unaware of the rapid progression of C. violaceum infection and its unexpected antibiotic resistance pattern, which contribute to the failure of patient management. Our review provides the clinical characteristics, possible sources of exposure, and comorbidities and determines factors associated with survival. We gathered information on 132 cases of C. violaceum causing disease in humans published between 1953 and 2020. Patients were predominantly male with a median age of 17.5, interquartile range (IQR) of 5.0–40.0 years, and a third of them were known to have immune deficiencies or comorbidities. Portals of entry were mainly through a wound in the leg and feet (28.0%), the torso (8.5%), or hands and arms (6.8%). It is not uncommon to acquire infection through unintended contact with contaminated water or dust through the mouth or inhalation. The median incubation period is 4.0 days (IQR 2.0–8.0 days) with a duration of clinical course of 17.5 days (IQR 8.0–30.8 days). The high rate of positive blood cultures (56.1%) and abscesses in internal organs (36.4%) shows the significant severity of this disease. Sepsis and Bacteremia were related to mortality with a risk ratio (RR) of 5.20 (95% CI, 0.831–32.58) and 2.14 (95% CI, 1.05–4.36), respectively. Appropriate antibiotic use prevented death at a RR 0.33 (95% CI, 0.21–0.52). Most patients who recovered and survived were treated with aminoglycosides, fluoroquinolones and carbapenems. This review shows the malignant nature of C. violaceum infection and the need for clinicians to be aware and provide prompt source management for patients. Appropriate empiric and targeted antibiotic regiment guided by susceptibility test results is of vital importance.
Keywords:
Introduction
Chromobacterium violaceum is a free-living, soil and freshwater gram-negative bacillus found in tropical and subtropical regions. Human infection with this organism is not common; therefore, its presence as a causative agent is mostly overlooked in clinical practice unless a patient presents with severe, rapidly deteriorating sepsis and a remarkable purplish lesion and colonies in culture.Citation1–3 C. vioaleceum was regarded as a saprophytic organism and non-pathogenic in humans. Wooley first reported its pathogenic characteristics in 1905, observing septicemia in water buffaloes caused by these bacteria.Citation4,Citation5 Later, more observations reported high human mortality with fatal septicaemia and skin lesions as well as internal organ abscesses. These manifestations have been reported from various parts of the world in the subtropic and the tropics, including Indonesia.Citation2,Citation4–Citation12 The organism has been characterized as an opportunistic pathogen causing severe and fatal manifestations in immunocompromised hosts.Citation2,Citation13–Citation16 However, this notion is challenged by other cases reporting fatal and severe clinical manifestations in non-immunocompromised hosts as well.Citation5,Citation17–Citation19
The earliest observation of the bacterium was made by French scientists Boisbaudran and Gasser in the 1870s, who found rice flower coloration.Citation20 Another independent observation was made by Bergonzini of Modena University, Italy, in 1880, who observed a contaminated flask in his experiments with a deep purple substance that after extraction was insoluble with many solvents. This bacterium was named Chromobacterium violaceum and later record it in the Bergey’s Manual of Systematic Bacteriology as C violaceum.Citation20 In 1976, two strains of C. violaceum with different colors of white and violet were isolated in Brazil and it was hypothesized that the violacein protected the bacterium against the sun.Citation21 There are nine other species of Chromobacterium genus that have been recognized to date, C. subtsugae, C. aquaticum, C. haemolyticum, C. piscinae, C. pseudoviolaceum, C. vaccinii, C. amazonense, C. alkanivorans, and C. rhizoryzae.Citation22
The purple pigment called violacein, which is expressed by the bacteria, has created great research interests. Violacein is thought to confer the virulence of the bacteria and also to have antibiotic-inhibiting properties.Citation23,Citation24 The violet pigment of the bacteria is assumed to be the causative agent of septicemia and fatal infections in humans and animals.Citation25 Bacteria with a dark violet color are resistant to various antibiotics, including vancomycin, ampicillin, and linezolid, and susceptible to colistin, oxacillin, gentamicin, norfloxacin, chloramphenicol, and amikacin.Citation26 Besides violacein, the bacteria also express metabolites that affect the growth of many gram-positive and -negative bacteria as well as produce an immunomodulatory effect.Citation25 Interestingly, these characteristics have been utilized to produce antibiotic attreonam.Citation27 Microbiologically, C. violaceum has biochemical characteristics similar to those of pseudomonas and aeromonas species,Citation24 which are related to the primary multi-drug characteristics that are challenging in treatment.Citation3,Citation22
Any part of the human body can serve as an inoculation site, with a traumatic break in the skin serving as the most common port of entry for infection in many reported cases, followed by regional lymphadenopathy, widespread bacteremia and abscess development in the visceral organs.Citation3,Citation5,Citation11,Citation17,Citation28,Citation29 The mortality rate of C. violaceum infection has been reported to be 60%–80% in disseminated infection, and it was difficult to eradicate in most cases. Our review aimed to describe the clinical characteristics, possible sources of exposure, and comorbidities and to determine the factors that are associated with survival.
Materials and Methods
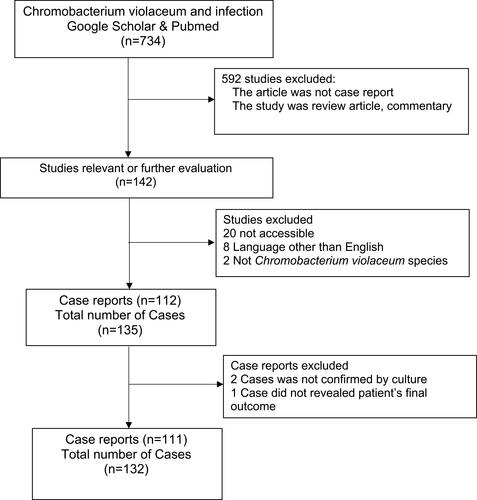
We carried out a literature search of published case reports or case series using two journal databases: PubMed and Google Scholar. The following search terms were used for searching relevant observational literature: “Chromobacterium violaceum” AND “infection” AND “Case Report.” Exclusion criteria were 1) the report did not contain reported cases, 2) the report was written in a language other than English, 3) suspected case of chromobacterium infection but no culture evidence, 4) study reporting chromobacterium culture other than Chromobacterium violaceum, and 5) no information regarding final outcome of the cases. All reviewers did article selection and assessment. The outcome of the searches is summarized in . De Siqueira reported three cases, but we included only one case, and the other two cases were not confirmed by bacterial isolation.Citation30 From the 111 studies reviewed, we collected information on 132 cases (). Most are single-case reports,Citation1,Citation2,Citation4,Citation5,Citation7–Citation9,Citation11,Citation15–Citation18,Citation28,Citation31–Citation80 except Huffam provided four cases,Citation81 Hagiya,Citation38 Karniyarakkal,Citation82 Macher,Citation83 Ma,Citation84 Tee,Citation85 and TeohCitation18 each reported three cases, Al Khalifa,Citation31 Banik,Citation86 Feldman,Citation36 Jitmuang,Citation28 Macher,Citation83 Ognibene,Citation87 Pant,Citation88 Ponte,Citation89 Simo,Citation90 Sorensen,Citation91 Starr,Citation92 Suarez,Citation93 and Ti,Citation94 each reported two cases. Of these reports, 28 have been reviewed by Yang et al.Citation95 Based on these reports we collected information about demographics, incubation period, predisposing factors, comorbidities, clinical presentations, laboratory and microbiologic data, antimicrobial therapy, clinical course and outcome for analysis.
Definition
Case report year referred to the time when the paper was published. Sepsis was considered if the patient fulfilled the sepsis definition according to Bone et al (1992) as having two or more of 1) temperature > 38 or < 36 °C, 2) heart rate > 90, 3) respiratory rate > 20 and PaCO2 < 32, and 4) white blood cell count > 12.000/mm3, or < 4000, or > 10% Immature (band) forms.Citation96 The location of the first suspected port of entry was classified as in legs and feet, hands and arms, head and neck, neck and trunk, ingestion, inhalation, urinary tract. Skin abscess and cellulitis were defined as any skin infection in the form of pustules, vesicles, ecthyma, or ulcers including lymphadenitis. Comorbidity is defined as having a previously known disease, diagnosed during the course of C. violaceum infection or under other clinical care. Internal organ abscess was noted if reports contained information about the presence of at least an abscess in the liver, spleen, lung, brain or other organ based on imaging or post-mortem studies. The clinical course was defined as the interval from the onset of symptoms to death or discharge from hospital. A patient was considered to have a particular exposure if the author provided information regarding the suspected way of acquiring the infection.
Antimicrobial susceptibility tests were obtained from the reviewed reports, if available. Interpretation of the results followed Clinical and Laboratory Standards Institute guidelines for non-Enterobacteriaceae gram-negatives).Citation97 An intermediate reaction was classified as non-susceptible. Antimicrobial therapy was considered appropriate if the treatment regimen included at least one antimicrobial agent described as an active agent against C. violaceum isolates in vitro. It was considered inappropriate if neither drug was sensitive against the isolated strain nor the patient did not receive the specified antimicrobial therapy. Healthcare-associated infection was defined if the infection occurred within hospital care or if the C. violaceum infection was related to a medical procedure. The year cut-off of 1990 was used as a reference year because of the general availability of new antibacterial agents, including ciprofloxacin in 2010 because of a previous review made by Yang et al in 2011.Citation95
Statistical Analysis
We presented data for dichotomous and categorical variables as frequencies and percentages. Continuous variables were presented as mean ± standard deviation and median and interquartile range (IQR). We used the Kolmogorov–Smirnov test to identify normal distributions. In the univariate analysis of variables associated with mortality, chi-square and Fisher's exact tests were used for binary variables and risk ratios were calculated. In multivariate analysis, binary logistics regression with backward method was employed to determine the factors most related to mortality outcome. The level of significance (α) was set at 0.05.
Results
We have listed and examined case reports from almost all continents, including India and its neighboring countries (n=31), Southeast Asia (n=20), North America (n=31), South America (n=10), Australia and New Zealand (n=8), Africa (n=8), Middle East (n=3) and Europe (n=6). Before 1990, most reports came from North America, but over the years, more reports came from all other countries (), including areas with cooler climate, such as Japan,Citation64 Korea,Citation56 China, Switzerland, the Czech Republic and Poland.
Table 1 Demographic, Clinical Characteristics, Culture Result and Outcome of 132 Chromobacterium violaceum Infection Cases Reviewed
Our C. violaceum patients were mostly males from all age groups with a median of 17.5 (IQR 5.0 to 40.0) years old. The highest number of patients was in the younger age group between 0–9 years old and 20–49 years old. The incubation period was mostly short (median of 4 days, ranging from 1 to 80 days). Most patients sought medical care because of rapidly developing fever after a localized wound infection. Skin lesions developed into dark or purple colored vesicle abscesses accompanied by necrosis (see ). In more than 75% of cases, routine hematologic examinations showed a pattern of ongoing bacterial infection with a leucocyte count of more than 11.000/mm3 (). As the disease progressed to sepsis, the leucocyte count gradually increased in parallel with decreasing haemoglobin and thrombocyte values.Citation98
Figure 2 Picture of a patient with black purplish abscess in the right cheek extending to the right ear (A) and Chromobacterium violaceum shown on a culture plate (B). Notes: Reproduced from Darmawan G, Kusumawardhani RY, Alisjahbana B, Fadjari TH. Chromobacterium violaceum: the Deadly Sepsis. Acta Med Indones. 2018;50(1):80–81.2 Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/legalcode).
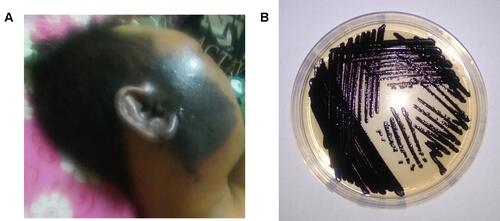
Following the initial infection, hematogenic spread occurred, as shown by the high proportion of positive blood culture in these patients (). The hematogenic dissemination also caused multiple small pustules to occur in other areas of the body.Citation99,Citation100 Severe sepsis and septic shock were the most common complications found, often followed by lung involvement with respiratory distress, requiring intensive care intervention.Citation18,Citation24 Some patients presented with developing abdominal pain, related abscess formation in the visceral organs, mainly the liver and spleen as shown by imaging studies. Abscess and necrosis also occurred in the lungs following disseminated infection. Skin and soft-tissue infection in the facial area also led to brain abscesses.Citation101 Culturing the specimen mainly reveals purple colonies of C. violaceum ().
Antibiotic usage was variable, and a large proportion of use was not appropriate as indicated by the antibiotic sensitivity pattern (). There was a wide range of clinical course duration with a median of 18 days (range 2–264 days). Surviving patients were also affected by relapse of C. Violaceum infection, especially those with underlying chronic granulomatous disease.Citation102 Elimination of existing visceral abscesses took several weeks to months.Citation81,Citation103 As guided by inflammatory markers, antibiotics needed to be taken until abscesses were fully cured.Citation28,Citation103–Citation105
These patients acquired C. violaceum infection mostly in the community because of environmental exposures. Injuries to the extremities were the most common source (), mainly due to recreational accidents,Citation33 puncture wounds,Citation106 or insect bites.Citation100 Surprisingly, ingestion or inhalation were not uncommon. Al Khalifa et al reported fatal cases in two boys who acquired infection from a contaminated water storage tank.Citation31 Recreational activity in a contaminated lake also affected all three children in a family but without skin injury.Citation30 Dust from contaminated ground was also suspected to cause primary pulmonary infection with C violaceum.Citation1,Citation107 In fact, five reported successful isolation of C. violaceum in the suspected lake, stream and soil sources. A more obscured mode of transmission was through the urinary tract in 12-year-old and 2-month-old children.Citation82,Citation108
Table 2 Location of Suspected Port of Entry and Comorbidity in Community Acquired and Health Care Associated C. violaceum Infection
C. violaceum healthcare-associated infections were also reported (). These patients were in hospital because of other illnesses or for a routine medical procedure. After a cervical spine surgery, one patient who remained febrile was shown to have C. violaceum infection in the femoral venous line.Citation18 Cases of urinary tract infection because of recurrent use of a catheter in a chronic kidney disease patients have also been reported.Citation88,Citation109 Two cases of infection were probably acquired through the use of a humidified Venturi mask while in transfer to or from an intensive care ward.Citation38
Antibiotic susceptibility testing revealed high rate of sensitivity to carbapenems, aminoglycosides, chloramphenicol, quinolones, tetracyclines and trimethoprim-sulfamethoxazole. Most first-line treatments for suspected gram-positive contamination of skin injuries, such as penicillin, beta-lactams, and clindamycin, were inactive against C. violaceum ().
Table 3 Antibiotic Susceptibility Pattern
Our serial cases showed that mortality decreased in the latter years (). It was almost 54.5% in the years before 1990 and 46.8% between 1990 and 2010, declining thereafter to 25.4%. Comparison of surviving patients versus fatal cases revealed that five variables were significant for the outcome: case report year, sepsis, bacteremia, appropriate antibiotic use, and duration of clinical course. We include all of these variables except the duration of the clinical course in an adjusted logistic regression model. Different categories of case report year were not associated with higher risk of death, while the other three variables remained important to note. Sepsis and bacteremia were associated with mortality with risk ratio of 5.20 (95% CI 0.83–32.58) and 2.14 (95% CI, 1.05–4.36), respectively. Appropriate antibiotic use prevented death at risk ratio 0.33 (95% CI, 0.21–0.52).
Table 4 Association Risk Factor with Mortality Outcome
Discussion
We reviewed cases of C. violaceum reported from five continents, showing the universal distribution of this successful species. It is known that C. violaceum are distributed in warmer climates of the world between 35° latitude in the north and south.Citation29 The earliest human case reports came from Malaysia,Citation110 followed by cases in the south-eastern United States among patients with chronic granulomatous disease and in healthy individuals.Citation83,Citation89 However, various new cases have been reported from colder regions, such as Japan and Europe.Citation38
Our C. violaceum infection cases are predominantly in young, male patients. This represents a group of people who are active outdoors and prone to injury. This pattern did not change much compared to case reviews by Yang et al in 2011Citation95 and the earlier reports in the United States, either among patients with chronic granulomatous diseaseCitation83 or non-immunocompromised individual.Citation89,Citation111
Infection with C. violaceum often occurred in a recreation or work environment. Contact with water through skin injury or ingestion is one of the most common routes to infection. In the water where C. violaceum was found, it was continuously detected through most of the observation time irrespective of human fecal contaminants as represented by E. coli.Citation112 The soil is also a natural habitat where C. violaceum can be isolated in abundant numbers but not in vegetables or other foods.Citation113 It is therefore reasonable to suspect that inhalation of dust outdoors could cause primary pulmonary infection with C. violaceum. It would also be reasonable to assume that C. violaceum may find an optimal environment in hospital equipment, such as cathetersCitation88 and Venturi masks.Citation38
Chronic granulomatous disease is a well-known predisposing factor to infection by C. violaceum.Citation83 Being immunocompromised may underlie other cases, as some reported history of recurrent infection and skin abscessCitation2,Citation81,Citation82 and diabetes mellitus.Citation114 Chronic granulomatous disease patients are susceptible to infection caused by catalase-producing bacteria such as C. violaceum because of their inability to carry out adequate oxidative metabolism during phagocytosis.Citation115 However, epidemiologically, we still cannot determine the strength of this association because of the small number of reported cases, and many reports did not confirm the immune status of their patients.Citation1,Citation33,Citation37 Other than the host immune condition, significant exposure to the bacteria seems to play a role as well. There are at least two reports where several healthy children acquired the infection from a shared community source.Citation30,Citation31
Once infection is established, C. violaceum rapidly spreads locally and through the bloodstream to internal organs, causing multiple abscesses and sepsis.Citation22,Citation110 If the mode of entry is inhalation, the patients rapidly develop upper respiratory tract infection followed by severe pneumoniaCitation31,Citation35 Diarrhea and abdominal pain could be manifestations if C. violaceum were transmitted through ingestionCitation116,Citation117 The virulence of these bacteria is shown as well by the high proportion of positive blood cultures in the case reports. The success of the invasion by these bacteria partly results from an immunomodulatory effect of secreted substancesCitation20 as well as a type III secretion system-mediated effect that damages host tissues, including hepatocytes.Citation22
The most critical factors for the bacterium’s high fatality rate are its severity, unawareness of the clinical characteristics of infection, and inappropriate antibiotic use. With the increasing knowledge of the disease and more antibiotic alternatives, we and others have shown that mortality has decreased.Citation22 Initial empirical antibiotic choice is important as C. violaceum are intrinsically resistant to many first-line antibiotics, mainly the penicillin group and beta-lactams.Citation1,Citation23,Citation32,Citation37 Although less common, some strains have also been reported to be resistant to imipenemCitation29 and aminoglycosides.Citation7,Citation9,Citation16,Citation107 Most patients who recovered and survived were treated with aminoglycoside, fluoroquinolone, and carbapenem antibiotic regimens as guided by culture and susceptibility test results.Citation10,Citation14,Citation15,Citation32
Conclusion
C. violaceum is a natural inhabitant of the water and soil and rarely causes disease. However, special care needs to be taken in dealing with patients with ulcers and abscesses and a history of environmental exposure, especially those with the disproportionately rapid development of sepsis, respiratory distress, and visceral pain. The organism shows high rate of susceptibility to aminoglycosides, carbapenems, quinolones, chloramphenicol, tetracyclines, and trimethoprim sulfamethoxazole. Early source control and prompt provision of the appropriate antibiotic regimen guided by culture and susceptibility test results are important in the management of this disease. Proper long-term follow-up care aimed at eliminating remaining bacteria is also vital to prevent relapses.
Disclosure
The authors report no conflicts of interest for this work.
References
- Ang YM. A very rare and rapidly fatal case of Chromobacterium violaceum septicemia. Med J Malaysia. 2004;59(4):535–537.
- Darmawan G, Kusumawardhani RY, Alisjahbana B, Fadjari TH. Chromobacterium violaceum: the Deadly Sepsis. Acta Med Indones. 2018;50(1):80–81.
- Fantinatti-Garboggini F, Almeida R, Portillo V, et al. Drug resistance in Chromobacterium violaceum. Genet Mol Res. 2004;3(1):134–147.
- Bottieau E, Mukendi D, Kalo J-R, et al. Fatal Chromobacterium violaceum bacteraemia in rural Bandundu, Democratic Republic of the Congo. NMNI. 2015;3:21–23. doi:10.1016/j.nmni.2014.10.007
- Sharmin S, Jahan AA, Kamal S, Sarker PJ. Fatal Infection Caused by Chromobacterium violaceum: a case report from a tertiary care hospital in Bangladesh. Case Rep Infect Dis. 2019;2019. doi:10.1155/2019/6219295
- Lin Y, Majumdar SS, Hennessy J, Baird RW. The spectrum of Chromobacterium violaceum infections from a single geographic location. AJTHAB. 2016;94(4):710–716. doi:10.4269/ajtmh.15-0862
- Pérez JAD, García J, Rodriguez VLA. Sepsis by Chromobacterium violaceum: first case report from Colombia. Braz J Infect Dis. 2007;11(4):441–442. doi:10.1590/s1413-86702007000400016
- Karthik R, Pancharatnam P, Balaji V. Fatal Chromobacterium violaceum septicemia in a South Indian adult. J Infect Dev Ctries. 2012;6(10):751–755. doi:10.3855/jidc.1866
- Kaufman SC, Ceraso D, Schugurensky A. First case report from Argentina of fatal septicemia caused by Chromobacterium violaceum. J Clin Microbiol. 1986;23(5):956–958. doi:10.1128/jcm.23.5.956-958.1986
- Ray P, Sharma J, Marak R, et al. Chromobacterium violaceum septicaemia from north India. Indian J Med Res. 2004;120(6):523–526.
- Slesak G, Douangdala P, Inthalad S, et al. Fatal Chromobacterium violaceum septicaemia in northern Laos, a modified oxidase test and post-mortem forensic family G6PD analysis. Ann Clin Microbiol Antimicrob. 2009;8(1):1–5. doi:10.1186/1476-0711-8-24
- Umadevi S, Kumar S, Stephen S, Joseph N. Chromobacterium violaceum: a potential nosocomial pathogen. Am J Infect Control. 2013;41(4):386. doi:10.1016/j.ajic.2012.05.004
- Midani S, Rathore MJ. Chromobacterium violaceum infection. South Med J. 1998;91(5):464–466. doi:10.1097/00007611-199805000-00011
- Saboo AR, Vijaykumar R, Save SU, Bavdekar SB. A rare nonfatal presentation of disseminated Chromobacterium violaceum sepsis. J Microbiol Immunol Infect. 2015;48(5):574–577. doi:10.1016/j.jmii.2012.11.002
- Sirinavin S, Techasaensiri C, Benjaponpitak S, Pornkul R, Vorachit M. Invasive Chromobacterium violaceum Infection in Children: case Report and Review. Pediatr Infect Dis J. 2005;24(6):559–561. doi:10.1097/01.inf.0000164761.81491.3f
- Lawrence H, Tucker AJ. Necrotizing External Otitis and Chromobacterium violaceum. Otorhinolaryngol Nova. 2001;11(6):306–307. doi:10.1159/000068299
- Ansari S, Paudel P, Gautam K, Shrestha S, Thapa S, Gautam RJ. Chromobacterium violaceum isolated from a wound sepsis: a case study from Nepal. Case Rep Infect Dis. 2015;2015. doi:10.1155/2015/181946
- Teoh AYB, Hui M, Ngo KY, Wong J, Lee KF, Lai PBS. Fatal septicaemia from Chromobacterium violaceum: case reports and review of the literature. Hong Kong Med J. 2006;12(3):228–231.
- Bilton BD, Johnson LW. Recurrent nonfatal Chromobacterium violaceum infection in a nonimmunocompromised patient. Infect Med. 2000;17(10):686–689.
- Durán M, Faljoni-Alario A, Durán N. Chromobacterium violaceum and its important metabolites. Folia Microbiol. 2010;55(6):535–547. doi:10.1007/s12223-010-0088-4
- Caldas LR. Photochemistry and photobiology in a virgin land. Photochem Photobiol. 1977;26(1):1–2. doi:10.1111/j.1751-1097.1977.tb07440.x
- Batista JH, da Silva Neto J. Chromobacterium violaceum pathogenicity: updates and insights from genome sequencing of novel Chromobacterium species. Front Microbiol. 2017;8:2213. doi:10.3389/fmicb.2017.02213
- Aldridge KE, Valainis GT, Sanders CV. Comparison of the in vitro activity of ciprofloxacin and 24 other antimicrobial agents against clinical strains of Chromobacterium violaceum. Diagn Microbiol Infect Dis. 1988;10(1):31–39. doi:10.1016/0732-8893(88)90124-1
- Kothari V, Sharma S, Padia D. Recent research advances on Chromobacterium violaceum. Asian Pac J Trop Dis. 2017;10(8):744–752. doi:10.1016/j.apjtm.2017.07.022
- Durán N, Menck C. Chromobacterium violaceum: a review of pharmacological and industrial perspectives. Crit Rev Microbiol. 2001;27(3):201–222. doi:10.1080/20014091096747
- Rajalakshmi G, Sankaravadivoo A, Prabhakaran S. Characterization of Chromobacterium violaceum isolated from spoiled vegetables and antibiogram of violacein. J Adv Lab Res Biol. 2011;2(1):18–22.
- Duma RJ. Aztreonam, the first monobactam. Ann Intern Med. 1987;106(5):766. doi:10.7326/0003-4819-106-5-766
- Jitmuang A. Human Chromobacterium violaceum infection in Southeast Asia: case reports and literature review. Southeast Asian J Trop Med Public Health. 2008;39(3):452.
- Moore CC, Lane JE, Stephens JL. Successful Treatment of an Infant with Chromobacterium violaceum Sepsis. Clinical Infectious Diseases. 2001;32(6):e107–e10. doi:10.1086/319356
- De Siqueira IC, Dias J, Ruf H, et al. Chromobacterium violaceum in siblings, Brazil. Emerg Infect Dis. 2005;11(9):1443. doi:10.3201/eid1109.050278
- Al Khalifa SM, Al Khaldi T, Alqahtani MM, Al Ansari AM. Two siblings with fatal Chromobacterium violaceum sepsis linked to drinking water. BMJ Case Rep. 2015;2015. doi:10.1136/bcr-2015-210987
- Bosch F, Badenhorst L, Le Roux J, Louw V. Successful treatment of Chromobacterium violaceum sepsis in South Africa. J Med Microbiol. 2008;57(10):1293–1295. doi:10.1099/jmm.0.2008/001883-0
- Carter E, Cain K, Rutland B. Chromobacterium violaceum cellulitis and sepsis following cutaneous marine trauma. Cutis. 2008;81(3):269.
- Chattopadhyay A, Kumar V, Bhat N, Rao P. Chromobacterium violaceum infection: a rare but frequently fatal disease. J Pediatr Surg. 2002;37(1):108–110. doi:10.1053/jpsu.2002.29439
- Cheong B. A fatal case of pulmonary Chromobacterium violaceum infection in an adult. Med J Malaysia. 2010;65(2):148–149.
- Feldman RB, Stern GA, Hood C. Chromobacterium violaceum infection of the eye: a report of two cases. Arch Opthalmol. 1984;102(5):711–713. doi:10.1001/archopht.1984.01040030567019
- Georghiou PR, O’Kane GM, Siu S, Kemp R. Near‐fatal septicaemia with Chromobacterium violaceum. Med J Aust. 1989;150(12):720–721. doi:10.5694/j.1326-5377.1989.tb136770.x
- Hagiya H, Murase T, Suzuki M, et al. Chromobacterium violaceum nosocomial pneumonia in two Japanese patients at an intensive care unit. J Infect Chemother. 2014;20(2):139–142. doi:10.1016/j.jiac.2013.10.001
- Ahmed M, Apte AA, Shukla V, Sarkar D, Shankar H. Chromobacterium violaceum bacteremia in a 2 years old male child. Pediatr Oncall J. 2008;39:452–460. doi:10.7199/ped.oncall.2017.17
- Anuradha K, Rodrigo P, Karunaratne G, De Silva R, Seneviratne SN, Wickramasinghe V. Chromobacterium violaceum sepsis in an infant with chronic granulomatous disease. J Postgrad Med. 2018;5(1):1–6. doi:10.4038/jpgim.8179
- Arosio M, Raglio A, Ruggeri M, et al. Chromobacterium violaceum lymphadenitis successfully treated in a Northern Italian hospital. New Microbiol. 2011;34(4):429–432.
- Ballal M, Kini P, Rajeshwari D, Shivananda P. Chromobacterium violaceum diarrhea. J Pediatr. 2000;67(5):388–389. doi:10.1007/bf02820697
- Bansie R, Harkisoen S, Lachman V, Fat ELA, Ramdhani N, van Laar JA. A rare infection in the tropics that is not uncommon in cases of chronic granulomatous disease. Access Microbiol. 2019;1:8. doi:10.1099/acmi.0.000039
- Blereau R, City M. Septicemia and death caused by Chromobacterium violaceum. South Med J. 1980;73:1093–1094. doi:10.1097/00007611-198008000-00046
- Bwire GM, Lema N, Makala R, et al. Pediatric Fatal Chromobacterium violaceum in Tanzania: the First Documented Case. SN Compr Clin Med. 2020;2(1):91–94. doi:10.1007/s42399-019-00177-z
- Dauphinais R, Robben G. Fatal Infection clue to Chromobacterium violaceum. Am J Clin Pathol. 1968;50(5):592–597. doi:10.1093/ajcp/50.5.592
- Dzupova O, Benes J. Serious imported infections: a focus on Chromobacterium violaceum. Bratisl Med J. 2019;120(10):730–733. doi:10.4149/bll_2019_121
- Fernandes G, Nascimento A, Borato E, da Silva N, Cunha R, Nascimento T. Chromobacterium Violaceum Sepsis in Minas Gerais. Int Arch Med. 2015;8. doi:10.3823/1691
- Fernandes M, Luz K, Dantas L, Melo M, Almeida D. Chromobacterium violaceum: a fatal case in the northeast of the Brazil. J Bras Patol Med Lab. 2014;50(4):278–279. doi:10.5935/1676-2444.20140027
- Frawley AA, Powell L, McQuiston JR, Gulvik CA, Bégué RE. Necrotizing Pneumonia Caused by Chromobacterium violaceum: report of a Rare Human Pathogen Causing Disease in an Immunodeficient Child. Am J Trop Med Hyg. 2018;99(1):164–167. doi:10.4269/ajtmh.18-0060
- Guo W, Li I, Li X, et al. Sequential Mycoplasma pneumoniae pneumonia and Chromobacterium violaceum skin abscess in a pediatric patient. J Infect Dev Ctries. 2017;11(08):656–661. doi:10.3855/jidc.8878
- Hodge RA. Non-chromogenic Chromobacterium violaceum in a urinary tract infection. Clin Microbiol Newsl. 2002;2(24):15. doi:10.1016/s0196-4399(02)80006-x
- Jędruszczak A, Węgrzyn-Bąk M, Budzyńska-Nosal R, Maciejewski M, Marczewski K. Sepsis caused by Chromobacterium violaceum-probably the first case in Europe, or Macbeth read anew. Ann Agric Environ Med. 2018;26(3):508–510. doi:10.26444/aaem/99295
- Kamat U, Ghodge R. Fatal Case of Chromobacterium violaceum Septicaemia in Goa. JKIMSU. 2021;10(1):111–114.
- Ke L, An K, Heng S, et al. Paediatric Chromobacterium violaceum in Cambodia: the first documented case. Trop Doc. 2012;42(3):178–179. doi:10.1258/td.2012.120054
- Kim M, Lee H, Suh J, Chang B, Cho K. A case of Chromobacterium infection after car accident in Korea. Yonsei Med J. 2005;46(5):700. doi:10.3349/ymj.2005.46.5.700
- Kumar M. Chromobacterium violaceum: a rare bacterium isolated from a wound over the scalp. IJABMR. 2012;2(1):70. doi:10.4103/2229-516x.96814
- Mamlok R, Mamiok V, Mills G, Daeschner III C, Schmalstieg F, Anderson D. Glucose-6-phosphate dehydrogenase deficiency, neutrophil dysfunction and Chromobacterium violaceum sepsis. J Pediatr. 1987;111(6):852–854. doi:10.1016/s0022-3476(87)80203-2
- Martinez R, Velludo M, Santos V, Dinamarco P. Chromobacterium violaceum infection in Brazil. A case report. Rev Inst Med Trop. 2000;42(2):111–113. doi:10.1590/s0036-46652000000200008
- Mazumder R, Sadique T, Sen D, et al. Agricultural Injury–Associated Chromobacterium violaceum Infection in a Bangladeshi Farmer. Am J Trop Med Hyg. 2020;103(3):1039–1042. doi:10.4269/ajtmh.20-0312
- Midani S, Rathore M. Chromobacterium violaceum infection. South Med J. 1998;91(5):464–466. doi:10.1097/00007611-199805000-00011
- Moretti E, Menozzi F, Elzi L, Lepori M. Chromobacterium violaceum bacteraemia: a new entity in Switzerland. Swiss Med Wkly. 2020;150:1718. doi:10.4414/smw.2020.20220
- Muharam N, Hussin A, Deris Z. Fatal Case of Chromobacterium violaceum Bacteraemia. Bangladesh J Med Sci. 2019;18(2):434–436. doi:10.3329/bjms.v18i2.40722
- Okada M, Inokuchi R, Shinohara K, et al. Chromobacterium haemolyticum-induced bacteremia in a healthy young man. BMC Infect Dis. 2013;13(1):1–4. doi:10.1186/1471-2334-13-406
- Piyasiri D, Samaranayake K, Chinthaka W, Wimalagunarathne N, Rizmy M. Abscess with parotid involvement caused by Chromobacterium violaceum: a rare pathogen. J Postgrad Med. 2014;1. doi:10.4038/jpgim.7333
- Roberts S, Morris A, Wieser B, McIvor N, Ellis-Pegler R. Chromobacterium violaceum infection of the deep neck tissues in a traveler to Thailand. CID. 1997;25(2):334–335. doi:10.1086/516913
- Salleh S, Zaki Z. Urinary tract infection by Chromobacterium violaceum. Int J Infect Dis. 2020;101:178–179. doi:10.1016/j.ijid.2020.09.479
- Shao P, Hsueh P, Lu C, Lee P, Lee C, Huang L. Chromobacterium violaceum infection in children: a case of fatal septicemia with nasopharyngeal abscess and literature review. Pediatr Infect Dis J. 2002;21(7):707–709. doi:10.1097/00006454-200207000-00022
- Shatalov A, Maianski Z. First Case of Chromobacterium violaceum as Urinary Tract Infection Agent in Angola. Open J Med Microbiol. 2019;9(1):37–40. doi:10.4236/ojmm.2019.91005
- Shenoy S, Baliga S, Wilson G, Kamath N. Chromobacterium violaceum septicemia. Indian O Pediatr. 2002;69(4):363–364. doi:10.1007/bf02723225
- Shobha K, Bairy I. Prostatitis by Chromobacterium violaceum A Case report. Int J Med Public Health. 2011;1:54–55. doi:10.5530/ijmedph.4.2011.12
- Thwe PM, Ortiz DA, Wankewicz AL, et al. The Brief Case: recurrent Chromobacterium violaceum Bloodstream Infection in a Glucose-6-Phosphate Dehydrogenase (G6PD)-Deficient Patient with a Severe Neutrophil Defect. J Clin Microbiol. 2020;58(2):e00312–19. doi:10.1128/jcm.00314-19
- Vincent D, Meghana C, Mohan V, Resmi K. Chromobacterium violaceum causing community-acquired urinary tract infection. Indian J Health Sci Biomed Res. 2017;10:97–99. doi:10.4103/2349-5006.198599
- Zhang P, Li J, Zhang Y-Z, Li X-N. Chromobacterium violaceum infection on lower limb skin: a case report. Medicine. 2021;100(6):e24696. doi:10.1097/MD.0000000000024696
- Richard K, Lovvorn J, Oliver S, Ross S, Benner K, Kong M. Chromobacterium violaceum sepsis: rethinking conventional therapy to improve outcome. Am J Case Rep. 2015;16:740–744. doi:10.12659/ajcr.894509
- Tiwari S, Pattanaik S, Beriha S. Nonpigmented strain of Chromobacterium violaceum causing neonatal septicemia: a rare case report. Indian J Pathol Microbiol. 2017;60(3):427–429. doi:10.4103/ijpm.ijpm_479_16
- Vikram K, Sanghai A, Vohra M, Das V. A case of Chromobacterium violaceum. J Microbiol Infect Dis. 2018;8(02):76–79.
- Way S, Sidbury R, Dooms K, Shors A, Qin X, Crane H. Chromobacterium violaceum causing sepsis and focal ulcer in a healthy child. Clin Infect Dis. 2007;15(4):281–283. doi:10.1097/ipc.0b013e31803097d9
- Wheat R, Zuckerman A, Rantz L. Infection due to Chromobacteria: report of eleven cases. Arch Intern Med. 1951;88(4):461–466. doi:10.1001/archinte.1951.03810100045004
- Winder M, Ingram D, Vaughan L, Warner H. Chromobacterium violaceum hepatic abscesses in a previously healthy child. Infect Dis Clin Pract. 2012;20(3):219–220. doi:10.1097/ipc.0b013e3182424189
- Huffam S, Nowotny M, Currie B. Chromobacterium violaceum in tropical northern Australia. MJA. 1998;168(7):335–337. doi:10.5694/j.1326-5377.1998.tb138962.x
- Kaniyarakkal V, Orvankundil S, Lalitha SK, Thazhethekandi R, Thottathil J. Chromobacterium violaceum septicaemia and urinary tract infection: case reports from a tertiary care hospital in South India. Case Rep Infect Dis. 2016;2016. doi:10.1155/2016/6795743
- Macher AM, Casale TB, Fauci AS. Chronic granulomatous disease of childhood and Chromobacterium violaceum infections in the southeastern United States. Ann Intern Med. 1982;97(1):51–54. doi:10.7326/0003-4819-97-1-51
- Ma T, Shi W, Cheng J, et al. Chromobacterium violaceum infection in China: three case reports and literature reviews. Afr J Microbiol Res. 2011;5(20):3096–3102. doi:10.5897/ajmr11.623
- Tee H, Francis A, How S. Chromobacterium violaceum infection. Br J Hosp Med. 2006;67(4):208–209. doi:10.12968/hmed.2006.67.4.20869
- Banik A, Sanjeev H, Kumar A, Snehaa K. Chromobacterium violaceum–an unusual pathogen. Perspectives to ponder! Eastern J Medical Sciences. 2018;6–10. doi:10.32677/ejms.2018.v03.i01.003
- Ognibene A, Thomas E. Fatal infection due to Chromobacterium violaceum in Vietnam. AJCP. 1970;54(4):607–610. doi:10.1093/ajcp/54.4.607
- Pant ND, Acharya SP, Bhandari R, Yadav UN, Saru DB, Sharma M. Bacteremia and urinary tract infection caused by Chromobacterium violaceum: case reports from a tertiary care hospital in Kathmandu, Nepal. J Med Case Rep. 2017;2017:1–4. doi:10.1155/2017/7929671
- Ponte R, Jenkins SG. Fatal Chromobacterium violaceum infections associated with exposure to stagnant waters. Pediatr Infect Dis J. 1992;11(7):583–586. doi:10.1097/00006454-199207000-00013
- Simo F, Reuman P, Martinez F, Ayoub E. Chromobacterium violaceum as a cause of periorbital cellulitis. Pediatr Infect Dis J. 1984;3(6):561–563. doi:10.1097/00006454-198411000-00017
- Sorensen R, Jacobs M, Shurin S. Chromobacterium violaceum adenitis acquired in the northern United States as a complication of chronic granulomatous disease. Pediatr Infect Dis J. 1985;4(6):701. doi:10.1097/00006454-198511000-00028
- Starr A, Cribbett L, Poklepovie J. Chromobacterium violaceum presenting as a surgical emergency. Pediatr Infect Dis J. 1981;74(9):1137–1139. doi:10.1097/00006454-198511000-00028
- Suarez A, Wenokur B, Johnson J, Saravolatz L. Nonfatal chromobacterial sepsis. South Med J. 1986;79(9):1146–1148. doi:10.1097/00007611-198609000-00027
- Ti T-Y, Tan WC, Chong AP, Lee E. Nonfatal and fatal infections caused by Chromobacterium violaceum. Clin Infect Dis. 1993;17(3):505–507. doi:10.1093/clinids/17.3.505
- Yang C, Li Y. Chromobacterium violaceum infection: a clinical review of an important but neglected infection. J Chin Med Assoc. 2011;74(10):435–441. doi:10.1016/j.jcma.2011.08.013
- Bone R, Balk R, Cerra F, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655. doi:10.1378/chest.101.6.1644
- Weinstein MP, Lewis IIJS, Bobenchik AM, et al. Performance Standards for Antimicrobial Susceptibility Testing. 27th. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
- Baker S, Campbell JI, Stabler R, et al. Fatal wound infection caused by Chromobacterium violaceum in Ho Chi Minh city, Vietnam. J Clin Microbiol. 2008;46(11):3853–3855. doi:10.1128/jcm.01068-08
- Brown KL, Stein A, Morrell D. Ecthyma gangrenosum and septic shock syndrome secondary to Chromobacterium violaceum. J Am Acad Dermatol. 2006;54(5):S224–S8. doi:10.1016/j.jaad.2005.07.016
- Lee T, Wright BD. Fulminating chromobacterial septicaemia presenting as respiratory distress syndrome. Thorax. 1981;36(7):557–559. doi:10.1136/thx.36.7.557
- Atapattu DN, Jayawickrama DP, Thevanesam V. An unusual bacterium causing a brain abscess. Emerg Infect Dis. 2001;7(1):159–160. doi:10.3201/eid0701.010127
- Sureisen M, Choon S, Tai C. Recurrent Chromobacterium violaceum infection in a patient with chronic granulomatous disease. Med J Malaysia. 2008;63(4):346–347.
- Orsetti A, Markiewicz P, Epstein M, Conceição O, D’Ippolito G, Ribeiro M. Liver abscesses by Chromobacterium violaceum: a case report of a rare disease. OA Case Reports. 2013;28:19–23. doi:10.13172/2052-0077-2-2-426
- Gao HN, Yuan WX, Yang MF, et al. Clinical significance of C-reactive protein values in antibiotic treatment for pyogenic liver abscess. World J Gastroenterol. 2010;16(38):4871. doi:10.3748/wjg.v16.i38.4871
- Law ST, Li K. Role of C-reactive protein in response-guided therapy of pyogenic liver abscess. Eur J Gastroenterol Hepatol. 2014;26(2):179–186. doi:10.1097/MEG.0b013e328365a3b7
- Parajuli NP, Bhetwal A, Ghimire S, et al. Bacteremia caused by a rare pathogen–Chromobacterium violaceum: a case report from Nepal. Int J Gen Med. 2016;9:441–446. doi:10.2147/ijgm.s125183
- Martinez P, Mattar S. Fatal septicemia caused by Chromobacterium violaceum in a child from Colombia. Rev Inst Med Trop Sao Paulo. 2007;49(6):391–393. doi:10.1590/s0036-46652007000600011
- Olalekan A, Itua F, Mutiu B, Egwuatu T, Akinloye O, Iwalokun B. Case Report on Pleural Empyema Thoracis and Urinary Tract Infection Caused by Chromobacterium violaceum from Lagos, Nigeria. J Med Case Rep. 2019;2019:1–5. doi:10.1155/2019/5321484
- Sachu A, Antony S, Mathew P, et al. Chromobacterium violaceum causing deadly sepsis. Iran J Microbiol. 2020;12(4):364. doi:10.18502/ijm.v12i4.3941
- Sneath PH, Whelan JP, Bhagwan Singh R, Edwards D. Fatal infection by Chromobacterium violaceum. Lancet. 1953;265(6780):276–277. doi:10.1016/s0140-6736(53)91132-5
- Meher-Homji Z, Mangalore RP, Johnson DR. Chromobacterium violaceum infection in chronic granulomatous disease: a case report and review of the literature. JMM Case Rep. 2017;4(1):e005084–e. doi:10.1099/jmmcr.0.005084
- Byamukama D, Kansiime F, Farnleitner AH, et al. Contrasting occurrence of Chromobacterium violaceum in tropical drinking water springs of Uganda. J Water Health. 2005;3(3):229–238. doi:10.2166/wh.2005.028
- Koburger J, May SJA. Isolation of Chromobacterium spp. from foods, soil, and water. Appl Environ Microbiol. 1982;44(6):1463–1465. doi:10.1128/aem.44.6.1463-1465.1982
- Yang CH. Nonpigmented Chromobacterium violaceum bacteremic cellulitis after fish bite. J Microbiol Immunol Infect. 2011;44(5):401–405. doi:10.1016/j.jmii.2010.04.004
- Goldblatt D, Thrasher A. Chronic granulomatous disease. Clin Exp Immunol. 2000;122(1):1–9. doi:10.1046/j.1365-2249.2000.01314.x
- Dromigny J-A, Fall AL, Diouf S, Perrier-Gros-Claude J. Chromobacterium violaceum: a case of diarrhea in Senegal. Pediatr Infect Dis J. 2002;21(6):573–574. doi:10.1097/00006454-200206000-00021
- Swain B, Otta S, Sahu KK, Panda K. Urinary tract infection by Chromobacterium violaceum. J Clin Diagn Res. 2014;8(8):DD01. doi:10.7860/JCDR/2014/9230.4703