Abstract
A 49-year-old male patient who had been diagnosed with variable hairy cell leukemia (HCL-V) was treated with interferon for half a year but exert no obvious effect. After two courses of chemotherapy with cladribine, he achieved remission, and splenomegaly significantly improved (the length in craniocaudal dimension decreased from 15.8cm to 11.8cm). Four years later, the patient got disease relapse and was recommended for another cycle of cladribine (6mg for 7 days). On the last day of cladribine, the patient developed fever with needle-like red rashes on the face, limbs, and trunk. At the very beginning, the rash was lighter in color, sparsely distributed, and without obvious itching. Three days later, the rash gradually darkened, expanded and merged, with itching. With the application of high dose gamma globulin and corticosteroids (prednisolone combined with dexamethasone), the rash finally faded, and the patient was discharged. Rash caused by cladribine is not uncommon, such serious and widespread drug-induced rash is rare, and there are few reports. This article reviewed relevant studies and treatments.
Introduction
Hairy cell leukemia (HCL) is a rare B cell chronic lymphoproliferative disease (B-CLPD), with a characteristic of villous processes on the cell surface and “fried egg-like” cytoplasm. It can be divided into classic hairy cell leukemia (HCL-C) and variable hairy cell leukemia (HCL-V).Citation1 Flow immunotyping showed mature B cell-associated antigen expression and high expression of CD20 and CD22,Citation2 CD 103 and Annexin A1 were specifically expressed in HCL.Citation3,Citation4 The current first-line treatment for HCL, whether HCL-C or HCL-V, is purine nucleoside analogs (PNA) cladribine,Citation5 which has a high complete remission rate, but also with high unpredictable side effects, such as bone marrow suppression, infection, skin reactions, and secondary malignant tumors. Herein, we report a rare case of HCL-V that showed severe denudation-like systemic rash after cladribine treatment. To the best of our knowledge, only five cases have been reported, two with HCL and the other three with chronic lymphocytic leukemia (CLL). Our case will help better understanding the development and prognosis of the severe rash in HCL during cladribine application.
Case Presentation
A 49-year-old male without underlying diseases presented abdominal distension and fatigue in January 2015. He was diagnosed with HCL-V by bone marrow aspiration, immunophenotyping, and related gene tests (BRAF-V600E). Two cycles of Cladribine (7 mg, d1–d5 (day, d)) were administered on August 8 and September 23, 2016, respectively. The patient did not present fever, rash, or other side effects during the process. Moreover, the size of the spleen was significantly reduced after treatment (with a length in craniocaudal dimension from 15.8cm to 11.8cm), and the case achieved disease remission.
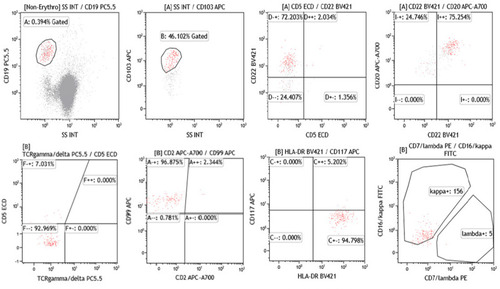
Four years later (On July 9th, 2019), the patient complaining of fatigue and recurrence of abdominal distension, and admitted to our hospital. Blood routine test showed white blood cell (WBC) 2.6×109/L, lymphocyte 41.7%, hemoglobin (Hb) 132 g/L, and platelet (PLT) 88×109/L. Lymphocyte subsets with flow cytometry showed 54.63% T cell (CD3+CD45), 31.06% B cell (CD19+) and 10.4% Treg (CD4+/CD25+/CD127-). Ultrasound indicated splenomegaly (with a length of 13.8cm in craniocaudal dimension), and lymphadenopathy in bilateral neck, supraclavicular, and inguinal. Bone marrow aspiration showed that lymphocytes accounted for 12.5%, and no obvious abnormality was detected in morphology. Flow immunophenotyping can detect abnormal B lymphocyte population, the phenotype was similar to the initial course and mainly expressed CD19++, CD45++, HLA-DR++, CD22++, CD20++, CD103++, CD99+, CD25-, CD117-, CD56-, and FMC7- (), which indicated the relapse of the disease.
Figure 1 Flow cytometric (FCM) immunophenotyping of the bone marrow. FCM indicated that the abnormal B lymphocyte population mainly expressed CD19, CD45, HLA-DR, CD22, CD20, CD103, CD99 and negative for CD25, CD117, CD56, and FMC7.
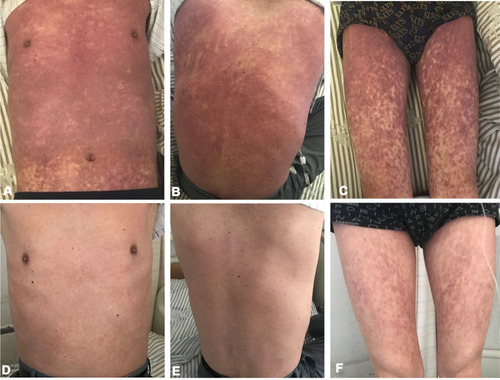
Four days later, the patient was initiated with cladribine at the dosage of 6 mg, from day (d) 1 to d7. On the last day of treatment, the patient developed a slight skin rash with itching on the chest, for which, anti-allergic drugs were administered. However, he had a high fever (over 39°C) and shivering with rash spreading throughout the body in the afternoon. The detection of C reaction protein (CRP) and procalcitonin (PCT) showed slight increase (CRP 30mg/L and PCT 0.35ng/mL), and Chest CT did not indicate any infection. On the next day, the rash still worsened without oral mucosa and genitalia involvement. The relative of the patient complained about the situation and reject to perform the skin biopsy. Considering the continued fever, the possibility of secondary infection could not be fully excluded. Moxalactam was further administered for anti-infection, and dexamethasone 5 mg×7 d combined with methylprednisolone 40 mg×10 d for anti-inflammatory. During the first two days of treatment, the patient still had a high fever with a systemic rash. Moreover, the rash on the chest and abdomen, back, and both lower limbs (–) darkened and was intensively distributed. Ascribe to the hematological toxicity of cladribine, the patient suffered from neutrophil deficiency, granulocyte colony-stimulating factor (G-CSF) was used to stimulate the hematopoiesis and the antibiotic was changed to Meropenem. Three days later, the patient’s body temperature returned to normal, but no significant decrease was observed in the systemic rash. Immunoglobulin 15g/day was added for 3 days to alleviate the immune response, ebastine was taken orally, and sodium thiosulfate was administered for anti-allergic treatment. Five days more, the rash began to fade, and disappeared 10 days later. Then, the patient was discharged (–).
Figure 2 The change of skin rash induced by cladribine in HCL, before (A–C) and after (D–F) rescue. The diffuse, exfoliated, and itchy erythema (throughout the chest, back and limbs) gradually faded after a combination treatment with corticosteroids, immunoglobulin, ebastine, and sodium thiosulfate.
Discussion
HCL accounts for 2% of adult leukemia, the median age of onset was 60–70 years.Citation6 Purine analogs are currently the standard first-line treatment for any type of HCL. Cladribine and pentostatin alone can achieve a high complete remission (CR) rate, but HCL is prone to relapse.Citation7 Rosenberg et alCitation8 reported the first-line treatment of cladribine in 88 young patients with HCL, among whom, the longest median overall survival (OS) was 231 months. Adverse reactions of cladribine including bone marrow suppression (neutropenia, thrombocytopenia and anemia), infection (bacteria, virus, fungus, and herpes simplex), skin reactions (rash, papules, and erythema with the incidence rate 21–51%),Citation2,Citation9 secondary malignant tumors, autoimmunity disorder, and rare toxicity.Citation10,Citation11 Among which, bone marrow suppression and infection are most common. The presentation of skin reactions was not rare during cladribine treatment, but most of them were mild rashes. Currently, limited data are available (3 papers with 5 cases) on extremely severe skin rash caused by cladribine (). In our case, the patient developed a severe systematic skin rash (except oral cavity and genitalia) on the last cladribine injection, which presented with high fever, diffuse, exfoliated, and itchy erythema throughout the body (face, limbs and trunk). With a timely multiple treatment for 10 days, including corticosteroids and immunoglobulin, the fever and rash got completely controlled and the occurrence of severe complication such as exfoliative dermatitis was avoided.
Table 1 Comparison of the Literature on Cases of Severe Rash During the Cladribine Treatment in HCL and CLL
The underling mechanism of severe rash after cladribine application is not clear. Some studies considering that a large part of the skin reaction is associated with allopurinol. When cladribine and allopurinol are used together, it will increase the chance of rash, and the skin reactions alleviated spontaneously after allopurinol withdrawal.Citation12,Citation13 Two patients with HCL who received cladribine treatment in the same center had severe skin reactions;Citation14 one received a combination of allopurinol and compound sulfamethoxazole, and on day 5 after treatment, the patient developed a severe rash with life-threatening bleeding and disseminated intravascular coagulation (DIC). Thus, the allopurinol and compound sulfamethoxazole were ceased, and hydrocortisone 200 mg/4h was administered. The rash disappeared gradually after 1 week.Citation15 Typically, allopurinol is used to treat hyperuricemia and tumor lysis,Citation13 and patients using cladribine for HCL should avoid it as much as possible, so as in our case. In addition, some studies reported that the skin reaction after cladribine treatment might be correlated to increased drug hypersensitivity caused by cladribine-induced T cell imbalance. Ganzel et alCitation9 collected 35 HCL patients treated with cladribine, and found that the frequency of rashes was quite high (18/35, 51%), which could be attributed to the simultaneous use of penicillin/trimethoprim-sulfamethoxazole (TMP-SMZ), G-CSF, and sulfonamides.Citation16 Meunier reported that 21% (7/33) had skin reactions within one month after cladribine, and 5 might be related to the use of antibiotics.Citation17 Also, in CLL patients treated with cladribine, the occurrence of skin lesions might be attributed to the simultaneous use of ciprofloxacin and fluconazole.Citation18,Citation19 In our case, we consider that the onset of body rash may ascribe to the accumulation of cladribine, and the early prophylactic anti-infection may contribute to the delayed allergy. Simultaneously, to mitigate bone marrow suppression, G-CSF was utilized to promote the hematopoiesis. The use of antibiotics and G-CSF might accelerate the induction or aggravate the occurrence of severe rash.
The incidence of rash in non-leukemia disease, such as multiple sclerosis, is quite low (~2.4%)Citation20 when compared with leukemia patient treated with cladribine. This reminds us that the primary disease may also be related to skin reactions. For severe and life-threatening skin reactions, we should intervene early to prevent the occurrence of toxic necrosis and dissolution. Desensitization schemes should be considered when simultaneous use with antibiotics, and the use of antibiotics and G-CSF should also be in caution when it is not determined whether the infection is bacterial or bone marrow suppression appeared agranulocytosis.
Abbreviations
HCL, hairy cell leukemia; CLPD, chronic lymphoproliferative disease; PNA, purine nucleoside analogs; CLL, chronic lymphocytic leukemia; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; CRP, C reaction protein; PCT, procalcitonin; G-CSF, granulocyte colony-stimulating factor; CR, complete remission; OS, overall survival; DIC, disseminated intravascular coagulation; TMP-SMZ, trimethoprim-sulfamethoxazole.
Ethics Statment
This study was approved by the ethical committee of the Zhejiang Provincial Hospital of TCM. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- Robak T. Management of hairy cell leukemia variant. Leuk Lymphoma. 2011;52(Suppl 2):53–56. doi:10.3109/10428194.2011.566392
- Tiacci E, Pettirossi V, Schiavoni G, Falini B. Genomics of hairy cell leukemia. J Clin Oncol. 2017;35(9):1002–1010. doi:10.1200/JCO.2016.71.155628297625
- Wang HY, Zu Y. Diagnostic algorithm of common mature B-cell lymphomas by immunohistochemistry. Arch Pathol Lab Med. 2017;141(9):1236–1246. doi:10.5858/arpa.2016-0521-RA28608720
- Dong HY, Weisberger J, Liu Z, Tugulea S. Immunophenotypic analysis of CD103+ B-lymphoproliferative disorders: hairy cell leukemia and its mimics. Am J Clin Pathol. 2009;131(4):586–595. doi:10.1309/AJCPL13YDUHFKPJU19289595
- Benz R, Arn K, Andres M, et al. Prospective long-term follow-up after first-line subcutaneous cladribine in hairy cell leukemia: a SAKK trial. Blood Adv. 2020;4(15):3699–3707. doi:10.1182/bloodadvances.202000216032777066
- Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood. 2017;129(5):553–560. doi:10.1182/blood-2016-01-68942227903528
- Bibi A, Java S, Chaudhary S, et al. BRAFV600E mutation in hairy cell leukemia: a single-center experience. Indian J Pathol Microbiol. 2018;61(4):532–536.30303143
- Rosenberg JD, Burian C, Waalen J, Saven A. Clinical characteristics and long-term outcome of young hairy cell leukemia patients treated with cladribine: a single-institution series. Blood. 2014;123(2):177–183. doi:10.1182/blood-2013-06-50875424192579
- Ganzel C, Gatt ME, Maly A, Ben-Yehuda D, Goldschmidt N. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53(6):1169–1173. doi:10.3109/10428194.2011.63586422035415
- Sigal DS, Miller HJ, Schram ED, Saven A. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood. 2010;116(16):2884–2896. doi:10.1182/blood-2010-02-24614020634380
- Wang TY, Li ZJ, Lyu R, et al. [Clinical analysis of 24 patients of hairy cell leukemia treated by cladribine]. Zhonghua Xue Ye Xue Za Zhi. 2018;39(6):491–495.30032567
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122(5):768–770. doi:10.1046/j.1365-2141.2003.04506.x12930387
- Espinosa Lara P, Quiros Redondo V, Aguado Lobo M, Jimenez-Reyes J. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96(7):1209–1210. doi:10.1007/s00277-017-2992-z28391438
- Grey MR, Flanagan NG, Kelsey PR. Severe skin rash in two consecutive patients treated with 2-chlorodeoxyadenosine for hairy cell leukaemia at a single institution. Clin Lab Haematol. 2000;22(2):111–113. doi:10.1046/j.1365-2257.2000.00283.x10792402
- Kreitman RJ. Hairy cell leukemia: present and future directions. Leuk Lymphoma. 2019;60(12):2869–2879. doi:10.1080/10428194.2019.160853631068044
- Johnston JB, Glazer RI, Pugh L, Israels LG. The treatment of hairy-cell leukaemia with 2ʹ-deoxycoformycin. Br J Haematol. 1986;63(3):525–534. doi:10.1111/j.1365-2141.1986.tb07530.x3488071
- Meunier P, Castaigne S, Bastie JN, Chosidow O, Aractingi S. Cutaneous reactions after treatment with 2-chlorodeoxyadenosine. Acta Derm Venereol. 1996;76(5):385–386.8891014
- Robak T, Sysa-Jedrzejowska A, Robak E, Dabkowski J, Blasinska-Morawiec M. 2-chlorodeoxyadenosine (cladribine) induced allergic cutaneous reactions with eosinophilia in a patient with B-cell chronic lymphocytic leukemia. J Med. 1997;28(3–4):199–209.9355024
- Rossini MS, de Souza EM, Cintra ML, Pagnano KB, Chiari AC, Lorand-Metze I. Cutaneous adverse reaction to 2-chlorodeoxyadenosine with histological flame figures in patients with chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2004;18(5):538–542. doi:10.1111/j.1468-3083.2004.00969.x15324388
- Cook S, Vermersch P, Comi G, et al. Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Mult Scler. 2011;17(5):578–593. doi:10.1177/135245851039134421228029
