Abstract
Purpose of Review
High-flow nasal oxygen and noninvasive ventilation are two alternative strategies to standard oxygen in the management of acute respiratory failure.
Discussion
Although high-flow nasal oxygen has gained major popularity in ICUs due to its simplicity of application, good comfort for patients, efficiency in improving oxygenation and promising results in patients with acute hypoxemic respiratory failure, further large clinical trials are needed to confirm its superiority over standard oxygen. Non-invasive ventilation may have deleterious effects, especially in patients exerting strong inspiratory efforts, and no current recommendations support its use in this setting. Protective non-invasive ventilation using higher levels of positive-end expiratory pressure, more prolonged sessions and other interfaces such as the helmet may have beneficial physiological effects leading to it being proposed as alternative to high-flow nasal oxygen in acute hypoxemic respiratory failure. By contrast, non-invasive ventilation is the first-line strategy of oxygenation in patients with acute exacerbation of chronic lung disease, while high-flow nasal oxygen could be an alternative to non-invasive ventilation after partial reversal of respiratory acidosis. Questions remain about the target populations and non-invasive oxygen strategy representing the best alternative to standard oxygen in acute hypoxemic respiratory failure. As concerns acute on-chronic-respiratory failure, the place of high-flow nasal oxygen remains to be evaluated.
Introduction
Respiratory failure is defined as the failure of the respiratory system in one or both of its gas exchange functions, ie, oxygenation of and/or elimination of carbon dioxide from mixed venous blood.Citation1 Oxygen therapy is usually the first-line treatment in acute respiratory failure and classically includes standard oxygen, noninvasive ventilation (NIV) and, more recently, high flow nasal cannula oxygen therapy (HFNC). Oxygen therapy delivered through a non-rebreathing face mask is the oldest non-invasive oxygen support, first described in 1946.Citation2 NIV was developed less than 10 years after, in 1952, in an initial study reporting NIV delivered through continuous positive airway pressure (CPAP) in cardiogenic pulmonary edema.Citation3 After the 1990s, NIV through CPAP or applied with pressure support (PS) and positive end expiratory pressure (PEEP) have been widely used with a strong level of evidence in cardiogenic pulmonary edemaCitation4 and COPD exacerbation.Citation5,Citation6 HFNC is another noninvasive oxygen support, first described in 1968, and is known for its ability to provide a high level of FiO2 due to high gas flow.Citation7 In the 2010s, its use spread among adult patients with acute hypoxemic respiratory failure, after first having being used in pre-term neonates and pediatric care, as a first-line treatment for respiratory distress syndrome, and apnea of prematurity. Contrary to NIV, most of the clinical studies on HFNC have preceded physiological studies and reported good comfort and a better prognosis in acute hypoxemic respiratory failure than with other noninvasive oxygenation supports, thereby justifying its widespread use in intensive care units.Citation8,Citation9
The expected aims of noninvasive oxygenation supports () are to provide comfort by preserving physiological pathways of airway protection (ie, cough and clearance of secretions)Citation10 and to avoid invasive mechanical ventilation and related morbidity or mortality. Adverse effects include ventilator-induced lung injury,Citation11,Citation12 ventilator-associated pneumonia,Citation13 and complications related to sedation and neuromuscular blockage.Citation14 Spontaneous breathing may have further benefits such as preventing diaphragm dysfunction and atrophy,Citation15 and it yields increased aeration of the dependent lung, which minimizes ventilation/perfusion mismatch.Citation16 However, maintenance of spontaneous breathing may expose the patient to prolonged inspiratory efforts and delayed intubation with potential impact on prognosis.Citation11,Citation17–Citation19
Table 1 Aims of Noninvasive Oxygen Supports in Acute Respiratory Failure
This review summarizes existing literature about the efficacy and safety of non-invasive oxygenation supports, ie, NIV and HFNC, in the management of acute respiratory failure.
Acute Hypoxemic Respiratory Failure
Acute hypoxemic respiratory failure or de novo respiratory failure is characterized by a gas exchange failure manifested by severe acute hypoxemia without hypercapnia. Hypoxemia results from lung failure due to an unequal ventilation/perfusion ratio, and increased shunt and/or diffusion impairment. The definition of acute hypoxemic respiratory failure is not clearly established. The level of hypoxemia varies from study to study with a PaO2/FiO2 ratio equivalent below or equal to 300 mm Hg or 200 mm Hg. The most common cause is pneumonia, and acute-on-chronic respiratory failure and cardiogenic pulmonary edema are ruled out.Citation8 Acute respiratory distress syndrome (ARDS) is a subset of acute hypoxemic respiratory failure. However, to fulfill the definition, it requires not only the presence of bilateral lung infiltrates on chest imaging but also a positive end-expiratory pressure (PEEP) level of at least 5 cmH2O assessed under positive pressure ventilation and hypoxemia not fully explained by fluid overload or cardiac dysfunction.Citation20
During acute hypoxemic respiratory failure, breathing patterns result in hyperventilation due to high respiratory drive and inspiratory effort leading to high tidal volumes, increased respiratory rates and, finally, to hypocapnia. However, spontaneous breathing in this setting can be potentially harmful and lead to a vicious circle generated by hypoxemia, dysregulated and high inspiratory effort, leading in turn to transpulmonary pressure swings and potential inhomogeneous redistribution of tidal volume generating local injurious forces. The concept of patient self-inflicted lung injury (P-SILI) helps to explain ventilation-induced lung injury during spontaneous breathing ().Citation17,Citation19
Figure 1 Physiological responses of high-flow nasal oxygen therapy and noninvasive ventilation to acute hypoxemic respiratory failure.
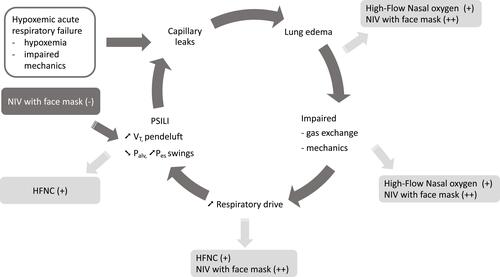
Physiological Responses of Non-Invasive Oxygen Support
Consequently, one of the objectives of non-invasive oxygen support is to unload respiratory effort and to mitigate the consequence of high inspiratory effort (). However, such a strategy should be conducted cautiously so as to avoid delayed intubation, which may increase risk of mortality.Citation18,Citation21 Since the end of the 90ʹs, NIV has shown contradictory results in acute hypoxemic respiratory failure,Citation6 while HFNC is currently on the rise after having delivered benefits in patients with acute hypoxemic respiratory failure.Citation8,Citation9
HFNC is an oxygen device able to deliver high flows of warmed humidified gas, up to 70 L/min with a temperature set from 33° to 37° C, with a FiO2 ranging from 21% to 100%. Physiological responses to HFNC include PEEP effect, due to low levels of positive pressure in the upper airways favoring an increase in end-expiratory lung volume and oxygenation, and also to ventilatory support due to the effects on dead-space washout, leading to a decreased respiratory rate and, finally, work of breathing (). During acute hypoxemic respiratory failure, the inspiratory effort leads to a high peak inspiratory flow reaching a mean of 30–40 L/min, which can exceed 60 and even reach 120 L/min in more severe patients.Citation22 The high flow generated by the HFNC system is able to provide high and controlled FiO2. Although the system is open, delivery of a high flow rate usually set at 50 L/min and able to reach 70 L/min in most cases exceeds the patient’s peak inspiratory flow rate and preserves from dilution of inhaled gas with room air. In a physiological study including healthy volunteers, Sim et al measured FiO2 in their pharynx during oxygen delivery through several devices such as a standard mask, a non-rebreathing mask and HFNC system.Citation23 With a standard mask, FiO2 did not exceed 0.6 despite a flow of 12 L/min, and it dropped below 0.5 when acute respiratory failure was simulated by thoracic contention. Although the non-rebreathing mask avoided such a FiO2 drop, the highest FiO2 obtained under an oxygen flow rate of 15 L/min was less than 0.7. By comparison, FiO2 reached 0.85 using the HFNC system set, with a flow rate of 40 L/min.Citation23 Another effect of the HFNC system is to generate a low level of positive pressure in the upper airway, which is directly proportional to the gas flow delivered. However, due to the open nature of the system (with air leakage), the pressure levels are quite variable and not controlled. Although positive pressure is markedly reduced when the patient opens his mouth, it generates a PEEP effect with alveolar recruitment that may help to improve gas exchange. In physiological studies assessing pulmonary volumes with electrical impedance tomography after cardiac surgery or during acute hypoxemic respiratory failure, increased end-expiratory lung volume directly proportional to gas flowCitation24 was found with HFNC as compared to standard oxygen therapy, suggesting alveolar recruitment reflecting a PEEP effect.Citation25,Citation26 Given these different physiological effects, HFNC can appear as a support for oxygenation able to decrease workof breathing. Many authors have highlighted in patients with acute respiratory failure treated first by standard oxygen through a non-rebreathing mask and then by HFNC, a decrease in work of breathing under HFNC assessed by swings of esophageal pressure (diminution of pressure–time product during inspiration).Citation26,Citation27 Mauri et al assumed that better working conditions could be partially due to improvement of inspiratory effort and pulmonary compliance possibly favored by increased ventilation homogeneity.Citation26 Otherwise, the high-flow rate of continuously delivered gas may flush the upper airways, generating a washout of dead space and then flushing out carbon dioxide.Citation28,Citation29 This effect, which is associated with mechanically improved thoracic properties, results in reduced inspiratory effort and minute ventilation requirement. This is consistent with the commonly reported finding of decreased respiratory rate and work of breathing with HFNC.Citation26,Citation27 Consequently, HFNC improves pattern of breathing by increasing oxygenation, decreasing inspiratory effort, respiratory rate and work of breathing, thereby possibly mitigating the consequences of self-inflicted lung injury ().
The main characteristic of NIV is assistance of spontaneous breathing by administration of preset positive pressure into the lungs through an external interface, ie, a commonly used face mask connected to a humidification system, a heated humidifier or a heat and moisture exchanger, and a ventilator. The most commonly used mode combines pressure support (PS) ventilation with PEEP,Citation30 or else simply applies continuous positive airway pressure (CPAP).Citation31 NIV through facemask is usually applied with low PEEP levels (5–8 cmH2O), because of the presence of air leaks potentially leading to discomfort and poor tolerance. NIV is able to increase oxygenation by providing high FiO2 up to 100% and through PEEP and to achieve gas exchange as effective as invasive ventilation.Citation10 In addition to improving oxygenation, PEEP alone or CPAP reduces inspiratory effort in spontaneous breathing patients with acute respiratory failure compared to standard oxygen, as attested by a reduction in esophageal pressure swings.Citation27 Moreover, Morais et al showed that increasing PEEP levels minimized lung injury, especially in dependent lung, in animals with ARDS and spontaneous breathing under mechanical ventilation.Citation32 In a physiological study including patients under NIV for mild ARDS, increased pressure support resulted in proportionally decreased inspiratory effort and dyspnea, as has been determined by changes in esophageal and diaphragmatic pressures.Citation33 Moreover, increasing pressure support resulted in proportionally increased tidal volumes and potential alveolar recruitment, as suggested by some authors ().Citation33
However, air leaks, discomfort and skin breakdown limit the tolerability of facemask NIV, rendering prolonged treatments with specific settings, ie high PEEP, difficult to apply, and it may even lead to a need for intubation in 10% of the cases.Citation34–Citation36 Several authors have proposed the helmet interface as an alternative interface for NIV delivery in hypoxemic patients.Citation37–Citation39 The helmet is a transparent hood that covers the entire head, sealed with a soft neck ring, thereby producing a breathing circuit semi-closed from the outside environment. NIV delivered through helmet has been shown to provide better tolerability and less air leaks, enabling the possibility to deliver prolonged treatments with high PEEP reaching 12 cm H2O.Citation40,Citation41 A physiological study comparing NIV delivered through helmet and face mask showed that with a helmet, improved breathing patterns, especially inspiratory muscle load, consisted in high PEEP and pressure support levels, which increased by 50% compared with the facemask settings.Citation42 Indeed, some of the pressure support is dissipated in the helmet and does not necessarily correspond to the pressure inside at the airway opening and in the alveoli. Therefore, the optimization of NIV delivery may involve changes in ventilator settings by increasing PEEP levels and changes in the interface improving comfort, with the aim of achieving prolonged sessions.
Clinical Impact and Limitations of Non-Invasive Oxygen Supports
As reported with invasive mechanical ventilation,Citation11 conventional NIV delivered with facemask may be deleterious in acute hypoxemic respiratory failure due to barotrauma favored by the high respiratory drive, leading to high inspiratory effort, and the synchronization with the pressure-support, which may result in high tidal volumes.Citation12 Accordingly, Tonelli et al have put forward the hypothesis of a relationship between excessive spontaneous patient effort and NIV failure.Citation43 In the 30 included patients with acute hypoxemic respiratory failure undergoing a trial of NIV, tidal changes in esophageal pressure were not reduced within 2 hours in patients who failed with NIV, whereas these changes were significantly reduced in those who succeed with NIV.Citation43 High expired tidal volumes, ie, exceeding 9.5 mL/kg of predicted body weight, were independently associated with NIV treatment failure. Similarly, two clinical studies had previously reported that tidal volumes exceeding 9 or 9.5 mL/kg of predicted body weight during NIV application were strongly associated with intubation and mortality in larger samples of patients treated for acute hypoxemic respiratory failure.Citation44,Citation45 In the study by Carteaux et al, the authors attempted to target a tidal volume from 6 to 8 mL/kg of predicted body weight under NIV, while nearly half of the patients had a tidal volume above 10 mL/kg. In our study, NIV settings between intubated and not intubated patients were similar, with pressure support around 8 and PEEP around 5 cm H2O, notwithstanding the higher tidal volumes generated in intubated patients.Citation45 Patients who needed intubation under NIV did not exhibit higher severity scores or higher respiratory rates than the others, ie the usual criteria to assess severity at bedside. Bellani et al showed that during spontaneous breathing in patients receiving invasive ventilation, a significant reduction of pressure support did not result in decreased patient-generated tidal volume and did not reduce transalveolar pressure.Citation46 Therefore, high tidal volumes might be considered as the consequence of the high respiratory drive leading to high inspiratory efforts due to hypoxemia, and thereby reflect the severity of the underlying respiratory disease. Moreover, they may further worsen a pre-existing lung insult by inducing ventilator-induced lung injury (VILI). The clinical benefits of conventional NIV delivered through facemask in patients with acute hypoxemic respiratory failure are uncertain,Citation8,Citation47–Citation51 as underlined in recent clinical practice guidelines on the use of NIV.Citation6 Indeed, international experts were unable to recommend NIV in this setting, given the low certainty of evidence concerning risks of intubation and mortality.Citation6
However, NIV delivered through helmet could change this paradigm with different settings and subsequent physiological effects. In a monocenter randomized controlled trial including 83 patients with ARDS treated first with NIV through facemask, NIV delivered secondarily through helmet seemed to be more beneficial than NIV continued through facemask, ie, lower intubation and mortality rates.Citation52 In addition to different interface application, NIV settings significantly differed between groups with higher positive end-expiratory pressure and lower pressure support levels in the helmet group. Benefits were probably due to the physiological advantages of helmet, namely delivery of higher PEEP in continuous sessions with enhanced comfort. Whether the benefits are due to decreased inspiratory effort or to optimized alveolar recruitment is not clear. In fact, measurement of tidal volumes under helmet, ie, a simple approach of inspiratory effort, is not routinely feasible and did not appear in the trial. As a result, the impact of settings and potential contribution to reduced transpulmonary pressure and lung injury (VILI) by decreasing ventilator assistance is difficult to analyze.Citation52
Whether HFNC is efficient in preventing superimposed lung injury in spontaneous breathing patients during acute hypoxemic respiratory failure remains to be established. In a randomized controlled trial comparing NIV, HFNC and standard oxygen in 313 patients with acute hypoxemic respiratory failure, we reported significant differences in favor of HFNC in terms of mortality (12% versus 23% and 28% with standard oxygen and NIV, respectively) and of intubation for severe hypoxemic patients (38%, 47% and 50%, respectively).Citation8 Considering these results, HFNC appeared superior to NIV and standard oxygen, given that it could prevent VILI (as compared to NIV) and also PSILI (as compared to standard oxygen). However, in another large randomized controlled trial including 778 immunocompromised patients with acute respiratory failure, the superiority of HFNC to standard oxygen was not confirmed as regards risk of intubation (39% versus 44%, respectively) or mortality (36% in both groups of treatment).Citation53 Accordingly, recent clinical practice guidelinesCitation54 and a meta-analysisCitation55 reported a reduced risk of intubation but not of mortality with HFNC as compared to standard oxygen. Currently, there is no strong evidence of the superiority of HFNC or conventional NIV delivered through facemask over standard oxygen in the management of acute hypoxemic respiratory failure whatever the severity. Although HFNC has gained popularity among intensivists thanks to its simplicity of application, good comfort for patients and efficiency in improving oxygenation, only one randomized controlled trial has reported benefits of HFNC as compared to NIV or standard oxygen,Citation8 whereas another randomized controlled trial did not show any superiority of HFNC as compared to standard oxygen.Citation53 Further studies are needed to confirm the place of HFNC in this setting and could help to determine the patient population, ie, mild, moderate or severe hypoxemic patients, most likely to benefit from HFNC in terms of intubation and mortality.
Experience of the Pandemic: Optimization of Non-Invasive Oxygen Support Strategies
During the COVID-19 pandemic, use of non-invasive oxygen supports was heterogeneous across countries; for instance, HFNC was less frequent in Italy or North America than in France (with use reaching 19%), while standard oxygen was the most frequent oxygen support applied.Citation56–Citation58 This was due to contradictory recommendations and a paucity of evidence-based management guidelines.Citation59 One of the major concerns was to limit the spread of infection among health-care workers, given that procedures liable to disperse viral particles, the so-called “aerosol-generating procedures”, were avoided whenever possible. Thereafter, several simulation studies using manikin model of exhaled air dispersion distances and analyzing concentrations of aerosol from the respiratory tract in room air showed that this risk was not higher under HFNC than NIV or standard oxygen devices.Citation60,Citation61 Moreover, barrier procedures and, specifically, the use of a surgical mask on HNFC helped to reduce the risk of bio-aerosol dispersion without impairment of patient blood oxygenation.Citation62
Whether or not the specificity of patients with COVID-19-induced respiratory failure might modify the efficiency of the different non-invasive oxygen supports has yet to be conclusively determined. Of note, these patients present with a remarkable disconnect at rest between profound hypoxemia without proportional signs of respiratory distress, no sensation of dyspnea or increased respiratory work, whatever the rapidity of deterioration. This is illustrated by the comparing patient populations with acute hypoxemic respiratory failure caused mainly by either bacterial pneumoniaCitation8 and with COVID-19.Citation38 Despite similar intubation rates, 38% and 34%, in the two respective populations, PaO2/FiO2 ratio at enrollment in non-COVID patients was higher (150–160 mm Hg) with a high respiratory rate (around 33 breaths/min), while patients with COVID-19 had more severe oxygen impairment (PaO2/FiO2 ratio of 102–105 mm Hg) with a surprisingly lower respiratory rate (28 breaths/min).Citation8,Citation38 This peculiar pattern of COVID-19-induced respiratory failure references the concept of “silent” or “happy” hypoxemia.Citation63,Citation64 Possible pathophysiological mechanisms include 1) intrapulmonary shunting, due to local interstitial edema, resulting in ventilation-perfusion ratio mismatch and in an alveolar to arterial oxygen gradient; 2) loss of lung perfusion regulation, with involvement of the renin-angiotensin system, intravascular microthrombi, favored by local acute inflammation; and 3) endothelial injury resulting in an imbalance between procoagulant and fibrinolytic activity; these different abnormalities lead to impaired diffusion capacity.Citation64
Silent hypoxemia during COVID-19-induced respiratory failure raises questions about the timing of initiation of non-invasive oxygen supports, whether before or after onset of tachypnea, and intubation to switch to invasive ventilation, based on level of hypoxemia or signs of respiratory distress. One potential risk of non-invasive oxygen supports is to delay intubation by masking signs of respiratory distress.Citation21 A North American study of six COVID-19-specific ICUs included 231 patients, of whom 175 were intubated after having received different non-invasive oxygen supports. Timing of intubation was not associated with mortality, and patients under HFNC intubated after 24 hours of treatment did not have a higher risk of mortality than those intubated earlier.Citation65 Two other observational studies have shown less intubation with HFNC as compared to standard oxygen, but no difference was reported in mortality rates.Citation66,Citation67 Up until now, most published studies in this setting have been observational and mainly showed better oxygenation under HFNC than standard oxygen, with intubation rates ranging from 30% to 50%. A recent trial compared HFNC with standard oxygen in 220 patients with severe COVID-19 for the need for intubation and time to clinical recovery until day 28. Authors reported that use of HFNC significantly decreased need for mechanical ventilation support and time to clinical recovery compared with standard oxygen therapy, but there was no difference in mortality between treatments, possibly due to an underpowered study.Citation68 Thereby, the question of the benefits of HNFC as a treatment for the management of COVID-19 induced respiratory failure remains incompletely unanswered.
A mega-trial pooling several trials from different countries aimed to evaluate whether HNFC optimized with sessions of awake prone position in patients with severe COVID-19-related acute hypoxemic respiratory failure could reduce either death or intubation.Citation69 Among the 1121 patients included in the intention-to-treat analysis, 94% were enrolled in three (Mexico, USA and France) of the six countries participating in the mega-trial. Results showed a favorable effect of awake prone position on the primary outcome, significant reduced intubation rates, while mortality rates did not differ between the two groups. However, there was heterogeneity among the different countries, with a significant effect of awake prone position in Mexico, which was not the case in the other countries. One explanation may be the longer duration of awake prone position, around 8 hours in Mexico as compared to fewer than 5 hours in the other countries. As a result, this approach requires confirmation in future studies as does the reference treatment for oxygenation.
Given the limitations of standard NIV delivered through facemask in acute hypoxemic respiratory failure, some authors have proposed protective NIV delivered through helmet, the objectives being to reduce inspiratory effort and to render spontaneous breathing less injurious by preventing the risk of VILI and P-SILI. Grieco et al showed in a physiological study that NIV delivered through helmet with a high level of PEEP (≥10 cm H2O) and pressure support around 10 cm H2O compared to HFNC could improve oxygenation, relieve dyspnea and reduce inspiratory effort.Citation39 In 2020, the same authors conducted a multicenter randomized controlled trial, including 109 patients with severe COVID-19-related respiratory failure randomly assigned to receive HFNC at 60 L/min or NIV through a helmet set as described above.Citation38 There was no difference in the primary outcome (days free of respiratory support at day 28). Although intubation rate was lower and invasive ventilation-free days at day 28 were higher after treatment by NIV through helmet, mortality did not differ compared to HFNC. In fact, the mortality rate was higher in patients who failed helmet NIV as compared those who failed HFNC.
As a result (), HFNC may represent an alternative to standard oxygen or conventional NIV through facemask in acute hypoxemic respiratory failure. However, there is no strong evidence favoring the use of HFNC in this setting, as it seems to reduce risks of intubation but not those of mortality. While the experience of COVID-19-related respiratory failure has highlighted the potential benefit of protective NIV delivered through a helmet with high levels of PEEP compared to HFNC, it once again seems to reduce risks of intubation but not those of mortality. We are conducting a trial in France with the aim at assessing the efficiency of HFNC versus standard oxygen and at determining the standard non-invasive oxygen strategy most apt to manage patients with acute hypoxemic respiratory failure, including those infected by COVID-19 (NCT 04468126). Further studies are necessary to confirm the possible superiority of HFNC over standard oxygen and the benefits of protective NIV through a helmet.
Table 2 Summary of Proposals for First-Line Therapies in Acute Respiratory Failure
Acute-on-Chronic Respiratory Failure
Acute respiratory failure resulting in acute hypercapnia appears in cases of underlying chronic respiratory failure, ie chronic obstructive pulmonary disease (COPD) exacerbation. Indeed, many of these patients may have hypercapnia at baseline and the development of acidosis indicates acute-on-chronic respiratory failure. Acute respiratory acidosis occurs when the respiratory muscles fail to achieve adequate alveolar ventilation contrasting with high levels of diaphragmatic activity. Consequently, pump failure leads to increased arterial carbon dioxide (CO2) and respiratory acidosis ensues.Citation1,Citation6 As of now, the recommended treatment includes NIV, which provides ventilatory support, avoids intubation and a need for invasive ventilation. Since 1990, NIV has shown benefits in the management of COPD patients. An initial physiological study conducted in 1990 reported decreased PaCO2 and increased pH after a 45-min NIV session.Citation70 Moreover, some authors have reported that diaphragmatic activity decreased significantly, when assessed either by transdiaphragmatic pressure changes or electromyographic signals.Citation70 Thereafter, a clinical study reported in a larger sample size of patients a reduced need for intubation and reduced hospital mortality.Citation71 Accordingly, the latest clinical practice guidelines including a large meta-analysis published in 2016 showed that with NIV, intubation rates have significantly decreased from around 40% with standard oxygen to 10–15% with NIV in patients treated for COPD exacerbation with hypercapnic acidosis, while mortality rates decreased from 20% to less than 10% with NIV.Citation6
As far as NIV outcomes are concerned, the current issue does not seem to further reduce the need of intubation or mortality, as these events are not common when using NIV, but rather to reduce the duration of NIV, and to offer another, more comfortable device to patients with poor tolerance to NIV or to patients with non-severe COPD exacerbation (). HFNC could be considered as ventilatory support in COPD patients, as physiological studies suggest its favorable effects on the work of breathing and gas exchange.Citation72–Citation74 HFNC delivers a humidified and heated gas through a nasal cannula at high flow rate, reducing anatomical dead space in the upper airways by clearing exhaled carbon dioxide.Citation28,Citation29 Moreover, the high flow rate generates a low level of positive pressure in the upper airways, which can provide a slight positive end expiratory pressure effect.Citation26,Citation75,Citation76 All of these physiological effects have been shown to provide help in decreasing PaCO2, respiratory rate and in improving breathing pattern with higher tidal volumes, in stable COPD patientsCitation72–Citation74,Citation77,Citation78 as well as in unstable patients.Citation26,Citation76,Citation79,Citation80 Accordingly, Spoletini et al assessed NIV interspaced with HFNC sessions in patients treated for acute respiratory failure.Citation81 However, this study, which included 57 patients, did not show any difference in the time spent on NIV or time on break between patients receiving standard oxygen in between NIV sessions and those receiving HFNC in between NIV sessions.Citation81 HFNC was associated with better comfort and improved respiratory rate and dyspnea compared to standard oxygen between NIV sessions. The authors were very cautious in their conclusions insofar as their study was a non-inferiority trial with a primary physiologic outcome, leaving uncertainty on stronger patient-related outcomes. One potential limitation in explaining the cause of the failed trial is a heterogeneous population including not only patients with severe acute exacerbation of COPD but also those with acute hypoxemic respiratory failure. In this latter group, the benefit of NIV is controversial and has been reported to be associated with poor prognosis.Citation6,Citation8 Another ongoing trial is focused on patients requiring NIV for hypercapnic acute respiratory failure due to severe COPD exacerbation. The primary outcome is to determine whether HFNC as compared with standard oxygen increases the number of ventilator-free days (NIV and invasive mechanical ventilation) and alive at day 28.Citation82
One use for HFNC may be in patients with non-severe acute exacerbation of COPD. A recent trial by Cortegiani et al reported non-inferior reduction of PaCO2 in patients with mild-to-moderate acute exacerbation of COPD treated with HFNC or NIV as initial ventilatory support.Citation83 However, one-third of the patients receiving HFNC required NIV within 6 hours, and intubation rates between groups were similar.Citation83 In less severe patients, a prospective randomized controlled trial including 320 patients with acute compensated hypercapnic respiratory failure showed a decreased intubation rate after treatment by HFNC as compared with conventional oxygen therapy, 10% versus 19%, respectively.Citation84
Bronchodilator therapy, the main pharmacological treatment in patients having COPD exacerbation,Citation85,Citation86 can be delivered through HFNC circuit. Clinical studies conducted in stable ambulatory COPD patients have shown that bronchodilator therapies are effectively delivered within a HFNC circuit, providing bronchodilation similar to standard mask jet nebulization.Citation87,Citation88 Braunlich et al reported in 26 stable COPD patients a 9% increase in FEV1 30 minutes after bronchodilator (salbutamol and ipratropium) nebulization using a jet-nebulizer adapted on a HFNC system.Citation88 Reminiac et al highlighted a 16% greater increase of FEV1 using a vibrating mesh nebulizer through HFNC system in 25 ambulatory patients with reversible obstructive pulmonary disease.Citation87 In a physiological crossover study including patients with severe acute exacerbation of COPD, we showed significantly improved FEV1 (9%), FVC (14%) and PEF (20%) after salbutamol vibrating-mesh nebulization through HFNC as compared to HFNC alone, a finding suggesting an effective bronchodilator effect.Citation89
As a result (), NIV remains the first-line therapy for severe COPD exacerbation with acidosis, whereas there is no high-level evidence favoring HFNC as first-line therapy or in less severe patients; that said, it can represent an alternative oxygen support in case of NIV intolerance with possible delivery of bronchodilator therapy.
Conclusion
Although HFNC is widely used in ICUs, further studies are needed to confirm its superiority over standard oxygen in acute hypoxemic or in acute-on-chronic respiratory failure, by determining the target population of patients likely to benefit from HFNC. Moreover, the benefits of NIV delivered through a helmet on HFNC in patients with acute hypoxemic respiratory failure reported in physiological studies have not been confirmed in clinical studies in terms of survival. Consequently, questions still remain about the best non-invasive oxygen strategy as an alternative to standard oxygen in acute hypoxemic respiratory failure; as concerns acute-on-chronic respiratory failure, the place of HFNC has to be evaluated.
Acknowledgments
We thank Jeffrey Arsham (CHU de Poitiers, Poitiers, France) for reviewing and editing the original English-language manuscript.
Disclosure
JPF reports grants from French Ministry of Health; travel expenses coverage to attend scientific meetings and consulting fees from Fisher & Paykel and SOS oxygen. SLP reports no conflicts of interest. AWT reports travel expenses coverage to attend scientific meetings and payment for lectures from Fisher & Paykel, Covidien, Maquet-Getinge, and General Electric Healthcare. RC reports travel expenses coverage to attend scientific meetings from Fisher & Paykel and MSD, grants from ERS and SRLF. The authors report no other conflicts of interest in this work.
References
- Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J Suppl. 2003;47:3s–14s. doi:10.1183/09031936.03.00038503
- Kent BS. Light-weight oxygen mask of plastic material. Lancet. 1946;2(6420):380. doi:10.1016/S0140-6736(46)90892-6
- Poulton EP, Oxon DM. Left-side heart failure with pulmonary edema. Lancet. 1936;228(5904):981–982. doi:10.1016/S0140-6736(00)47948-1
- Masip J, Roque M, Sanchez B, Fernandez R, Subirana M, Exposito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA. 2005;294(24):3124–3130. doi:10.1001/jama.294.24.3124
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185. doi:10.1136/bmj.326.7382.185
- Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi:10.1183/13993003.02426-2016
- Lomholt N. Continuous controlled humidification of inspired air. Lancet. 1968;2(7580):1214–1216. doi:10.1016/S0140-6736(68)91695-4
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi:10.1056/NEJMoa1503326
- Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408–413.
- Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339(7):429–435. doi:10.1056/NEJM199808133390703
- Laffey JG, Kavanagh BP. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi:10.1056/NEJM200005043421801
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi:10.1056/NEJMra1208707
- Girou E, Schortgen F, Delclaux C, et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284(18):2361–2367. doi:10.1001/jama.284.18.2361
- Chanques G, Constantin JM, Devlin JW, et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46(12):2342–2356. doi:10.1007/s00134-020-06307-9
- Goligher EC, Dres M, Fan E, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197(2):204–213. doi:10.1164/rccm.201703-0536OC
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49. doi:10.1164/ajrccm.164.1.2001078
- Brochard L. Ventilation-induced lung injury exists in spontaneously breathing patients with acute respiratory failure: yes. Intensive Care Med. 2017;43(2):250–252. doi:10.1007/s00134-016-4645-4
- Carrillo A, Gonzalez-Diaz G, Ferrer M, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38(3):458–466. doi:10.1007/s00134-012-2475-6
- Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–442. doi:10.1164/rccm.201605-1081CP
- Ferguson N, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi:10.1007/s00134-012-2682-1
- Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–632. doi:10.1007/s00134-015-3693-5
- Katz JA, Marks JD. Inspiratory work with and without continuous positive airway pressure in patients with acute respiratory failure. Anesthesiology. 1985;63(6):598–607. doi:10.1097/00000542-198512000-00008
- Sim MA, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008;63(9):938–940. doi:10.1111/j.1365-2044.2008.05536.x
- Basile MC, Mauri T, Spinelli E, et al. Nasal high flow higher than 60 L/min in patients with acute hypoxemic respiratory failure: a physiological study. Crit Care. 2020;24(1):654. doi:10.1186/s13054-020-03344-0
- Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107(6):998–1004. doi:10.1093/bja/aer265
- Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–1215. doi:10.1164/rccm.201605-0916OC
- Vargas F, Saint-Leger M, Boyer A, Bui NH, Hilbert G. Physiologic effects of high-flow nasal cannula oxygen in critical care subjects. Respir Care. 2015;60(10):1369–1376. doi:10.4187/respcare.03814
- Moller W, Celik G, Feng S, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol. 2015;118(12):1525–1532. doi:10.1152/japplphysiol.00934.2014
- Moller W, Feng S, Domanski U, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122(1):191–197. doi:10.1152/japplphysiol.00584.2016
- Demoule A, Chevret S, Carlucci A, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med. 2016;42(1):82–92. doi:10.1007/s00134-015-4087-4
- Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284(18):2352–2360. doi:10.1001/jama.284.18.2352
- Morais CCA, Koyama Y, Yoshida T, et al. High positive end-expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med. 2018;197(10):1285–1296. doi:10.1164/rccm.201706-1244OC
- L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med. 2005;172(9):1112–1118. doi:10.1164/rccm.200402-226OC
- Gregoretti C, Confalonieri M, Navalesi P, et al. Evaluation of patient skin breakdown and comfort with a new face mask for non-invasive ventilation: a multi-center study. Intensive Care Med. 2002;28(3):278–284. doi:10.1007/s00134-002-1208-7
- Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27(11):1718–1728. doi:10.1007/s00134-001-1114-4
- Demoule A, Girou E, Richard JC, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32(11):1756–1765. doi:10.1007/s00134-006-0324-1
- Patel BK, Hall JB, Kress JP. Face mask vs helmet for noninvasive ventilation-reply. JAMA. 2016;316(14):1497. doi:10.1001/jama.2016.13858
- Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731–1743. doi:10.1001/jama.2021.4682
- Grieco DL, Menga LS, Raggi V, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201(3):303–312. doi:10.1164/rccm.201904-0841OC
- Antonelli M, Pennisi MA, Pelosi P, et al. Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease: a feasibility study. Anesthesiology. 2004;100(1):16–24. doi:10.1097/00000542-200401000-00007
- Tonnelier JM, Prat G, Nowak E, et al. Noninvasive continuous positive airway pressure ventilation using a new helmet interface: a case-control prospective pilot study. Intensive Care Med. 2003;29(11):2077–2080. doi:10.1007/s00134-003-1925-6
- Vargas F, Thille A, Lyazidi A, Campo FR, Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37(6):1921–1928. doi:10.1097/CCM.0b013e31819fff93
- Tonelli R, Fantini R, Tabbì L, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. A pilot study. Am J Respir Crit Care Med. 2020;202(4):558–567. doi:10.1164/rccm.201912-2512OC
- Carteaux G, Millan-Guilarte T, De Prost N, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44(2):282–290. doi:10.1097/CCM.0000000000001379
- Frat JP, Ragot S, Coudroy R, et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med. 2018;46:208–215. doi:10.1097/CCM.0000000000002818
- Bellani G, Grasselli G, Teggia-Droghi M, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care. 2016;20(1):142. doi:10.1186/s13054-016-1290-9
- Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1585–1591. doi:10.1164/ajrccm.160.5.9903015
- Ferrer M, Esquinas A, Leon M, Gonzalez G, Alarcon A, Torres A. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168(12):1438–1444. doi:10.1164/rccm.200301-072OC
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481–487. doi:10.1056/NEJM200102153440703
- Martin TJ, Hovis JD, Costantino JP, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161(3 Pt 1):807–813. doi:10.1164/ajrccm.161.3.9808143
- Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure. A randomized comparison with conventional therapy. Chest. 1995;107(3):761–768. doi:10.1378/chest.107.3.761
- Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435–2441. doi:10.1001/jama.2016.6338
- Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320(20):2099. doi:10.1001/jama.2018.14282
- Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226–2237. doi:10.1007/s00134-020-06312-y
- Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):1–12.
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi:10.1007/s00134-020-06294-x
- Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi:10.1001/jama.2020.5394
- Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi:10.1016/S0140-6736(20)31189-2
- Raoof S, Nava S, Carpati C, Hill NS. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158(5):1992–2002. doi:10.1016/j.chest.2020.07.013
- Gaeckle NT, Lee J, Park Y, Kreykes G, Evans MD, Hogan CJ. Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med. 2020;202(8):1115–1124. doi:10.1164/rccm.202006-2309OC
- Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020;55(5):2000892. doi:10.1183/13993003.00892-2020
- Montiel V, Robert A, Robert A, et al. Surgical mask on top of high-flow nasal cannula improves oxygenation in critically ill COVID-19 patients with hypoxemic respiratory failure. Ann Intensive Care. 2020;10(1):125. doi:10.1186/s13613-020-00744-x
- Serrano Ricardo, Corbella X, Jordi R. Management of hypoxemia in SARS-CoV-2 infection: lessons learned from one year of experience, with a special focus on silent hypoxemia. J Intens Med. 2021;1(1):26-30.
- Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21(1):198. doi:10.1186/s12931-020-01462-5
- Hernandez-Romieu AC, Adelman MW, Hockstein MA, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48(11):e1045–e1053. doi:10.1097/CCM.0000000000004600
- Demoule A, Vieillard Baron A, Darmon M, et al. High flow nasal canula in critically ill severe COVID-19 patients. Am J Respir Crit Care Med. 2020;202(7):1039–1042. doi:10.1164/rccm.202005-2007LE
- Bonnet N, Martin O, Boubaya M, et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care. 2021;11(1):37. doi:10.1186/s13613-021-00825-5
- Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(21):2161–2171. doi:10.1001/jama.2021.20714
- Ehrmann S, Li J, Ibarra-Estrada M, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi:10.1016/S2213-2600(21)00356-8
- Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990;323(22):1523–1530. doi:10.1056/NEJM199011293232204
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi:10.1056/NEJM199509283331301
- Rittayamai N, Phuangchoei P, Tscheikuna J, Praphruetkit N, Brochard L. Effects of high-flow nasal cannula and non-invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: a preliminary study. Ann Intensive Care. 2019;9(1):122. doi:10.1186/s13613-019-0597-5
- Pisani L, Fasano L, Corcione N, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. 2017;72(4):373–375. doi:10.1136/thoraxjnl-2016-209673
- Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71(8):759–761. doi:10.1136/thoraxjnl-2015-207962
- Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103(6):886–890. doi:10.1093/bja/aep280
- Braunlich J, Mauersberger F, Wirtz H. Effectiveness of nasal highflow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm Med. 2018;18(1):14. doi:10.1186/s12890-018-0576-x
- Atwood CW, Camhi S, Little KC, et al. Impact of heated humidified high flow air via nasal cannula on respiratory effort in patients with chronic obstructive pulmonary disease. Chron Obstruct Pulmon Dis. 2017;4(4):279–286. doi:10.15326/jcopdf.4.4.2017.0169
- Braunlich J, Seyfarth HJ, Wirtz H. Nasal High-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med. 2015;10(1):27. doi:10.1186/s40248-015-0019-y
- Braunlich J, Kohler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1077–1085. doi:10.2147/COPD.S104616
- Pilcher J, Eastlake L, Richards M, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology. 2017;22(6):1149–1155. doi:10.1111/resp.13050
- Spoletini G, Mega C, Pisani L, et al. High-flow nasal therapy vs standard oxygen during breaks off noninvasive ventilation for acute respiratory failure: a pilot randomized controlled trial. J Crit Care. 2018;48:418–425. doi:10.1016/j.jcrc.2018.10.004
- Ricard JD, Dib F, Esposito-Farese M, Messika J, Girault C. Comparison of high flow nasal cannula oxygen and conventional oxygen therapy on ventilatory support duration during acute-on-chronic respiratory failure: study protocol of a multicentre, randomised, controlled trial. The ‘HIGH-FLOW ACRF’ study. BMJ open. 2018;8(9):e022983. doi:10.1136/bmjopen-2018-022983
- Cortegiani A, Longhini F, Madotto F, et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020;24(1):692. doi:10.1186/s13054-020-03409-0
- Li XY, Tang X, Wang R, et al. High-flow nasal cannula for chronic obstructive pulmonary disease with acute compensated hypercapnic respiratory failure: a randomized, controlled trial. Int J Chron Obstruct Pulmon Dis. 2020;15:3051–3061. doi:10.2147/COPD.S283020
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
- Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791. doi:10.1183/13993003.00791-2016
- Reminiac F, Vecellio L, Bodet-Contentin L, et al. Nasal high-flow bronchodilator nebulization: a randomized cross-over study. Ann Intensive Care. 2018;8(1):128. doi:10.1186/s13613-018-0473-8
- Braunlich J, Wirtz H. Oral versus nasal high-flow bronchodilator inhalation in chronic obstructive pulmonary disease. J Aerosol Med Pulm Drug Deliv. 2018;31(4):248–254. doi:10.1089/jamp.2017.1432
- Beuvon C, Coudroy R, Bardin J, et al. β agonist delivery by high-flow nasal cannula during COPD exacerbation: a prospective physiological study. Respir Care. 2021. doi:10.4187/respcare.09242
