Abstract
Background
This study aimed to investigate the improvement of pulmonary function in heart failure patients with restrictive patterns undergoing transcatheter aortic valve replacement (TAVR).
Methods
A total of 80 patients with heart failure and restrictive patterns undergoing TAVR due to severe aortic stenosis were included in this study. Spirometry and gas diffusion were assessed before and 4–6 months after TAVR. Pre- and post-TAVR measures were compared using paired t-tests.
Results
Spirometry demonstrated increased absolute and percentage predicted total lung capacity (TLC), forced vital capacity (FVC), residual volume (RV), forced expiratory volume in the first second (FEV1), and forced vital capacity (FVC). FEV1/FVC decreased due to a pronounced increase in FVC. Additionally, the diffusing capacity for carbon monoxide (DLCO) increased significantly.
Conclusion
Pulmonary function improves in heart failure patients with restrictive patterns undergoing TAVR.
Introduction
The prevalence of left ventricular dysfunction is between 6% and 11% when the ejection fraction (EF) falls below 30% in patients undergoing transcatheter aortic valve replacement (TAVR) for severe aortic stenosis. It can rise to 46% when the EF is between 30% and 50%.Citation1–Citation3 A negative prognostic impact of TAVR was reported in the FRANCE 2 registry for patients with clinical signs of heart failure.Citation4
Two distinct spirometric patterns are described by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). The common abnormality in these patterns is a forced expiratory volume in the first second (FEV1) of <80%. The restrictive pattern was defined as FEV1/forced vital capacity (FVC) ratio (FEV1/FVC) >70%, whereas the obstructive pattern was defined as FEV1/FVC <70%.Citation5 Decreased total lung capacity due to reduced lung compliance was the main component of restriction. It is associated with an impaired functional status and frailty in older patients.Citation5 Obstructive patterns are well documented, especially in chronic obstructive pulmonary disease (COPD). However, restrictive patterns in heart failure have not been well characterized in the literature. The mortality is higher in older adults with airflow restriction. Therefore, more attention should be paid to the diagnosis and prognosis of this condition.
Patients with heart failure develop pulmonary functional abnormalities ranging from minimal restriction to mixed restriction/obstruction.Citation6 Several pathophysiological consequences of heart failure, including increased left ventricular filling pressure and pulmonary edema, may provoke these spirometric alterations. A restrictive pattern may emerge in the presence of decreased lung volume and due to the diffusing capacity for carbon monoxide (DLCO). However, the FEV1/FVC remained within the normal range. Bronchial edema may cause an obstructive pattern (low FEV1/FVC) in heart failure. The pulmonary function in heart failure patients with restrictive patterns undergoing TAVR has not been well studied. Moreover, the mutual interaction between heart failure with severe aortic stenosis and pulmonary function needs to be elucidated. There is mounting evidence that cardiac causes play an incontrovertible role in pre-TAVR pulmonary dysfunction in severe aortic stenosis.
Hence, we hypothesized that TAVR improves static and dynamic lung parameters, gas diffusion, and functional status. To our knowledge, there are no studies in the literature that have evaluated pulmonary function in pure heart failure patients undergoing TAVR. We sought to investigate the pulmonary function in this setting.
Materials and Methods
Study Location
The institution at which the work was performed: Bezmialem Foundation University, Faculty of Medicine, Department of Cardiology, Istanbul, Turkey.
Study Population
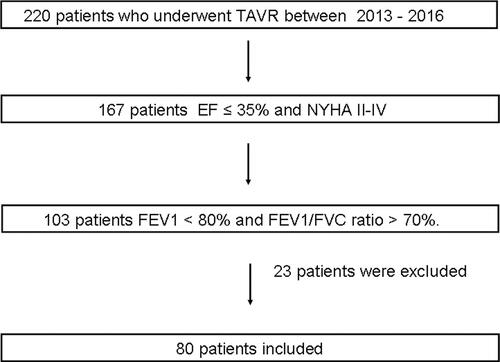
A total of 220 patients who underwent transfemoral TAVR due to severe aortic stenosis between 2013 and 2016 were retrospectively analyzed. A flowchart of the search strategy is shown in . A total of 167 patients had a New York Heart Association (NYHA) class II to IV and an EF ≤ 35%. Among these, 103 patients had an FEV1 <80% and FEV1/FVC ratio >70%. Twenty-three patients were excluded from the study. Finally, 80 consecutive patients (mean age: 79.79 ± 8.47 years) were enrolled in the study. Blood pressure higher than 140/90 mmHg and prior antihypertensive drug use were diagnosed as hypertension. The measurement should be verified at least three times in all patients. A fasting blood glucose level of 7.0 mmol/L or higher and prior antidiabetic drug use were diagnosed as diabetes mellitus. Total cholesterol levels of 5.2 mmol/L or higher and prior statin use were diagnosed as hyperlipidemia. The main diagnostic criteria for coronary artery disease were as follows: a) Previous coronary angiography showed ≥70% stenosis in at least one major epicardial coronary vessel; b) The patient had a history of post-coronary artery bypass graft or percutaneous coronary intervention. The components of stroke included transient ischemic attack and stroke (ischemic or hemorrhagic). Smoking status was defined as smoking prior to the hospitalization. Peripheral vascular disease was defined as claudication, carotid stenosis, planned or completed vascular surgery, or X-ray intervention. We calculated the estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation. Patients with any of the following were excluded: obstructive lung disease, intrinsic restrictive lung impairment (ie, interstitial lung diseases such as pneumoconiosis, pulmonary fibrosis, sarcoidosis, idiopathic pulmonary fibrosis, hypersensitivity pneumonitis causing inflammation and scarring of the lung tissue), extrinsic restrictive lung impairment (ie, neuromuscular disease affecting respiratory muscle function such as muscular dystrophy, phrenic neuropathy, and other nerve and muscle disorders, musculoskeletal system abnormalities such as scoliosis, kyphosis, and chest wall deformities cause incomplete expansion of the lungs), malignancy, end-stage hepatic and renal failure, and acute cardiovascular or cerebrovascular events within the preceding three months. Study was approved by Bezmialem University Ethics Committee and it was conducted in accordance with ethical principles described by the Declaration of Helsinki. The research involves no more than minimal risk to subjects hence patient consent to review their medical records was not required by the Bezmialem University Ethics Committee. Additionally, patient data confidentiality is adequately protected.
Data Collection
Demographic, clinical, and laboratory data were recorded from a local database. The medical history, physical findings, and laboratory data were evaluated at every clinical visit. Patients were regularly followed up in the cardiology outpatient clinic at Bezmialem University. All patients had spirometric data. Pulmonary function measurements, echocardiography, and electrocardiography were recorded at baseline 1–2 days before TAVR. They were repeated 4–6 months after TAVR.
Echocardiography
Transthoracic echocardiography (VIVID 7 Dimension Cardiovascular Ultrasound System) (Vingmed-General Electric, Horten, Norway) was performed at least twice (before the procedure and 4–6 months after the procedure) for each patient. The left ventricular diameters were measured by M-mode, and the left atrial area was calculated using the apical four-chamber view. Additionally, the Simpson method was the method of choice for calculating the EF. The aortic valve area and aortic mean gradient were calculated using valve planimetry and Doppler echocardiography. If the planimetric area was smaller than 1 cm2 and the mean transaortic gradient was greater than 40 mmHg, the stenosis was classified as severe. All echocardiographic examinations were performed by two experienced cardiologists in the Bezmialem University echocardiography laboratory.
Device and Procedure
The procedure was performed via the transfemoral route under local anesthesia with conscious sedation. All patients received supplemental oxygen by face mask to maintain arterial oxygen saturation higher than 90%. A Medtronic CoreValve prosthesis (Medtronic, Minneapolis, MN, USA) was implanted through an Amplatz Extra Stiff guidewire under temporary pacing at a rate of 90–120 beats/min. Pre- and post-implantation balloon valvuloplasty was undertaken at the discretion of an interventional cardiologist. Valve position, paravalvular leakage, rhythm disturbances, and peripheral complications were evaluated comprehensively using fluoroscopy. Aspirin and clopidogrel were administered as antiplatelet drugs for one year after the procedure.
Pulmonary Function
A spirometer (SensorMedics Corporation, Yorba Linda, CA, USA) was used to evaluate pulmonary function in the Pulmonary Medicine Department of Bezmialem University. The same device was used for all patients. Recent myocardial infarction and cranial, ophthalmological, thoracic, and abdominal surgery were absolute contraindications for performing spirometry. NHANES III described age-, sex-, and race-specific normalized reference values for spirometric parameters. Additionally, we calculated the FEV1 and FVC from the NHANES III equations.Citation7 Abnormal lung functions included % FEV1/FVC or FVC below the lower limit of normal (ie, 5th percentile) and were further classified into obstructive and restrictive patterns. The obstructive pattern was defined as FEV1 <80% and FEV1/FVC <70%. On the other hand, the restrictive pattern was defined as FEV1 <80% and FEV1/FVC >70%.Citation8 Total lung capacity, FEV1, FVC, and residual volume were measured. We collected DLCO measurements according to the standards of the American Thoracic Society.Citation9
Statistical Analysis
Data were analyzed using SPSS 17 (SPSS Inc., Chicago, IL, USA). Continuous parameters were expressed as mean ± standard deviation, and categorical parameters were expressed as numbers and percentages. After testing the normality of distribution using the Shapiro–Wilk test, continuous variables were evaluated using either the paired sample t-test or the Mann–Whitney U-test. Moreover, the chi-squared test was performed for categorical variables. Statistical significance was set at p < 0.05.
Results
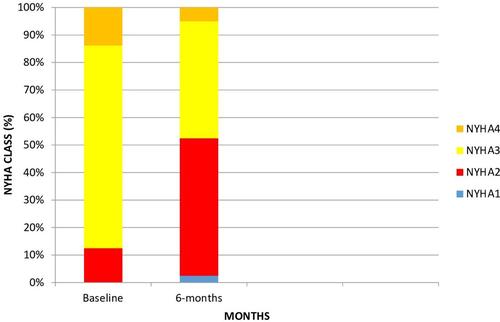
The study population consisted of 80 patients with a mean age of 79.79 ± 8.47 years. The gender distribution was nearly equal (m/f = 0.95). They were predominantly in the NYHA class III (73.8%), with a low EF (29.41 ± 4.86%), markedly dilated left ventricle (62.71 ± 6.59 mm) and pulmonary hypertension (45.89 ± 14.75 mmhg). The baseline characteristics are summarized in . The pre-TAVR and post-TAVR clinical and echocardiographic parameters are shown in . There were significant improvements in left ventricular function, pulmonary artery pressure, and NYHA status. shows pre- and post-TAVR NYHA functional class improvement (3.01 ± 0.51, 2.50 ± 0.63, 0.001). Spirometry after TAVR demonstrated an increased absolute and percentage predicted total lung capacity (3.90 ± 0.37 vs 4.61 ± 0.26 L, p < 0.001 and 64.56 ± 2.84 vs 80.20 ± 4.19%, p < 0.001), FVC (1.90 ± 0.34 vs 2.09 ± 0.63 L, p < 0.001 and 67.50 ± 4.63 vs 74.46 ± 5.74%, p < 0.001), residual volume (1.47 ± 0.7 vs 1.69 ± 0.53 L, p < 0.001 and 64.43 ± 3.85 vs 75.73 ± 5.26%, p < 0.001), FEV1 (1.45 ± 0.30 vs 1.49 ± 0.28 L, p < 0.001 and 59.54 ± 3.43 vs 60.73 ± 3.19%, p < 0.001), FVC (1.90 ± 0.34 vs 2.09 ± 0.63 L, p < 0.001 and 67.50 ± 4.63 vs 74.46 ± 5.74%, p < 0.001) ( and ). Spirometry revealed decreased FEV1/FVC (91.13 ± 5.46 vs 88.33 ± 5.33%, p = 0.027) due to a pronounced increase in FVC (). DLCO increased significantly (10.04 ± 0.51 vs 10.84 ± 0.39 mL/min/mmHg, p < 0.01 and 42.40 ± 4.62 vs 46.74 ± 2.00%, p < 0.001) ( and ).
Table 1 Baseline Characteristics
Table 2 Clinical and Echocardiographic Response to TAVR
Table 3 Pulmonary Function Tests (Absolute Values) (n=80)
Table 4 Pulmonary Function Tests (Percentage Predicted Values) (n=80)
Discussion
The main finding of our study is that TAVR improves static (ie, total lung capacity, residual volume) and dynamic (ie, FEV1 and FVC) pulmonary functions, in accordance with increased lung conductance and volumes in heart failure patients with restrictive patterns. FEV1/FVC decreased due to a pronounced increase in FVC. Additionally, the DLCO significantly increased. Spirometry showed minimal restrictive to mixed restrictive/obstructive pulmonary functional abnormalities in patients with heart failure.Citation7–Citation10 The inclusion criteria for the enrollment in this retrospective study were LVEF ≤ 35%, NYHA class II to IV despite medical therapy, and FEV1 <80% and FEV1/FVC ratio >70% in heart failure with reduced ejection fraction patients without obstructive pulmonary functions. The initial pulmonary function revealed a restrictive pattern with severely depressed lung volumes.
Increased left ventricular end-diastolic pressure leads to pulmonary edema and congestion in heart failure. In the acute phase, these changes result in cardiac decompensation. However, chronic elevation of the left ventricular filling pressure causes progressive pulmonary hypertension. Several pathophysiological consequences of heart failure, including low cardiac output,Citation13 cardiomegaly,Citation14 respiratory muscle weakness,Citation12 chronic pulmonary congestion, and hypertension,Citation11 may provoke abnormal pulmonary function. Additionally, left ventricular hypertrophy and left atrial enlargement may deepen the effect of heart failure, causing diastolic dysfunction in severe aortic stenosis. TAVR breaks this vicious cycle by increasing the forward transmission through the prosthetic valve.
Traditionally, decreased DLCO and FEV1/FVC ≥70% have been used to detect restrictive pulmonary physiology, as observed in patients with pure heart failure. However, the exact mechanism that explains the association between decreased lung volumes and TAVR has not been characterized in the literature. Magee et al postulated a moderate improvement in COPD severity in surgical aortic valve replacement (SAVR) and TAVR.Citation15 They observed increased FEV1 and decreased brain natriuretic peptide (BNP) levels. The main reason for these alterations was the reversibility of airway obstruction due to edema in heart failure. It is noteworthy that this study was conducted in patients with a typical obstructive pattern. Another study with COPD patients undergoing TAVR demonstrated decreased BNP and increased EF in all COPD categories, suggesting improved overall cardiac function.Citation16 However, no change in DLCO has been reported.Citation16 The main proposal was that restrictive lung physiology was not altered after TAVR. In contrast, we found a 4.34% increase in the DLCO. The main mechanism underlying this improvement could be related to the reversibility of the restrictive pattern under specific conditions.
Pulmonary artery systolic pressure (PASP) strongly predicts death and provides incremental and clinically relevant prognostic information among patients with heart failureCitation17 and severe aortic stenosis.Citation18 Conditions such as aortic stenosis, left ventricular dysfunction, mitral regurgitation, and diastolic dysfunction lead to increased LV end-diastolic pressure, resulting in elevated pressures in the pulmonary venous circulation and consecutively inducing arterial vasoconstriction and pulmonary arterial remodeling.Citation17,Citation19 Recent studies have reported that TAVR leads to systolic pulmonary artery pressure reduction with baseline pulmonary hypertension. In concordance with other studies, we found a significant decrease in the PASP. This may have a positive effect on pulmonary function.
We demonstrated a significant improvement in the NYHA class at six months in our study (−0.51 class). Eight studiesCitation20–Citation27 qualitatively reported improvements in the NYHA class after TAVR. In most studies, there was an average improvement of ≥1 NYHA class after TAVR at 6–11 months (range: −0.8, −2.1 class), 12–23 months (−0.8, −2.1 class), 24–35 months (−1.2, −2.6 class), and ≥ 36 months (−1.2, −1.6 class). However, several studiesCitation28–Citation31 showed a mean change of < 1 NYHA class and the lower end of the 95% confidence interval near 0, indicating that a large proportion of patients failed to improve after TAVR. We found only −0.51 class improvement at six months. This relatively low increase could be attributed to heart failure.
The average improvement in the NYHA class and pulmonary function does not necessarily mean that every individual derives the same benefit. Identifying patients who are most likely to have functional and pulmonary benefits is crucial to achieve optimal health outcomes and prevent avoidable harm.
Additionally, there is an increased usage of diuretics, beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in post-TAVR patients. This has a positive impact on the NYHA class, ejection fraction, and pulmonary function.
This study was designed as a single-center, observational, retrospective study. Information was obtained from the electronic medical data. The limitations of this study include the subjectivity of pre- and post-TAVR pulmonary function tests and selection bias for TAVR. Further, large prospective studies may allow a better assessment of restrictive pulmonary function in patients with heart failure undergoing TAVR.
Conclusions
Pulmonary functions significantly improved in heart failure patients with a restrictive pattern undergoing TAVR.
Disclosure
The authors reported no conflicts of interest for this work.
Additional information
Funding
References
- Hamm CW, Möllmann H, Holzhey D, et al. GARY-executive board. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J. 2014;35(24):1588–1598. doi:10.1093/eurheartj/eht381
- Di Mario C, Eltchaninoft H, Moat N, et al. The 2011–12 pilot European sentinel registry of transcatheter aortic valve implantation: in-hospital results in 4571 patients. Eurointervention. 2013;8:1362–1371. doi:10.4244/EIJV8I12A209
- Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom transcatheter aortic valve implantation) registry. J Am Coll Cardiol. 2011;58(20):2130–2138. doi:10.1016/j.jacc.2011.08.050
- Iung B, Laouenan C, Himbert D, et al. FRANCE 2 investigators. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100(13):1016–1023. doi:10.1136/heartjnl-2013-305314
- Kurth L, Hnizdo E. Change in prevalence of restrictive lung impairment in the U.S. population and associated risk factors: the National Health and Nutritional Examination Survey (NHANES) 1988–1994 and 2007–2010. Multidiscip Respir Med. 2015;10:7. doi:10.1186/s40248-015-0003-6
- Guder G, Brenner S, Stork S, et al. Chronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatment. Eur J Heart Fail. 2014;16(12):1273–1282. doi:10.1002/ejhf.183
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi:10.1164/ajrccm.159.1.9712108
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi:10.1183/09031936.05.00035205
- Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. doi:10.1183/13993003.00016-2016
- Roversi S, Fabbri LM, Sin DD, et al. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med. 2016;194(11):1319–1336. doi:10.1164/rccm.201604-0690SO
- Ayres SM. Mechanisms and consequences of pulmonary edema: cardiac lung, shock lung, and principles of ventilatory therapy in adult respiratory distress syndrome. Am Heart J. 1982;103(1):97–112. doi:10.1016/0002-8703(82)90536-1
- Daganou M, Dimopoulou I, Alivizatos PA, et al. Pulmonary function and respiratory muscle strength in chronic heart failure: comparison between ischaemic and idiopathic dilated cardiomyopathy. Heart. 1999;81(6):618–620. doi:10.1136/hrt.81.6.618
- Mancini DM, Henson D, LaManca J, et al. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation. 1992;86(3):909–918. doi:10.1161/01.CIR.86.3.909
- Olson TP, Beck KC, Johnson JB, et al. Competition for intrathoracic space reduces lung capacity in patients with chronic heart failure: a radiographic study. Chest. 2006;130(1):164–171. doi:10.1378/chest.130.1.164
- Magee MJ, Herbert MA, Roper KL, et al. Pulmonary function tests overestimate chronic pulmonary disease in patients with severe aortic stenosis. Ann Thorac Surg. 2013;96(4):1329–1335. doi:10.1016/j.athoracsur.2013.04.123
- Gilmore RC, Thourani VH, Jensen HA, et al. Transcatheter aortic valve replacement results in improvement of pulmonary function in patients with severe aortic stenosis. Ann Thorac Surg. 2015;100(6):2167–2173. doi:10.1016/j.athoracsur.2015.06.008
- Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure. J Am Coll Cardiol. 2012;59(3):222–231. doi:10.1016/j.jacc.2011.06.076
- Malouf JF, Enriquez-Sarano M, Pellikka PA, et al. Severe pulmonary hypertension in patients with severe aortic valve stenosis: clinical profile and prognostic implications. J Am Coll Cardiol. 2002;40(4):789–795. doi:10.1016/S0735-1097(02)02002-8
- Park MH, Mehra MR. Pulmonary hypertension: the great leveler. J Am Coll Cardiol. 2012;59(3):232–234. doi:10.1016/j.jacc.2011.09.052
- Ussia GP, Barbanti M, Colombo A, et al. Impact of coronary artery disease in elderly patients undergoing transcatheter aortic valve implantation: insight from the Italian CoreValve Registry. Int J Cardiol. 2013;167(3):943–950. doi:10.1016/j.ijcard.2012.03.089
- Abdel-Wahab M, Mostafa AE, Geist V, et al. Comparison of outcomes in patients having isolated transcatheter aortic valve implantation versus combined with preprocedural percutaneous coronary intervention. Am J Cardiol. 2012;109(4):581–586. doi:10.1016/j.amjcard.2011.09.053
- Buellesfeld L, Gerckens U, Schuler G, et al. 2-year follow-up of patients undergoing transcatheter aortic valve implantation using a self-expanding valve prosthesis. J Am Coll Cardiol. 2011;57(16):1650–1657. doi:10.1016/j.jacc.2010.11.044
- D’Onofrio A, Gasparetto V, Napodano M, et al. Impact of preoperative mitral valve regurgitation on outcomes after transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2012;41(6):1271–1277. doi:10.1093/ejcts/ezr236
- Ewe SH, Muratori M, Delgado V, et al. Hemodynamic and clinical impact of prosthesis–patient mismatch after transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;58(18):1910–1918. doi:10.1016/j.jacc.2011.08.027
- Gautier M, Pepin M, Himbert D, et al. Impact of coronary artery disease on indications for transcatheter aortic valve implantation and on procedural outcomes. EuroIntervention. 2011;7(5):549–555. doi:10.4244/EIJV7I5A90
- Gotzmann M, Lindstaedt M, Bojara W, et al. Clinical outcome of transcatheter aortic valve implantation in patients with low-flow, low gradient aortic stenosis. Catheter Cardiovasc Interv. 2012;79(5):693–701. doi:10.1002/ccd.23240
- Hutter A, Bleiziffer S, Richter V, et al. Transcatheter aortic valve implantation in patients with concomitant mitral and tricuspid regurgitation. Ann Thorac Surg. 2013;95(1):77–84. doi:10.1016/j.athoracsur.2012.08.030
- Gotzmann M, Bojara W, Lindstaedt M, et al. One-year results of transcatheter aortic valve implantation in severe symptomatic aortic valve stenosis. Am J Cardiol. 2011;107(11):1687–1692. doi:10.1016/j.amjcard.2011.01.058
- Ye J, Cheung A, Lichtenstein SV, et al. Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg. 2010;139(5):1107–1113. doi:10.1016/j.jtcvs.2009.10.056
- Chodor P, Wilczek K, Przybylski R, et al. Immediate and 6-month outcomes of transapical and transfemoral Edwards-Sapien prosthesis implantation in patients with aortic stenosis. Kardiol Pol. 2010;68(10):1124–1131.
- Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34(9):684–692. doi:10.1093/eurheartj/ehs304