Abstract
Nosocomial candidiasis remains a potential risk in intensive care units (ICUs), wherein Candida albicans is most responsible for its occurrence. Equally, non-C. albicans species, especially C. glabrata, are also involved. These infections are frequently associated with biofilms that contaminate medical devices, such as catheters. These biofilms constitute a significant clinical problem, and cause therapeutic failures, because they can escape the immune response and considerably decrease sensitivity to antifungal therapy. The diagnosis of catheter-related candidiasis is difficult; however, the differentiation between an infection of the catheter (or other medical implant) and a simple contamination is essential to start an antifungal treatment. Among the methods used for this type of study is the Brun-Buisson method, but this method only examines the infectivity of catheters caused by bacteria. For this reason, we wanted to adapt this method to the yeast cells of Candida spp. To assess the various types of infectivity of catheters (contamination, colonization, or infection) and their corresponding rates, as well as the responsible yeast species, we conducted our study, between February 2011 and January 2012, in the ICU at the University Hospital Center of Sidi Bel Abbes, Algeria; during this study, we took photographic images of the tongue of one patient and of that patient’s implanted orobronchial catheter. In addition, catheters contaminated by C. albicans biofilms were observed by scanning electron microscopy.
Keywords:
Introduction
Nosocomial invasive candidiasis is a major problem, associated with significant mortality,Citation1,Citation2 that affects 15% of nosocomial infections in patients hospitalized in intensive care units (ICUs).Citation3 Clinical signs of such infections appear late, rendering them difficult to diagnose.Citation3,Citation4 Many yeasts are opportunistic pathogens,Citation5 and Candida albicans is the most common of these to colonize, but non-albicans species of Candida are increasingly involved in infection,Citation6–Citation8 in particular, C. glabrata, an emerging species associated with high mortality.Citation9 Compared with C. albicans fungemia, the impact of C. glabrata fungemia on patients in the ICU is not well known.Citation10
Candidemia is frequently associated with the colonization of medical devices, such as catheters.Citation11 In addition, a factor that is generally predisposing to invasive candidiasis in the ICU is the alteration of skin and mucus barriers caused by the use of medical devices (catheters, probes, intubation, etc).Citation12 Further, the resistance of Candida spp. to antifungal agents increases with the formation of biofilms into medical implants.Citation13–Citation15 Indeed, Candida colonization appears to be a prerequisite for Candida infection;Citation16 therefore, it is important to distinguish between simple contamination, colonization, and infection of catheters,Citation6,Citation17 because this differentiation remains crucial in initiating treatment.Citation18 A threshold of significance, ≥103 colony-forming units (CFU)/mL with or without the appearance of systemic symptoms (fever > 38°C) or local symptoms (purulent opening, insertion-site or route inflammation), determines the type of infectivity of the catheter.Citation19 The removal of the catheter is preferable when it is a gateway to candidemia. Analysis of the culture of the catheter after its ablation is necessary in confirming infection.Citation20,Citation21
Since there is no specific technique by which to study the different types of infectivity of catheters contaminated by Candida spp., our study in the ICU at the University Hospital Center of Sidi Bel Abbe, Algeria aimed to adapt the Brun-Buisson technique and previous specific bacterial definitions for the assessment of the different types of fungal infectivity of medical implants, and also to isolate and identify the involved species and calculate their corresponding rates. In addition to this, we aimed to investigate the formation of biofilms on catheter surfaces, using electronic imagery.
Materials and methods
Based on previous results obtained from a prevalence survey that was done in June 2009 by the Epidemiology Department of the University Hospital Center of Sidi Bel Abbes (unpublished data), we conducted this study in the ICU between February 2011 and January 2012. The samples of the implanted medical devices were taken from patients hospitalized for 48 hours or more. Candidemia is considered nosocomial when it first occurs 48 hours or more after hospital admission.Citation7,Citation22 Additionally, other samples were taken by swabbing the surface of the tongue. To transport the samples to the laboratory, a refrigerant bag was used.
Patients
The samples were taken directly from patients who presented the most important candidiasis risk factors (implanted medical devices, broad-spectrum antibiotic therapy, long stay in the ICU, etc). For every patient, the following data were registered: age, sex, antibiotic treatment, body temperature during the sampling, and presence or absence of inflammation and/or pus along the route of the catheter.
One patient agreed to photography of the tongue; this was a diabetic woman of 64 years who suffered from meningoencephalitis. She was treated by antibiotics, gentamicin and cephalosporin three times a day at dose levels of 100 mg/day and 3 g/day, respectively, and acyclovir (900 mg × 2/day), vitamin K (10 mg), paracetamol (1 g × 3/day), and antidiabetic treatment (insulin, 3 × 8 IU/day). This patient died of a cardiac attack.
Samples and yeast isolates
We took samples based on the method of Boucherit-Atmani et al.Citation15 Briefly, the implanted medical devices (catheters and other invasive medical devices) were taken directly from patients and placed in sterile Sabouraud liquid medium. After cutting the distal ends of the catheters, the tubes were opened in front of a torch, then rapidly flamed and sealed to avoid contamination. The tubes were then agitated in a vortex mixer for 1 minute, and purified strains were identified by a yeast identification system (API 32-C System; bioMériux, Marcy L’Etoile, France) and microculture (germ-tube and chlamydospore formation).
Tongue samples were collected by scraping the dorsum of the tongue with a sterile cotton swab, which was returned into the tube immediately after sampling. Then, the tube was agitated, and the different forms (yeast cells, hyphae, and pseudohyphae) were verified using light microscopy. In fact, scraping the surface of the tongue is a reliable method for detecting Candida spp.Citation23
Types of catheter infectivity
The type of infectivity of each collected medical implant was assessed using the quantitative method of Brun-BuissonCitation24 with some modifications. For this reason, the samples were vortexed for 1 minute, and then the yeasts were counted using a Thoma cell. To this end, a volume of the sample (after vortexing) was placed between the Thoma cell and the coverslip, and yeast cells were counted by light microscopy (magnification × 40). Then, the calculation was reported in CFU/mL. When cell counting was difficult due to high density of the milieu, dilutions were performed.
The different types of catheter infectivity were determined by referring to the significance threshold, ≥103 CFU/mL, and to the systemic and/or local clinical symptoms.Citation19
Biofilms observation with SEM
In order to highlight the formation of biofilms on the catheter surfaces, a central vascular catheter (CVC) and an orobronchial catheter (OBC) were removed from the aforementioned female patient after exposure times of 8 and 3 days, respectively. Biofilm samples were randomly selected and preserved with glutaraldehyde fixative (Sigma-Aldrich, St Louis, MO, USA), and sent to the SEM laboratory.
Catheter-segment fixation was done according to the methods of Chandra et al.Citation25 Briefly, biofilms of C. albicans on catheter segments were fixed with 2.5% (v/v) glutaraldehyde in phosphate-buffered saline (PBS) (0.1 M, pH 7.4) and were introduced in sterile Eppendorf tubes that were sealed with an adhesive film, numbered, placed in another vial, and left for 12 hours at 4°C before shipment to the scanning electron microscope (SEM) laboratory for observations.
SEM preparation of the catheter’s pieces was performed according to Chandra et al.Citation25 The samples were examined using a Hitachi S-4800 (Hitachi Ltd, Tokyo, Japan) field-emission SEM by Dennis Kunkel Microscopy Inc (Kailua, HI, USA).
Results and discussion
The prevalence survey, which was conducted in June 2009 by the Epidemiology Department of the University Hospital Center of Sidi Bel Abbes, showed that 60% of the patients hospitalized in the ICU were victims of nosocomial infection, and the principal cause was the use of any type of catheter. In addition, this survey revealed widespread use of antibacterial agents, while antifungal agents were prescribed only in rare cases.
According to Avila-Aguero et al,Citation11 previous use of antibiotics, endotracheal intubation, and use of central devices constitute the risk factors for candidemia infection. Effectively, catheters are the leading cause of bloodstream infection in patients in the ICU;Citation26 half of all cases of candidemia occur in the ICU.Citation27
In the present study, a total of 63 samples were taken from different implanted medical devices at the ICU during the study period. Twelve strains (19.04%) of Candida spp. were isolated during the study period; nine strains of C. glabrata and only three strains of C. albicans were identified ().
Table 1 Results of the counting and identification of yeast strains isolated from implanted devices in the ICU
Based on the results displayed in , C. glabrata was predominant compared with C. albicans; a ratio of 3:1 of C. glabrata versus C. albicans was observed.
The strains of C. albicans were isolated from the OBC and tongue of the female patient and from the CVC of another patient. The strains of C. glabrata were taken from different medical devices implanted into other patients.
In general, fungal infection in critically or seriously ill patients is a significant problem,Citation16 and the use of indwelling devices, such as catheters, is a source of nosocomial infection.Citation5 In addition, the vast majority of the cases of candidemia occur in hospitalized patients;Citation28 commonly affect patients in the ICU; and carry a high mortality risk.Citation29
C. glabrata was previously thought to be a primarily nonpathogenic organism, but, in the past couple of decades, it has been increasingly recognized as a pathogen of problematic etiological origin in bloodstream infections.Citation30–Citation32 Additionally, this yeast has recently emerged as an important nosocomial pathogen,Citation9,Citation33,Citation34 yet little is known about its epidemiology.Citation33 In fact, C. glabrata has been suggested to be the second most important human pathogen responsible for candidemia.Citation35
According to Develoux and Bretagne,Citation6 among the episodes of ICU candidemia, C. glabrata was the second most common causative species.Citation10 However, a recent study found that no difference in mortality was associated with C. albicans and C. glabrata bloodstream infections.Citation32,Citation36 Trick et alCitation37 have demonstrated that, among the Candida spp., the rate of C. glabrata has increased as a cause of bloodstream infection in American ICUs since 1993.
Types of catheter infectivity
shows the results of the types of catheter infectivity. In total, three different types were observed: six (9.52%) cases were contaminations, two (3.17%) cases were colonizations, and three (4.76%) cases were infections. The distinction between these different infectivity types was made according to previous definitions,Citation19,Citation24 and the counting of the yeast cells was accomplished using Thoma cells and the collected clinical data. These same observations were also distinguished in all the other medical departments of the University Hospital Center mentioned above (Seddiki et al, unpublished data, 2012).
Figure 1 Rates of different types of infectivity of implanted medical devices observed in the intensive care unit.
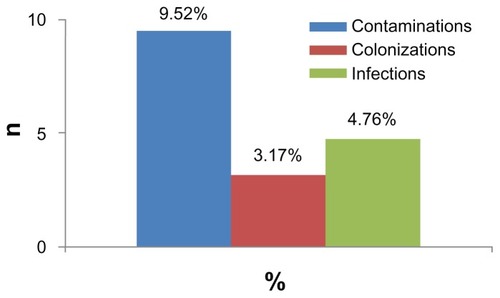
Similarly to Carrière and Marchandin,Citation19 three different types of catheter infectivity were reported: contamination, colonization, and infection. The rate of contamination of catheters was higher than the rates of colonization and infection; this explains, in part, the difficulty in diagnosis of catheter-related candidiasis.
The increase in acquired fungal infections in hospital, particularly in candidiasis, has been attributed to the ability of fungi to adhere to the invasive devices that are used for treatment.Citation38 According to Bleichner et al,Citation39 the frequency of isolation of Candida spp. in catheter-related infections ranges from 2% to 5%.Citation39 However, catheter-related infection is preceded by the colonization of the distal end of the catheter, and quantitative culture must be used to confirm that the catheter is the source of the signs of infection.Citation19
shows the OBC colonized by C. albicans in the previously described patient. The count of yeast cells isolated from this catheter was higher than 103 cells/mL (Brun-Buisson technique). The patient’s temperature during the sampling was 37.1°C; however, the patient was given an infusion solution of paracetamol, which prevented an increase of temperature, and the same patient was also treated with broad-spectrum antibiotics. We also observed a white carpet on the patient’s tongue, from which the isolate strain revealed C. albicans.
Figure 2 Microbial propagation between the patient’s tongue and the implanted orobronchial catheter.
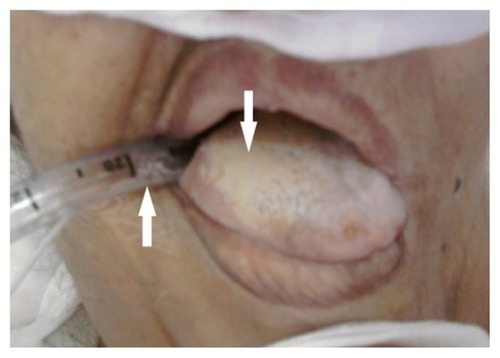
The observation of the sample (swabbing of the tongue surface) under an optical microscope revealed the existence of yeast cells and elongated hyphal forms. Interestingly, these different forms exist in the biofilms of C. albicans;Citation25 thus, it is possible that this carpet was a biofilm of C. albicans, and it may possibly have indicated a dissemination of this yeast between the tongue and the OBC implanted in the neighboring area.
Fungi have the ability to adhere not only to human tissues, but also to any invasive devices.Citation38 In fact, the acquisition of C. albicans in the ICU seems to be mainly endogenous.Citation40,Citation41 Oropharyngeal candidiasis is a common opportunistic infection occurring in immunocompromised patients.Citation42 It appears as a white, elevated, mossy substance attached to the tongue surface or oral mucosa.Citation43,Citation44 It has been reported that oral C. albicans colonization increases remarkably in diabetic patients, especially in women with diabetes.Citation45 In addition, biofilms contribute to the pathogenesis of oral candidiasis,Citation46 and its presence on the surface of medical implants may constitute a risk factor for persistent candidiasis.Citation15,Citation47
In our study, most of the patients (including this one) had been treated with broad-spectrum antibacterial agents. Candida colonization, indeed, is strongly linked to the receipt of antibiotics, particularly broad-spectrum ones.Citation48
Biofilm observation with SEM
C. albicans biofilm from the inner surface of the CVC segments was examined and imaged with SEM.
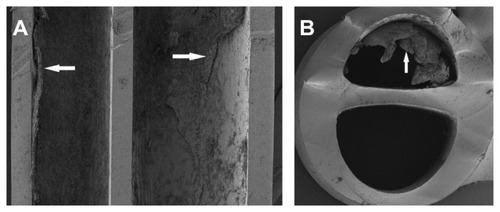
Longitudinal and lateral cuts performed on the CVC (, respectively) revealed a dense layer of C. albicans biofilm attached to the inner surface of the CVC; however, its external surface was not affected by the biofilm.
Figure 3 SEM of (A) longitudinal and (B) lateral cuts performed on the CVC.
Abbreviations: SEM, scanning electron microscope; CVC, central venous catheter.
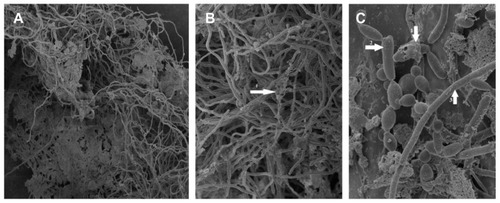
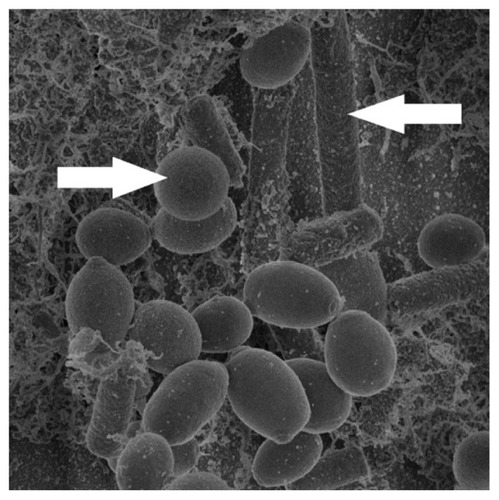
With higher magnifications of SEM, the structure of the biofilm could be demonstrated. shows a mixture of morphological forms of C. albicans biofilm. This biofilm revealed a dense network of yeasts, pseudohyphae, and hyphae. The extracellular matrix was also observed; it coated cells and hyphae.
Figure 4 SEM of a mixture of morphological forms of Candida albicans biofilm developed on the inner surface of the CVC. Magnification: (A) ×450; (B) ×1100; (C) ×4500.
Abbreviations: SEM, scanning electron microscope; CVC, central venous catheter; ECM, extracellular matrix.
The OBC of the female patient was contaminated by C. albicans biofilm. This is shown clearly in , which also shows the complex structure of the biofilm. C. albicans biofilm was formed by yeast cells, pseudohyphae, hyphae, and a visible matrix.
Figure 5 SEM of the contaminated OBC showing Candida albicans biofilm.
Abbreviations: SEM, scanning electron microscope; OBC, orobronchial catheter.
In Andes et al,Citation49 the SEM of the biofilm on catheter segments demonstrated more clearly the heterogeneous architecture of the biofilm structure. In their work, SEM of in vivo C. albicans biofilm showed a mature biofilm that consisted of a dense network of yeasts cells, hyphae, and pseudohyphae. Further, the same results were observed by Hawser and DouglasCitation50 10 years before.
In addition, biofilms of C. albicans consist of matrix-enclosed microcolonies containing yeasts, hyphae, and pseudohyphae. The biofilm is arranged in a bilayer structureCitation51 consisting of a thin yeast layer and an upper hyphal layer.Citation52 In a recent study, the structure of the biofilm was investigated using SEM, and this study, indeed, revealed an extracellular matrix material coating the cells.Citation53 In fact, adjacent to the catheter surface, yeast cells were densely embedded in an extracellular matrix.Citation48
Conclusion
Progress in the medical field is constantly evolving; however, clinical, epidemiological, and microbiological studies have so far failed to prevent nosocomial infections and, in particular, those of fungal origin. The current study is, to the authors’ knowledge, the first survey of an ICU in Algeria. It highlights the nosocomial candidemia related to medical implants (catheters, probes, intubations, etc) by calculating their rates and by determining the causative strains and the various types of catheter infectivity. These infections, in fact, are serious additional risks acquired by critically ill patients who require significant intensive care.
According to the literature, there is no accepted method by which to evaluate the types of catheter fungal infectivity. Within this frame, our study has shown that the Brun-Buisson method seems to be suitable for these studies. Our results revealed three different types of catheter infectivity: contaminations (9.52%), infections (4.76%), and colonizations (3.17%). In addition, the results prove that C. glabrata was strongly involved in the contamination of catheters; this species was more frequently isolated from the implanted medical devices than C. albicans.
On the other hand, SEM revealed the formation of biofilms of C. albicans on the surfaces of the catheters. Consequently, biofilms constitute a major problem in the diagnosis of candidiasis.
To conclude, it is highly recommended that vigilance be exercised when using medical implants to prevent fungal infections, especially in ICUs. To this end, in developing countries, the surveillance of nosocomial infections caused by fungi remains an open discussion, and further studies are warranted in this field.
Acknowledgments
The authors express great appreciation for the support provided by Prof Bertrand Dupont. We also wish to thank Mr Brahim Yala, teacher of English, for reviewing and correcting this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- VandijckDBlotSLabeauSCandidemia in critically ill patients: An analysis of daily antifungal therapy related costsJ Mycol Med20081829699
- FalagasMERoussosNVardakasKZRelative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic reviewInt J Infect Dis20101411e954e96620797887
- EloyOMarqueSMourvilliersBContribution of the Pittet’s index, antigen assay, IgM, and total antibodies in the diagnosis of invasive candidiasis in intensive care unitJ Mycol Med2006163113118
- EggimannPPittetDCandidoses en réanimationCandidiasis in the ICURéanimation200211209221 French
- SeabraRBhogalNHospital infections, animal models and alternativesEur J Clin Microbiol Infect Dis200928656156819096886
- DevelouxMBretagneSCandidiasis and yeast infectionsEMC-Maladies Infectieuses200523119139
- GürcüoğluEAkalınHEnerBNosocomial candidemia in adults: Risk and prognostic factorsJ Mycol Med2010204269278
- MakniaFSellamiaATrabelsiHYeast species isolated in the CHU of Sfax, TunisiaJ Mycol Med20102014247
- MoranCGrussemeyerCASpaldingJRBenjaminDKJrReedSDComparison of costs, length of stay, and mortality associated with Candida glabrata and Candida albicans bloodstream infectionsAm J Infect Control2010381788019836856
- RuanSYLeeLNJerngJSYuCJHsuehPRCandida glabrata fungaemia in intensive care unitsClin Microbiol Infect200814213614018042196
- Avila-AgueroMLCanas-CotoAUlloa-GutierrezRCaroMAAlfaroBParisMMRisk factors for Candida infections in a neonatal intensive care unit in Costa RicaInt J Infect Dis200592909515708324
- PicazoJJGonzález-RomoFCandelFJCandidemia in the critically ill patientInt J Antimicrob Agents200832Suppl 2S83S8519013345
- RamageGSavilleSPThomasDPLópez-RibotJLCandida biofilms: an updateEukaryotic Cell20054463363815821123
- KhanSAlamFAzamAKhanAUGold nanoparticles enhance methylene blue-induced photodynamic therapy: a novel therapeutic approach to inhibit Candida albicans biofilmIntl J Nanomedicine2012732453257
- Boucherit-AtmaniZSeddikiSMLBoucheritKSari-BelkharoubiLKunkelDCandida albicans biofilms formed into catheters and probes and their resistance to amphotericin BJ Mycol Med2011213182187
- SoniNWagstaffAFungal infectionCurr Anaesth Crit Care2005164231241
- VeyssierPDomartYLiebbeAMInfections NosocomialesNosocomial Infections2nd edParisCollection Abrégés de Médecine1998 French
- PittetDMonodMSuterPMFrenkEAuckenthalerRCandida colonization and subsequent infections in critically ill surgical patientsAnn Surg199422067517587986142
- CarrièreCMarchandinHInfections liées aux cathéters veineux centraux: diagnostic et definitionsInfection linked to central venous catheters: diagnosis and definitionsNéphrologie2001228433437 French
- TimsitJFWolffMMourvillierBSchortgenFRégnierBDiagnosis and management of catheter-related infections in the ICUMéd Mal Infect20033312619627
- GallienSSordetFEnache-AngoulvantATreatment of catheter-related candidemiaJ Mycol Med20071714249
- BlotSIVandewoudeKHHosteEAColardynFAEffects of nosocomial candidemia on outcomes of critically ill patientsAm J Med2002113648048512427497
- KadirTPisiricilerRAkyüzSYaratAEmekliNIpbükerAMycological and cytological examination of oral candidal carriage in diabetic patients and non-diabetic control subjects: thorough analysis of local aetiologic and systemic factorsJ Oral Rehabil200229545245712028493
- Brun-BuissonCAbroukFLegrandPHuetYLarabiSRapinMDiagnosis of central venous catheter-related sepsis: Critical level of quantitative tip culturesArch Intern Med198714758738773555377
- ChandraJMukherjeePKGhannoumMAIn vitro growth and analysis of Candida biofilmsNat Protoc20083121909192419180075
- TimsitJFDuboisYMinetCNew materials and devices for preventing catheter-related infectionsAnn Intensive Care201113421906266
- AlmiranteBRodríguezDParkBJBarcelona Candidemia Project Study GroupEpidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003J Clin Microbiol20054341829183515815004
- KettaniABelkhadirZHMosadikAAntifungal treatment of systemic candidiasis in intensive care unitJ Mycol Med20061611625
- ChalmersCMBalAMManagement of fungal infections in the intensive care unit: a survey of UK practiceBr J Anaesth2011106682783121504935
- ClarkTAHajjehRARecent trends in the epidemiology of invasive mycosesCurr Opin Infect Dis200215656957412821832
- FidelPLJrVazquezJASobelJDCandida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicansClin Microbiol Rev199912180969880475
- RuanSYHuangYTChuCCYuCJHsuehPRCandida glabrata fungaemia in a tertiary centre in Taiwan: antifungal susceptibility and outcomesInt J Antimicrob Agents200934323623919361960
- HajjehRASofairANHarrisonLHIncidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance programJ Clin Microbiol20044241519152715070998
- TortoranoAMCaspaniLRigoniALBiraghiESicignanoAVivianiMACandidiasis in the intensive care unit: a 20-year surveyJ Hosp Infect200457181315142710
- PfallerMAMesserSAHollisRJTrends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United StatesDiagn Microbiol Infect Dis199933421722210212747
- KlevayMJErnstEJHollanbaughJLMillerJGPfallerMADiekemaDJTherapy and outcome of Candida glabrata versus Candida albicans bloodstream infectionDiagn Microbiol Infect Dis200860327327718024053
- TrickWEFridkinSKEdwardsJRHajjehRAGaynesRPNational Nosocomial Infections Surveillance System HospitalsSecular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999Clin Infect Dis200235562763012173140
- VerstrepenKJReynoldsTBFinkGROrigins of variation in the fungal cell surfaceNat Rev Microbiol20042753354015197389
- BleichnerGBeaucaireGGottotSInfections liées aux cathéters veineux centraux en reanimationInfections related to central venous catheters in intensive careRéan Urg19943321330 French
- AbbesSSellamiHSellamiAVariability of Candida albicans strains in ICU in Tunisia hospitalJ Mycol Med20081811015
- BouzaEMuñozPEpidemiology of candidemia in intensive care unitsInt J Antimicrob Agents200832Suppl 2S87S9119013346
- NairyHMCharyuluNRShettyVAPrabhakaraPA pseudo-randomised clinical trial of in situ gels of fluconazole for the treatment of oropharngeal candidiasisTrials2011129921504616
- HoshiNMoriHTaguchiHManagement of oral candidiasis in denture wearersJ Prosthodont Res2011551485220381447
- YangYLLoHJHungCCLiYEffect of prolonged HAART on oral colonization with Candida and candidiasisBMC Infect Dis20066816423306
- JavedFKlingsporLSundinUAltamashMKlingeBEngströmPEPeriodontal conditions, oral Candida albicans and salivary proteins in type 2 diabetic subjects with emphasis on genderBMC Oral Health200991219435501
- TheinZMSamaranayakeYHSamaranayakeLPCharacteristics of dual species Candida biofilms on denture acrylic surfacesArch Oral Biol200752121200120817681271
- MukherjeePKChandraJCandida biofilm resistanceDrug Resist Updat200474–530130915533767
- HaJFItalianoCMHeathCHShihSReaSWoodFMCandidemia and invasive candidiasis: a review of the literature for the burns surgeonBurns201137218119520395056
- AndesDNettJOschelPAlbrechtRMarchilloKPitulaADevelopment and Characterization of an in vivo central venous catheter Candida albicans biofilm modelInfect Immun200472106023603115385506
- HawserSPDouglasLJBiofilm formation by Candida species on the surface of catheter materials in vitroInfect Immun19946239159218112864
- DouglasLJMedical importance of biofilms in Candida infectionsRev Iberoam Micol200219313914312825991
- DouglasLJCandida biofilms and their role in infectionTrends Microbiol2003111303612526852
- NettJESanchezHCainMTRossKMAndesDRInterface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulationEukaryotic Cell201110121660166921666076