Abstract
Objective
This study aims at assessing the potential benefits of observation of monocyte-to-albumin ratio (MAR) and neutrophil percentage-to-hemoglobin ratio (NPHR) in the detection of non-small cell lung cancer (NSCLC).
Methods
This study retrospectively involved 195 NSCLC patients and 204 healthy volunteers. The correlations between the clinicopathological characteristics of NSCLC and the two ratios including MAR and NPHR were assessed. The diagnostic efficiency of NSCLC patients by MAR and NPHR, alone or in combination with carcinoembryonic antigen (CEA), was assessed by receiver operating characteristic (ROC) curve. The risk factors for NSCLC were analyzed with binary logistic regression.
Results
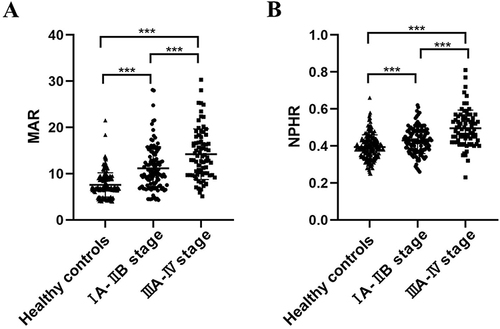
Compared to healthy controls, the levels of MAR and NPHR in NSCLC patients were elevated. MAR and NPHR were related to clinicopathologic characteristics and increased significantly along with the progression of NSCLC. The area under the curve (AUC) for 95% confidence interval (95% CI) of MAR and NPHR in the diagnosis of NSCLC was 0.812 (0.769–0.854) and 0.724 (0.675–0.774), respectively. The combination of MAR, NPHR, and CEA achieved the highest diagnostic utility compared to each individually or combined markers (AUC, 0.86; 95% CI, 0.824–0.896; sensitivity, 72.8%; specificity, 87.3%). Further analysis showed that MAR combined with NPHR presented the potential to detect early-stage (IA–IIB) NSCLC (AUC, 0.794; 95% CI, 0.743–0.845; sensitivity, 55.1%; specificity, 87.7%). The result indicated that MAR and NPHR might be risk factors for NSCLC.
Conclusion
MAR and NPHR could be novel and effective auxiliary indexes in the detection of NSCLC, especially when combined with CEA.
Introduction
Lung cancer has become the second most common cancer on earth, which has also been the main cause of the cancer-related deaths, with 21% as its overall 5-year survival rate.Citation1 The risk factors for lung cancer include smoking, asbestos and other occupational carcinogens, radon, and environmental tobacco smoke, among which smoking has been the most significant risk factor.Citation2 In the lung carcinomas, non-small cell lung cancer (NSCLC) is the commonest form, taking up about 85% of the total lung carcinomas. Unfortunately, most NSCLC patients could not be diagnosed until an advanced stage.Citation3 Considering the above conditions, early detection and diagnosis are of great importance in reducing the mortality of NSCLC patients. There are many methods to treat lung cancer, such as surgery, radiotherapy, chemotherapy, the latest molecular targeted therapy, and cancer immunotherapy. However, the long-term prognosis of NSCLC has not been significantly improved due to the high risk of local recurrence and distant metastases.Citation4 Therefore, early detection and diagnosis are vital to reducing the mortality of the patients with NSCLC.
The methods of detecting lung cancer include vary from imaging examinations to pathological biopsy. Pathological biopsy has been considered standardized method of diagnosis in lung cancer up till now. However, it has been widely limited due to its high cost, strong invasiveness, and prone to complications.Citation5 At the same time, low-dose spiral computed tomography (CT) has also become a routine screening in the detection of lung cancer.Citation6 However, the implementation in the population is limited due to its high cost, low accessibility, and false positives. Besides, long-term exposure to low-dose radiation might also increase the probability of having cancer.Citation7 Carcinoembryonic antigen (CEA) as a serum tumor marker is an important indicator for the diagnosis, prognosis and efficacy evaluation of NSCLC, being widely used in clinical practices due to its simple and accurate detection and high repeatability.Citation8 In NSCLC, cancer cells secrete a large amount of CEA into the blood, resulting in remarkable increases in serum CEA levels.Citation9 However, serum CEA levels are affected by smoking and increase along with the smoking time.Citation10 In addition, a single CEA index cannot meet the clinical needs due to its poor sensitivity and lack of organ specificity. Consequently, seeking biomarkers that are simple, easily obtained and reproducible has become a hotspot in NSCLC detection.
An increasing number of studies have proved that the interactions between tumor microenvironment and immune mechanism offered important reference basis in diagnosis and treatment of tumors. A large number of inflammatory cells such as neutrophils and macrophages exist in the tumor microenvironment. They accelerate tumor growth, invasion and metastasis by secreting cytokines and interacting with tumor cells.Citation11 Tumor-associated neutrophils (TAN) are elevated in the peripheral serum of patients with several cancers, such as lung cancer,Citation12 nasopharyngeal carcinomaCitation13 and colon cancer.Citation14 TAN could promote tumor growth, angiogenesis, extracellular matrix remodeling and curb anti-tumor immune responses. Tumor-associated macrophages (TAM) are the major component of tumor microenvironment. Monocytes in peripheral circulation infiltrate into tumor tissues through vascular endothelial cells, and differentiate into TAM under the tumor microenvironment effects.Citation15 TAM could accelerate the proliferation, metastasis and invasion of tumor cells by affecting normal angiogenesis, promoting the polarization of M2 macrophages, and promoting epithelial–mesenchymal transition.Citation15
The nutritional and metabolism status is related to tumor progression and metastasis. Tumor-related inflammation could release a large number of inflammatory cytokines including tumor necrosis factor, interleukin-1, interferon-γ, which inhibit the production of erythropoietin and reduce the utilization rate of ferritin in red blood cells, causing cancer-related anemia.Citation16 Serum albumin is synthesized in the liver, the falling level of which representing both malnutrition and ongoing systemic inflammation.Citation17 As important indicators of malnutrition and inflammation, anemia and hypoproteinemia are generally associated with poor prognosis and high mortality in cancer patients.Citation18 However, the problem of the above indicators is that each of them reflects either inflammatory or nutritional. The research also found that, compared to single indicator, the combination of inflammatory and nutritional indicators could help better diagnose cancer and predict prognosis.Citation16 Monocyte-to-albumin ratio (MAR) and neutrophil-to-hemoglobin ratio (NPHR) consist of inflammatory markers and nutritional indicator, reflecting the systemic inflammatory and nutritional status. However, few studies have evaluated the clinical significance of MAR and NPHR in NSCLC. This study aimed to explore the additional value of MAR and NPHR in the diagnosis of NSCLC, including at the early stage.
Materials and Methods
Patients
The patients diagnosed with NSCLC were selected into this retrospective monocentric study according to medical records at the Affiliated Hospital of Jiangsu University (Zhenjiang, China) from June 2014 to June 2021. A total of 204 healthy subjects were enrolled. Included patients with NSCLC were those who were newly diagnosed without receiving chemoradiotherapy, immune targeted therapy or surgery, and those who were diagnosed as NSCLC by cytology or histopathology. To assess the severity of NSCLC and complete the clinical data, patients underwent magnetic resonance imaging, chest and abdominal CT or contrast-enhanced CT, positron emission tomography/CT and bone scans. Included healthy volunteers were participants without suspicious lesion found in the chest CT. The exclusion criteria for all participants were as follows: (1) cardiovascular disease; (2) chronic obstructive pulmonary disease; (3) diabetes mellitus; (4) ischemic stroke; (5) hepatitis and cirrhosis; (6) hematological diseases; (7) renal system diseases; (8) autoimmune diseases; (9) history of malignant tumor; (10) acute and chronic infectious diseases; (11) incomplete, unavailable, or obvious abnormal clinical and laboratory data. The stages of the disease were classified according to the Tumor-Node-Metastasis (TNM) classification of the Union International for Cancer Control, 8th edition.Citation19 Depending on patients’ conditions, NSCLC was divided into early-stage NSCLC (IA-IIB) and advanced NSCLC (IIIA-IV).Citation20 The study has been approved by the Ethics Committee of the Affiliated Hospital of Jiangsu University. This study was performed under the Declaration of Helsinki with confidential data. Broad consent was obtained from all participants included in this retrospective study.
Method
Baseline characteristics and laboratory measurements for each group were obtained from the hospital’s electronic medical record system. The peripheral blood samples of participants were gathered in the early morning after fasting for at least 8 hours. Routine blood tests were performed through an automated hematology analyzer SYSMEX XN3000 (Sysmex Corporation). Serum albumin was detected through a BEKMAN AU5800 automatic biochemical analyzer (Beckman Coulter, Inc.). Serum CEA levels were determined with the ABBOTT ARCHITECT i2000sr (Abbott Pharmaceutical Co., Ltd.), by means of chemiluminescent immunoassay. The normal range of all indicators was recorded according to the instructions of the manufacturer. MAR was calculated as monocyte (number/mm3) to albumin (g/L) ratio and NPHR was calculated as neutrophil percentage (%) to hemoglobin (g/L) ratio.
Statistical Analysis
Statistical analyses were performed, and graphs were generated through the Statistical Package for Social Science (SPSS) software version 22.0 (IBM Corp., Armonk, NY, USA),Citation21 MedCalc software version 19.0.4 (MedCalc Software bvba, Ostend, Belgium)Citation22 and GraphPad Prism software version 8.0 (GraphPad Software Inc., San Diego, CA, USA).Citation23 The distribution characteristics of the data were assessed by the Kolmogorov–Smirnov test. The normal distribution of continuous variables was presented as mean ± standard deviation (SD), and the skewed distribution of continuous variables was presented as median and interquartile range [Median (Q1, Q3)]. Age was presented as median and range. Categorical variables were expressed as quantity and percentage with Chi-square Test (χ2 Test) used to analyze these variables. The Student-t-test (unpaired) was used to compare normally distributed data. Except for the normal distributed data, the other two groups were compared by Mann–Whitney U-test. Binary logistic regression was taken to analyze the risk factors for NSCLC, and the receiver operating characteristic (ROC) curve was applied to compute the area under the curve (AUC) with 95% confidence interval (95% CI) of MAR, NPHR and CEA to determine their diagnostic accuracy. Additionally, the areas under the ROC curve of the individual indicators were compared using DeLong test. Odds ratios (ORs) with 95% CI were calculated by logistic regression. In the logistic regression analysis, the effect of NPHR changes was enhanced by performing reciprocal conversion of the NPHR and reversing the correlation. Both the correlation between monocytes and albumin and that between neutrophil percentage and hemoglobin were assessed by correlation analysis.
Results
Baseline Characteristics of All Participants
A total of 399 volunteers were enrolled in this study, including 195 NSCLC patients and 204 subjects with no diseases detected (). The NSCLC patients consist of 157 adenocarcinomas, 23 squamous cell carcinomas, and 15 other NSCLC patients. Additionally, 42.0% of patients suffered from lymph node metastases, 26.7% of them got distant metastases, and 39.4% of them were in the IIIB-IV stage of TNM (). No significant differences were found in age (P=0.955), gender composition (P=0.312), smoking history (P=0.071) and pack-year (P=0.696) between the NSCLC group and the healthy control group. Compared with healthy volunteers, leukocytes, neutrophils, monocytes, and neutrophil percentages of the NSCLC patients remarkably increased (P<0.001), while hemoglobin and albumin decreased in the healthy subjects (P<0.001). The levels of MAR and NPHR rose in the NSCLC patients (P<0.001). MAR and NPHR of patients in the IIIA–IV stage were markedly elevated compared to that of patients in the IA–IIB stage. MAR and NPHR of patients in the IA-IIB stage were much higher than that of healthy volunteers ().
Table 1 Clinical Baseline Characteristics of the NSCLC Group and the Healthy Controls
Relationships Between MAR and NPHR Levels, and Clinicopathological Characteristics in NSCLC Patients
The correlations between the levels of MAR and NPHR and the baseline characteristics (age, sex, and smoking history) and clinicopathological characteristics (tumor size, lymph node metastasis, distant metastasis, and clinical stage) of NSCLC patients are shown in . Age, gender and smoking history were the confounding factors of MAR, adjusted by Binary Logistic regression. According to the data analysis, compared to NSCLC patients with low MAR level, those with higher MAR level had larger tumor tissue (P=0.027), higher lymph node metastasis (P=0.025), and further metastasis (P=0.137). However, there was no significant difference between MAR and TNM stage. Similarly, except for age, sex and smoking history, NPHR was strongly associated with tumor size (T1-T2) (P<0.001), lymph node metastasis (N1-N3) (P<0.001), distant metastasis M1 (P<0.001), and stage of TNM (IIIA–IV) (P<0.001).
Table 2 Associations Between MAR and NPHR Levels and Clinicopathological Features in the NSCLC Group
Diagnostic Efficiency of MAR, NPHR, and Combined Indexes in NSCLC
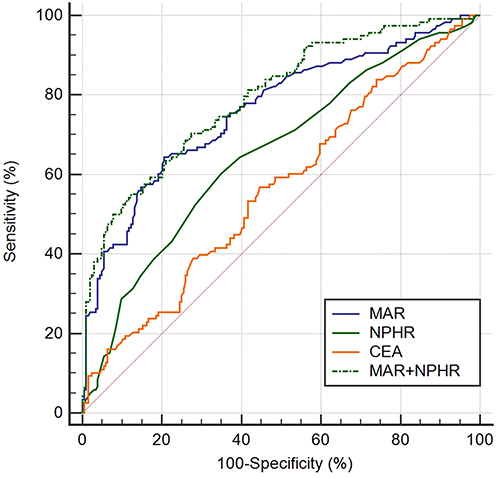
In this part, the diagnostic efficiency of MAR, NPHR and the combined markers were evaluated to discriminate NSCLC patients from healthy subjects ( and , ). The results proved that MAR was superior to NPHR and CEA (AUC, 0.812; 95% CI, 0.769–0.854) in diagnostic utility, with its sensitivity and specificity being 66.2% and 85.3%, respectively. MAR and NPHR were higher in sensitivity (66.2% and 53.3%, respectively) to differentiate patients with NSCLC from the healthy controls, while CEA got greater specificity (93.6%). Sensitivity of CEA increased when combined with either MAR or NPHR (68.2% and 59.5%, respectively). When the three of MAR, NPHR and CEA were combined for detecting NSCLC, maximum diagnostic efficiency and high sensitivity and specificity were obtained (AUC, 0.86; 95% CI, 0.824, 0.896; sensitivity, 72.8%; specificity, 87.3%).
Table 3 Diagnostic Efficiency of MAR, NPHR, Alone or Combined, in the NSCLC Patients and the Healthy Controls
Figure 2 (A) ROC curve analysis of the value of MAR, NPHR and CEA alone in the diagnosis between the NSCLC group and the healthy controls. (B) ROC curve analysis of the value of MAR, NPHR and CEA combined in diagnosis between the NSCLC group and the healthy controls.
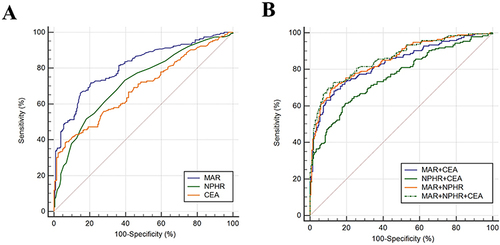
Subsequently, risk factors were analyzed for NSCLC with binary logistic regression. MAR (Odds ratio (OR), 1.412; 95% CI, 1.292–1.544; P<0.001), reciprocal-transformed NPHR (OR, 0.183; 95% CI, 0.096–0.349; P<0.001) and CEA (OR, 0.182; 95% CI, 1.016–1.418; P=0.032) could be possible risk factors for NSCLC (). To further evaluate the diagnostic utility of MAR and NPHR in the early-stage NSCLC group, 204 healthy subjects and 118 NSCLC patients in their early-stage were analyzed when age (P=0.135), composition of gender (P=0.105), smoking history (P=0.198) and pack years (P=0.056) were comparable. MAR (AUC, 0.763, sensitivity, 64.4%, specificity, 79.4%) was superior in diagnostic efficiency to NPHR (AUC, 0.656, sensitivity, 62.7%, specificity, 72.6%) and CEA (AUC, 0.565, sensitivity, 56.8%, specificity, 55.4%). When MAR and NPHR were in combination, AUC (95% CI) rose to 0.794 (0.743–0.845) with higher specificity (87.7%) but lower sensitivity (55.1%) ( and ).
Table 4 Binary Logistic Regression Analysis of Potential Risk Factors for NSCLC
Table 5 Diagnostic Efficiency of MAR and NPHR, Alone or Combined in the Early-Stage NSCLC Group and the Healthy Controls
Discussion
Lung cancer is a common cancer: only patients with early diagnosis of NSCLC have the chance to be cured. Therefore, it is of great significance seeking for screening and diagnostic measures. At present, pathological biopsy is the standardized method of lung cancer detection, but it is not a routine physical examination.Citation1 Increasing numbers of studies have been carried out to analyze the effects of hematologic parameters, including monocytes, neutrophils, albumin, and hemoglobin in the detection and prognosis of malignant tumors. However, the clinical significance of MAR and NPHR in NSCLC, alone or in combination with CEA, has not been fully investigated.
The tumor environment can mediate the constant inflammatory response, which plays a significant role in tumor cell multiplication, angiogenesis, and metastatic invasion.Citation24 Thus, the interactions between systemic inflammatory processes and the tumor microenvironment help the occurrence and development of the tumors. Monocytes play a vital part in the progression and metastasis of tumor, by promoting immunosuppression, remodeling the extracellular matrix, angiogenesis, and tumor cell transmission to make connections between innate and adaptive immune responses.Citation25 Elevated serum monocytes have been reported to be related to TAM production and high tumor burden, which may lead to poor prognosis.Citation26 Some studies have found that monocytes and cytokines secreted by monocytes in lung cancer patients remarkably increased in comparison with those of healthy volunteers,Citation27 and the high levels before treatment were connected to the lower survival rates of lung adenocarcinoma.Citation28 Albumin is a functional serum protein that can reflect nutritional status. Hypoalbuminemia, which is usually the result of insufficient nutrient absorption and excessive tumor consumption, has some influence on the metabolism and immune function, and also induces the activation of cytokines such as tumor necrosis factor-α, interleukin-6, and interleukin-1, leading to adverse reactions of the anticancer agents.Citation29 C. VARIM et al have found that in patients with NSCLC, albumin levels decreased with a reduction in nutrition along with cancer progression.Citation30 There was no correlation between monocytes and albumin in healthy controls. In the NSCLC group, there was a certain negative correlation between monocytes and albumin, indicating a negative regulatory effect between monocytes and albumin (Figure S1). MAR is the ratio of monocytes to albumin. The increase of MAR reflects both the increase of monocytes and the decrease of albumin, which could more accurately reflect the inflammatory and nutritional status of NSCLC patients. Previous studies have proved that MAR could take part in the prediction of long-term negative effects of the patients who had taken percutaneous coronary intervention.Citation31 However, it is not significantly related to the overall and recurrence-free survival of rectal cancer.Citation32 Few studies have assessed the value of MAR in the detection and prognosis of NSCLC.
Neutrophils, derived from bone marrow stem cells, are the main immune cells that remove pathogenic microorganisms. Plenty of studies have shown that neutrophils interacted with cancer and immune cells in blood and the tumor microenvironment by releasing reactive oxygen species or elastase to promote tumor growth, proliferation, metastasis, and spread, to further contribute to cancer developing and occurring.Citation33 Previous studies suggested that neutrophils increased with disease progression, and high neutrophil counts were associated with aggressive behavior in NSCLC.Citation30 The reduction of hemoglobin could induce hypoxia of tumor cells, thereby stimulating tumor growth. It could also increase chemoresistance by regulating gene expression and cell cycle, thus leading to cancer progression and shortening survival time.Citation34 Patients with anemia before treatment were found to be connected to a poor prognosis of various malignancies.Citation35,Citation36 Both neutrophils and hemoglobin could be affected by a variety of diseases other than cancer, with no clear correlation between the neutrophil percentage and hemoglobin in the NSCLC group (Figure S2). Based on these phenomena, a new index NPHR has been created, which has not been observed before. It was noticeable that the NPHR is normally distributed.
In this retrospective study, the hematologic parameters such as MAR and NPHR were applied for the first time in the progress of NSCLC. MAR and NPHR remarkably rose in the NSCLC patient group in comparison with the control group. Further analysis found that MAR and NPHR might have potential values in identifying NSCLC patients from the healthy population. The area under the ROC curve showed that the cut-off value of MAR presented better accuracy than NPHR in the detection of NSCLC patients. The sensitivity and specificity are equally important for these indexes when it comes to disease detection. When MAR and NPHR were combined, the sensitivity and specificity could be improved.
With the increase of prevalence and morbidity of the NSCLC patients, the researchers have highly focused on the prevention and cure of NSCLC in recent years, and more studies have been carried out on the prognosis of NSCLC.Citation37 In recent years, liquid biopsies and genetic biomarkers have become the new potential biomarkers to assess the prognosis of NSCLC. The liquid biopsy is a non-invasive method of detecting and monitoring cancer by finding biomarkers in blood or other body fluids, such as circulating tumor cells or circulating tumor DNA. The liquid biopsy has the advantages of less invasiveness, abilities to detect tumor-specific genetic and epigenetic abnormalities to evaluate patient condition in time. For example, the combination of classical standard assay (SA) with extended cytokeratin spectroscopy (EA) could quantify the expression of EML4-ALK fusion protein in circulating tumor cells, and predict the prognosis of patients with NSCLC.Citation38 Genetic biomarkers such as GLUT-1 and p16, are clinical tools for monitoring disease evolution, which are associated with poor prognosis in NSCLC patients.Citation39 However, liquid biopsy and genetic biomarkers have high requirements for technology with high detection cost. According to the above conditions, they could not be widely promoted in clinical practice. A number of research has been proving that systemic inflammation in NSCLC is closely related to long-term prognosis. Higher inflammatory markers such as platelet-to-lymphocyte ratio, prognostic nutritional index, hemoglobin, albumin, lymphocyte, platelet score, neutrophil-to-lymphocyte ratio, systemic immune inflammation index and advanced lung cancer inflammation index generally predict poor prognosis of NSCLC. Meanwhile, high levels of hemoglobin and albumin are positively correlated with the prognosis of NSCLC.Citation40 Therefore, further investigation has been carried out in this study on whether MAR and NPHR levels could be used as potential biomarkers to predict the disease progression of NSCLC. Interestingly, the results showed that MAR and NPHR were both significantly associated with tumor size, lymph node metastasis, and distant metastasis, which might be a partial suggestion to tumor progression. Considering the possible confounding factors of age, gender and smoking history, logistic regression was used to control the effects. After the adjustment, the notable correlations between MAR and NPHR and clinicopathological characteristics were still clearly observed. Based on the above findings, this study hypothesized that MAR and NPHR might be used as potential prognostic factors for NSCLC patients.
CEA is an important serum biomarker of tumor often used in the detection of lung cancer.Citation41 However, the low sensitivity of CEA limits its clinical application in lung cancer screening, besides hematological indicators in NSCLC detection are easily affected by numerous factors. Therefore, this study considered that the combination of blood biomarkers and tumor markers could improve the reliability in the detection of NSCLC. The findings in this study proved that MAR and NPHR could improve the diagnostic efficacy of CEA and obtain better sensitivity, while suggesting that combined detection of CEA, MAR or NPHR might be a better biomarker in the detection of NSCLC. When MAR, NPHR and CEA were applied in combination, it could lead to superior diagnostic efficiency, larger AUC, and improved sensitivity and specificity than when the markers were applied individually. MAR had certain advantages in the early detection of NSCLC. When MAR and NPHR were combined, they can obtain better diagnostic efficiency and specificity with limited sensitivity. Therefore, when MAR and NPHR were used as markers for the early diagnosis of NSCLC, other biomarkers (including serum tumor markers) would be used to enhance the ability of MAR and NPHR to monitor the early-stage NSCLC to reduce the rate of misdiagnosis and missed diagnosis.
This study still got some limitations. First, this is a retrospective, single-center, single-cut study: the small sample size might have influenced the reported results. Therefore, multi-center and larger-scale studies are necessary in the future. Secondly, this study is a retrospective case–control study. The results might be influenced by specific confounding factors, thus prospective cohort studies are further needed. Third, this study has not evaluated the association between MAR and NPHR and overall survival in NSCLC patients, which limits the generalization of the findings. Therefore, larger, multicenter, and prospective studies are still needed to examine the efficacy and to learn more about the relationship between these markers and diagnosis and prognosis in NSCLC cases.
Conclusions
MAR and NPHR could be effective novel auxiliary biomarkers in the diagnosis of NSCLC patients, particularly combined with CEA.
Data Sharing Statement
The study has retrospective characteristics. The data in favor of the study results could be provided by the corresponding author if needed.
Ethics Approval and Consent to Participate
The present study was approved by The Ethical Review Committee of Jiangsu University Affiliated Hospital (Zhenjiang, China). The broad consent of all participants was obtained.
Author Contributions
All authors have made significant contributions to the work reported, whether in conception, study design, execution, acquisition of data, analysis and interpretation, or in all of them; all have taken part in drafting, revising or critically reviewing the article; all have given final approval of the version to be published; all have agreed on the journal to which the article has been submitted; and all have agreed to be accountable for all aspects of the work.
Disclosure
The authors did not report any exist potential conflict of interest associate with this study.
Acknowledgments
The National Natural Science Foundation of China (81370119) was in support of the study.
References
- Nooreldeen R, Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci. 2021;22(16):8661. doi:10.3390/ijms22168661
- Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23(14):3175–3185. doi:10.1200/JCO.2005.10.462
- Planchard D, Popat S, Kerr K, et al; ESMO guidelines Committee. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi:10.1093/annonc/mdy275
- Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198(6):897–907. doi:10.1007/s00408-020-00407-5
- Pillai RN, Ramalingam SS. Advances in the diagnosis and treatment of non-small cell lung cancer. Mol Cancer Ther. 2014;13(3):557–564. doi:10.1158/1535-7163.MCT-13-0669
- Toyoda Y, Nakayama T, Kusunoki Y, et al. Sensitivity and specificity of lung cancer screening using chest low-dose computed tomography. Br J Cancer. 2008;98(10):1602–1607. doi:10.1038/sj.bjc.6604351
- Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol. 2002;20(4):911–920. doi:10.1200/JCO.2002.20.4.911
- Cho WC. Potentially useful biomarkers for the diagnosis, treatment and prognosis of lung cancer. Biomed Pharmacother. 2007;61(9):515–519. doi:10.1016/j.biopha.2007.08.005
- Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76(2):138–143. doi:10.1016/j.lungcan.2011.11.012
- Chevinsky AH. CEA in tumors of other than colorectal origin. Semin Surg Oncol. 1991;7(3):162–166. doi:10.1002/ssu.2980070309
- Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. doi:10.1155/2016/6058147
- Chen JL, Wu JN, Lv XD, et al. The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS One. 2020;15(8):e0237947. doi:10.1371/journal.pone.0237947
- Lin Z, Zhang X, Luo Y, et al. The value of hemoglobin-to-red blood cell distribution width ratio (Hb/RDW), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) for the diagnosis of nasopharyngeal cancer. Medicine. 2021;100(28):e26537. doi:10.1097/MD.0000000000026537
- Li X, Guo D, Chu L, et al. Potential diagnostic value of combining inflammatory cell ratios with carcinoembryonic antigen for colorectal cancer. Cancer Manag Res. 2019;11:9631–9640. doi:10.2147/CMAR.S222756
- Chen Y, Song Y, Du W, et al. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26(1):78. doi:10.1186/s12929-019-0568-z
- Xu SS, Li S, Xu HX, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. 2020;26(8):828–838. doi:10.3748/wjg.v26.i8.828
- Zhang J, Li SQ, Liao ZH, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget. 2017;8(43):75195–75205. doi:10.18632/oncotarget.20661
- Zhang L, Qin S, Lu L, et al. Diagnostic value of combined prealbumin-to-fibrinogen and albumin-to-fibrinogen ratios in Hp-negative gastric cancer. Int J Biol Markers. 2022;37(1):66–73. doi:10.1177/17246008211072875
- Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi:10.1016/j.chest.2016.10.010
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi:10.1378/chest.12-2359
- Gouda MA. Common pitfalls in reporting the use of SPSS software. Med Princ Pract. 2015;24(3):300. doi:10.1159/000381953
- Schoonjans F, Zalata A, Depuydt CE, et al. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48(3):257–262. doi:10.1016/0169-2607(95)01703-8
- Berkman SJ, Roscoe EM, Bourret JC. Comparing self-directed methods for training staff to create graphs using GraphPad Prism. J Appl Behav Anal. 2019;52(1):188–204. doi:10.1002/jaba.522
- Orozco-Morales M, Soca-Chafre G, Barrios-Bernal P, et al. Interplay between cellular and molecular inflammatory mediators in lung cancer. Mediators Inflamm. 2016;2016:3494608. doi:10.1155/2016/3494608
- Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322. doi:10.1002/JLB.4RI0818-311R
- Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21(34):9966–9973. doi:10.3748/wjg.v21.i34.9966
- Yin W, Lv J, Yao Y, et al. Elevations of monocyte and neutrophils, and higher levels of granulocyte colony-stimulating factor in peripheral blood in lung cancer patients. Thorac Cancer. 2021;12(20):2680–2690. doi:10.1111/1759-7714.14103
- Kumagai S, Marumo S, Shoji T, et al. Prognostic impact of preoperative monocyte counts in patients with resected lung adenocarcinoma. Lung Cancer. 2014;85(3):457–464. doi:10.1016/j.lungcan.2014.06.015
- Almasaudi AS, Dolan RD, Edwards CA, et al. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers. 2020;12(7):1986. doi:10.3390/cancers12071986
- Varim C, Celik FD, Sunu C, et al. The role of neutrophil albumin ratio in predicting the stage of non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2022;26(8):2900–2905. doi:10.26355/eurrev_202204_28621
- Zhang ZL, Guo QQ, Tang JN, et al. Monocyte-to-albumin ratio as a novel predictor of long-term adverse outcomes in patients after percutaneous coronary intervention. Biosci Rep. 2021;41(7):BSR20210154. doi:10.1042/BSR20210154
- Yamamoto T, Kawada K, Hida K, et al. Combination of lymphocyte count and albumin concentration as a new prognostic biomarker for rectal cancer. Sci Rep. 2021;11(1):5027. doi:10.1038/s41598-021-84475-4
- Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–2167. doi:10.1182/blood-2018-11-844548
- Huang Y, Wei S, Jiang N, et al. The prognostic impact of decreased pretreatment haemoglobin level on the survival of patients with lung cancer: a systematic review and meta-analysis. BMC Cancer. 2018;18(1):1235. doi:10.1186/s12885-018-5136-5
- Mori K, Janisch F, Mostafaei H, et al. Prognostic value of hemoglobin in metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Clin Genitourin Cancer. 2020;18(4):e402–e409. doi:10.1016/j.clgc.2019.12.002
- Magrowski Ł, Masri O, Ciepał J, et al. Pre-treatment hemoglobin concentration and absolute monocyte count as independent prognostic factors for survival in localized or locally advanced prostate cancer patients undergoing radiotherapy. Biomedicines. 2022;10(10):2514. doi:10.3390/biomedicines10102514
- Dong Y, Wang H, Shan D, et al. Research progress on the relationship between blood lipids and lung cancer risk and prognosis. Zhongguo Fei Ai Za Zhi. 2020;23(9):824–829. doi:10.3779/j.issn.1009-3419.2020.102.36
- Rossi E, Aieta M, Tartarone A, et al. A fully automated assay to detect the expression of pan-cytokeratins and of EML4-ALK fusion protein in circulating tumour cells (CTCs) predicts outcome of non-small cell lung cancer (NSCLC) patients. Transl Lung Cancer Res. 2021;10(1):80–92. doi:10.21037/tlcr-20-855
- Pezzuto A, D’Ascanio M, Ricci A, et al. Expression and role of p16 and GLUT1 in malignant diseases and lung cancer: a review. Thorac Cancer. 2020;11(11):3060–3070. doi:10.1111/1759-7714.13651
- Mazzella A, Maiolino E, Maisonneuve P, et al. Systemic inflammation and lung cancer: is it a real paradigm? Prognostic value of inflammatory indexes in patients with resected non-small-cell lung cancer. Cancers. 2023;15(6):1854. doi:10.3390/cancers15061854
- Khanmohammadi A, Aghaie A, Vahedi E, et al. Electrochemical biosensors for the detection of lung cancer biomarkers: a review. Talanta. 2020;206:120251. doi:10.1016/j.talanta.2019.120251