Abstract
Synaptic plasticity is the capacity of synaptic transmission between neurons to be strengthened or weakened. There are many signal molecules accumulated in the presynaptic and postsynaptic membranes that can lead to the regulation of synaptic plasticity and involvement in numerous of neurological and psychiatric diseases, including anxiety disorder. However, the regulatory mechanisms of synaptic plasticity in the development of anxiety disorder have not been well summarized. This review mainly aims to discuss the biological functions and mechanisms of synaptic plasticity-related molecules in anxiety disorder, with a particular focus on the metabotropic glutamate receptors, brain-derived neurotrophic factor, hyperpolarization-activated cyclic nucleotide–gated channels, and postsynaptic density 95. The summarized functions and mechanisms of synaptic plasticity-related molecules in anxiety will provide insight into novel neuroplasticity modifications for targeted therapy for anxiety.
Introduction
Anxiety disorder is the most common psychological disorder, affecting about 7.3% of the world’s population, including generalized anxiety disorder, agoraphobia, posttraumatic stress anxiety disorder, social anxiety disorder, and panic anxiety disorder.Citation1 In the pathological anxiety state, the patient falls into a persistent high degree of unhappiness and discomfort. There are often symptoms of vegetative nerve dysfunction. such as palpitation, chest tightness, gastrointestinal and discomfort, and other anxiety, such as restlessness and panic.Citation2 About 75% of anxiety disorders are combined with depression, personality bipolar disorder, and alcohol and drug abuse, which increases the prevalence of hypertension, coronary heart disease, gastrointestinal disease, and even cancer.Citation3,Citation4 With the acceleration of the pace of modern life, the number of people suffering from anxiety disorders is increasing. However, the cure rate of anxiety disorder through psychotherapy and drug treatment is only 50%, mainly because the molecular mechanisms of anxiety are poorly understood.Citation5
Synaptic plasticity is the activity-dependent change in neuronal connection strength.Citation6 In 1973, Bliss first reported the long-term potentiation (LTP) phenomenon during the synaptic transmission between performing fibers and hippocampal granule cells of rabbits, which was the prelude to the study of synaptic plasticity.Citation7 Then, the corresponding long-term depression (LTD) of synaptic plasticity was reported by Ito in 1982.Citation8 Although synaptic plasticity also includes post-tetanic stimulation, short-term potentiation, short-term depression, and two-wave facilitation, the focus of related research is on long-term change in LTP/LTD, which can be divided into NMDAR-dependent LTP/LTD in the hippocampus CA1 region and NMDAR-independent LTP/LTD in the CA3 region.Citation9,Citation10
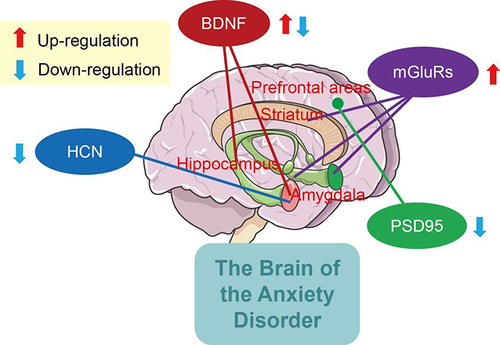
Recent studies have shown anxiety disorder is characterized by synaptic pathological changes, including abnormal morphology of dendritic spines, synaptic loss, and dysregulation of signal transduction and plasticity.Citation11,Citation12 These pathological features may involve abnormal expression of synaptic plasticity-related molecules, such as metabotropic glutamate receptors (mGluRs), BDNF, hyperpolarization-activated cyclic nucleotide–gated (HCN) channels, and postsynaptic density 95 (PSD95). These molecules are pivotal for the development of the central nervous system and the key regulators of synaptic plasticity, which makes them particularly attractive drug targets in neurological and psychiatric disorders, such as anxiety and depression. For example, PSD is a dense localized area in excitatory synapses and is comprised of receptors and signaling molecules associated with synaptic plasticity. mGluRs are family G protein–coupled receptors that express in PSD and regulate long-term plasticity in synaptic transmission in the striatum, hippocampus, and cerebellum. Much progress has been made in determining the mechanisms by which mGluRs are activated during synaptic transmission throughout the central nervous system.Citation13 Notably, the expression levels of PSD95 are higher than mGluRs within PSD. PSD95 is involved in the stabilization, recruitment, and trafficking of NMDAR to the postsynaptic membrane, and elevated PSD95 results in higher dendritic branching complexity and spine density in amygdala of male mice through abnormal activation of the MEK/ERK signals.Citation14,Citation15 Hence, spatial expression patterns of PSD related molecules, such as mGluRs and PSD95, and the molecules interacting with them need to be explored to widen our understanding of the mechanisms underlying current therapeutic options for anxiety.
In 2006, Duman summarized the functional role of neurotrophic factors in neurogenesis and proposed a neurotrophic theory during the progression of anxiety and depression. According to neurotrophic theory, emotional abnormalities may be caused by abnormal adaptations of the neural networks to changes in the external environment. Neurotrophic factors, including BDNF, may directly induce the onset of anxiety and influence the effects of anxiolytics.Citation16 BDNF is an essential molecule for axonal growth, synaptic function, and mood regulation. DNA methylation and histone modification are involved in regulating BDNF transcripts. Changes in BDNF gene and protein levels have been found to impair memory and induce anxiety.Citation17,Citation18 Subsequent evidence from numerous animal studies supports the conclusion that social isolation, social frustration, forced swimming stress, maternal deprivation, and chronic unpredictable stress (CUS) both reduce hippocampal BDNF protein and mRNA levels. Subsequent evidence from numerous animal studies supports the conclusion that social isolation, social frustration, forced swimming stress, maternal deprivation, and CUS reduce both hippocampal BDNF protein and mRNA levels.Citation16 Preclinical studies indicated that anxiolytics and selective serotonin-reuptake inhibitors elevated BDNF levels in the brain.Citation18 However, Larsen’s study found CUS was not correlated with a decrease but rather an increase in BDNF mRNA levels in the dentate gyrus of the dorsal hippocampus, suggesting that anxiety-like behavior was not simply related to BDNF expression.Citation19 It has also been reported that injection of BDNF into the ventral tegmental area augments anxiety-like behaviors. Interestingly, despite the inconsistent changes observed in stress-induced BDNF, further antianxiety and antidepressant treatment resulted in a significant increase in BDNF mRNA levels in the dorso dentate gyrus, implicating a potential therapeutic application in novel anti-anxiety drug development.Citation20 HCN channels are active at the resting membrane potential in CA1 pyramidal neurons, which is critical for controlling neuronal excitability and regulating synaptic inputs. Previous studies have shown that HCN channels are highly expressed in CA1 pyramidal neurons and knocking out the HCN channels induce an antianxiety-like phenotype in mice, suggesting HCN channels hold promise as a therapeutic biomarker of anxiety.Citation21 Immunoinflammatory-related molecules can also induce anxiety-like behavior by modulating synaptic plasticity in the basolateral amygdala (BLA).Citation22 A novel study showed that while noise stress reduced the protein expression of NMDA-receptor subunits, occludin and claudin 5 in the anxiety animal model, nanocurcumin administration downregulated these molecules.Citation23 Hence, a thorough understanding of biochemical characteristics and molecular mechanisms of synaptic plasticity-related molecules is of great significance to the prevention and targeted treatment of anxiety disorder. This article aims to review the regulatory molecules and their molecular mechanisms that are closed to synaptic plasticity during the initiation and development of anxiety disorder.
mGluRs
mGluRs are coupled to second messenger pathways via G proteins and modulate neurotransmission of glutamatergic and γ-aminobutyric acid, which is necessary for LTP activity.Citation24,Citation25 The group 1 of mGluRs (1, 5) is coupled with phospholipase C through Gq protein to activate inositol triphosphate receptor, leading to the release of intracellular Ca2+ and the induction of LTP.Citation26 The mGluR antagonist α-methyl-4-carboxyphenylglycine (MCPG) can inhibit LTP of hippocampal CA1 region.Citation27 Interestingly, MCPG can block the production of the first LTP, but cannot block the second LTP at the same synapse. Therefore, it is speculated that the mGluRs are activated at the first high-frequency conditional stimulation, and will be maintained for a long time as the intracellular “molecular switches”, and hence, in the second conditional stimulation, it can also induce LTP even if there is MCPG. A low-frequency stimulation can close the “molecular switches”, and MCPG can block the generation of LTP.Citation28 A further mechanism identifies an important role for Fmr1 protein in synaptic plasticity regulated by mGluRs.Citation29 Hippocampal mGluR-dependent LTD was enhanced in adolescent mice with Fmr1 knockout, which was related to the differential expression of Cav1. CAV1 expression in Fmr1 KO mice induced excessive endocytosis of the α-amino-3-hydroxyl-5-methyl-4-isoxazole propionate (AMPA) receptor. This process relied on mGluR1/5 activation rather than NMDAR. Importantly, injection of the cholesterol scavenger methyl-β-cyclodextrin recovered AMPA-receptor trafficking and significantly alleviated hippocampus-dependent anxiety-like behavior.Citation29 Group 2 of mGluRs (2–4) is located at the presynaptic terminals of the cortex and striatum. Khodagholi reported that the levels of mGluR2 and oxytocin receptor were decreased, while mGluR5 increased in hippocampus, prefrontal cortex and amygdala of anxiety mice.Citation30,Citation31 Oxytocin treatment ameliorated anxiety-like behavior through increasing oxytocin receptor, mGluR2, and glutathione, and decreasing mGluR5 in studied regions.Citation32 mGluR2 allosteric agonist inhibited anxiety-like behavior in wild-type male mice: 12-month-old male mice with mGluR2 knockdown had higher levels of anxiety in the water maze, while female mice had lower levels of anxiety in the maze and enhanced rotarod performance.Citation33 Similarly, anxiety showed some sex-dependence in the mice with mGluR4 knockdown. The 12-month-old male mice with mGluR4 knockdown indicated increased anxiety, while the female mice showed decreased anxiety in the water maze. Additionally, both 12-month-old male and female mice with mGluR4 knockdown had hippocampus-dependent cognitive impairments.Citation34 Duvoisin reported that male mice with mGluR8 knockdown indicated higher measures of anxiety in the elevated plus maze and open field than age-matched wild-type mice. Acute pharmacological stimulation induced by an mGluR8 agonist restrained anxiety in wild-type male mice.Citation35,Citation36 In conclusion, these results demonstrated that effects of mGluRs on anxiety disorder were strongly associated with sex and age, and mGluRs are potential targets for anxiety treatment due to their roles in regulating synaptic plasticity.
BDNF
Neurotrophic factors include nerve growth factor, neurotrophic factor and BDNF, in which the relationship between BDNF and synaptic plasticity have received more special attention.Citation37,Citation38 BDNF supports the survival of neurons and promotes the differentiation of dendrites and axons, which can further regulate synaptic plasticity.Citation39 BDNF also acts on the whole nervous system together with 5-hydroxytryptamine, epinephrine, and acetylcholine, and participates in the regulation of important neurotransmitters.Citation40 It is found that BDNF can specifically induce the phosphorylation of the NMDA receptor and enhance the activation of NMDA-receptor channel, thus participating in the process of LTP.Citation40 The decrease in BDNF expression made the hippocampus particularly vulnerable, and slight trauma would cause edema, degeneration, or necrosis of hippocampal neurons.Citation41 Numerous studies have confirmed the close relationship between anxiety disorder and BDNF expression level. Chiba demonstrated anxiety-like behavior restrained glucocorticoid receptor level and decreased glutamate release induced by BDNF in the prefrontal cortex.Citation42 Moonat found that alcohol-preferring rats were more prone to anxiety disorders, and BDNF expression was in a state of inhibition.Citation43 The low expression of BDNF in the central amygdala (CeA), medial amygdala (MeA) and BLA of the rat brain showed high measures of anxiety disorder. The anxiety of rats was relieved after injecting sufficient BDNF into the MeA, indicating that the level of BDNF in the amygdala was closely related to anxiety-like behavior.Citation43 Popoli reported that acute and chronic trauma or psychological stress could reduce the number of BDNF neurons in the hippocampus, thus increasing the frequency of anxiety-related behaviors.Citation44 Zhu demonstrated that the upregulation of BDNF could suppress the sensitivity of the neural structure in the hippocampus to various stresses, and further alleviate the anxiety disorder.Citation45 Notably, Casagrande reported that an obesogenic diet withdrawal induced anxiety-like behaviors and promoted BDNF expression in the dentate gyrus and the CA1 of the hippocampus. The increase in BDNF likely occurred in the mature forms (14 kDa and 28 kDa), which revealed a possible new role for mature BDNF. Although it was generally associated with beneficial features, it could also induce anxiety-like behaviors.Citation46
Trkb is a BDNF-specific receptor belong to the member of the neurotrophic tyrosine kinase receptor family.Citation47 In the field of anxiety disorders, Trkb has been a significant target for which knockout of its gene in mice resulted in depression and anxiety disorders.Citation48 BDNF first promotes Trkb homodimerize and induces the self-phosphorylation of the binding site, which can activate intracellular signal transduction mediated by BDNF and promote the functional changes in synaptic connection of neurons.Citation49,Citation50 Related study indicated the phosphorylation level of BDNF and Trkb binding site was significantly temporarily increased in the hippocampus of anxiety rats, and after a period of time, it continued to decline and finally was lower than the control group.Citation51 The higher level of soluble sortilin in patient serum is correlated with an increase in BDNF levels.Citation52 A recent study found that mice with sortilin knockdown promoted Trkb action deficiency and indicated a corticosterone-independent anxiety-like behavior, indicating that the serum soluble sortilin could be a potential biomarker of anxiety state.Citation53
BDNF gene self-mutation and epigenetic modification can also contribute to anxiety disorders. The BDNF gene has three mutation forms (Met-Mets type, Val-Vals type and Val-Mets type), and any variant genotype can cause anxiety disorder of different degrees.Citation54 The activity of hypothalamus–pituitary–adrenal (HPA) axis of individuals carrying Val-Vals gene is significantly higher than that of Met/Met gene carriers when coping with psychological stress.Citation55 A clinical study based on the state-trait anxiety inventory score of 340 patients showed that the Val-Vals genotype had the highest anxiety score among the three variant genotypes.Citation56 Gujral reported a higher score of anxiety-like behavior in the patients with the allele of Met66.Citation57 Additionally, maternal antenatal anxiety is a risk factor for child emotional development. DNA methylation, may contribute to the embedding of maternal distress into emotional outcomes.Citation58,Citation59 Nazzari reported that higher maternal antenatal anxiety was associated with greater negative emotionality of infant, and further contributed to methylation of the BDNF gene in male infants but not in female infants. These results may provide information for developing strategies to promote the emotional health of mothers and infants.Citation60
HCN
HCN channels have important physiological functions in the regulation of synaptic plasticity and membrane voltage regulation.Citation61 The inappropriate activity of HCN can lead to neurological and psychological disorders, including pain, epilepsy, addiction and anxiety.Citation62,Citation63 Studies also show that blocking HCN can promote the development of anxiety or depression behaviors.Citation64,Citation65 Therefore, HCN is beneficial for exploring potential drug targets for anxiety therapy. At present, four mammalian subtypes (HCN1–4) have been identified, which are all composed of tetramers.Citation66 Each subtype contains an N-terminal transmembrane region, a transmembrane core region, and a C-terminal intracellular region. The transmembrane core consists of six transmembrane helical domains (S1–S6), of which S1–S4 constitute the voltage sensor domain and connect to the pore domain (spiral S5 and S6).Citation67 The positively charged voltage sensor (S4) carries the glycine–tyrosine–glycine motif between S5 and S6, forming a filter with high selectivity for K+.Citation67 There is a cyclic nucleotide-binding domain (CNBD) at the C-terminal. CNBD is connected to the S6 transmembrane region through a C-linker of 80 amino acids, which is regulated by cyclic adenosine monophosphate.Citation68
The inappropriate activity of HCN can lead to neurological and psychological disorders, and the mainly emotional change is likely to be anxiety-like behavior. Cerebrovascular lesions could induce affective disorders, and the increase of HCN1 and decrease of potassium channel subunit 3 expression in amygdale may be factors to blame for anxiety-like symptom caused by cerebrovascular lesions.Citation65 Studies also showed that the change in synaptic ultrastructure might be caused by lower BDNF, while the high expression of HCN1 downregulated the synthesis of BDNF mRNA in the brain.Citation69 Blocking HCN could alleviate anxiety-like behavior of rats by improving learning ability, which might be associated with the overexpression of BDNF–mTOR signaling pathways and synaptic plasticity.Citation70 The anxiety signal caused by chronic pain was mediated by the pre-LTP of the anterior cingulate cortex (ACC).Citation71,Citation72 Inhibiting the signal transduction of HCN in ACC in vivo and reducing the synaptic expression of LTP can reduce the anxiety effect under chronic pain conditions.Citation72 Injecting a small amount of ZD7288 into ACC can not only prevent anxiety but also relieve pain.Citation73 Retigabine, an FDA-approved potassium-channel agonist for the treatment of epilepsy, has been found to reduce anxiety-like behavior by regulating the interaction of voltage-gated K+ channels and HCN.Citation74 The BLA plays a pivotal role in anxiety by the excitability of BLA neurons. The activation of BLA–ventral hippocampus synapses significantly induces anxiety-like behavior, while inhibition of BLA–ventral hippocampus synapses relieves anxiety-like behavior.Citation75 HCN1 is widely expressed in BLA neurons, and HCN knockdown enhances the excitability of BLA neurons by changing the input resistance and synaptic plasticity of BLA neurons.Citation76 Shao found that Tmem74 knockout in BLA pyramidal neurons led to anxiety-like behavior in mice.Citation68 Further data demonstrated that there was a direct interaction between Tmem74 and HCN1, and mice with Tmem74 knockout showed the same anxiety-like behavior with HCN1 knockout.Citation77
PSD95
PSD is a homogeneous dense substance on the inner cytoplasmic surface of the postsynaptic membrane that contains neurotransmitter receptors, cytoskeleton and signal molecules, and plays an important role in synaptic plasticity.Citation78,Citation79 PSD95 is a member of the membrane-associated guanosine kinase (MAGUK) family and the most abundant protein in PSD.Citation80 PSD95 contains three PDZ regions, an SH3 region. and a homologous guanosine acid region. The PDZ region is a molecule with 90-residue length, which binds the short C-terminal peptide chain motif of other molecules.Citation80 The PDZ region has two main ligands on its peptide chain: one is the serine or threonine residue of PDZ-2, and the other contains a hydrophobic residue at the same position. The PDZ region is an important structural domain of intracellular signal transduction.Citation80 On the dendritic spines of neurons, glutamate receptors and their coupled signal-transduction pathways form PSD95 through various skeletal proteins, which are independent units that receive presynaptic signals and perform biochemical processing.Citation81 The PSD-MAGUK protein family plays an important role in the synaptic localization of glutamate receptor and the receptor activity of downstream signal connection. PSD95 may play a physiological and pathological role in synaptic function and plasticity by affecting the dynamics of the glutamate receptor.Citation82,Citation83 Wang reported that NMDA receptors could bind to PSD95 and anchor in PSD, which further transmit signals of synapses.Citation84 Hence, PSD95 is a potential drug target for the treatment of neurological diseases, including anxiety disorder. Li reported that endocrine-disrupting chemicals induced anxiety-like behavior and could promote alterations in synaptic plasticity, including decrease in dendritic spine density and PSD thickness. Moreover, inhibition of NMDA receptors aggravates the effect of endocrine-disrupting chemicals on neuronal synapse-related molecules (PSD95 and synapsin 1).Citation85 α-amino-3-hydroxy-5-methyl-4-isoxazole-propionicacid receptor (AMPAR) mediates rapid excitatory synaptic transmission in the central nervous system, and its expression in the postsynaptic membrane is related to the induction and maintenance of LTP and LTD.Citation86 Chronic pain patients often have anxiety disorders, and some of them suffers from anxiety even after analgesic administration. Chen demonstrated that S-nitrosylation is necessary for AMPAR-mediated synaptic transmission in the ventromedial prefrontal cortex under chronic pain-induced anxiety disorder. However, administration of ZL006, a small-molecule inhibitor of PSD95, significantly reduced the expression of S-nitrosylation of AMPAR-interacting molecules in the ventromedial prefrontal cortex, inducing anxiety-like behavior in the mice after ibuprofen treatment.Citation87
Discussion
Neurons are connected to one another by synapses, forming a complex brain information-processing network.Citation88 With the deepening of scientific research, there have been significant advances in the development and maintenance of mechanisms, physiological functioning, and treatment of synaptic plasticity-related diseases. Related regulatory molecules lead to changes in the morphology of key brain regions (eg, hippocampus and amygdala) and further the individual’s emotional responses to environmental stimuli, and hence anxiety disorder. The findings from preclinical studies indicated that mGluRs, BDNF, HCN channels and PSD95 hold promise as diagnostic biomarkers of anxiety (). However, the exact regulatory mechanisms of synaptic plasticity-related molecules underlying the pathogenesis of anxiety disorders are not fully understood, and the identification of therapeutic targets has considerable limitations. In current clinical studies remains several key questions, such as insufficient sample sizes and varying patient recruitment. Additionally, synapses are sensitive parts in synaptic plasticity, and presynaptic and postsynaptic membrane accumulates many signal molecules that can lead to the regulation of synaptic plasticity. There is a need to better explore the effects of the aforementioned molecules in different regions, which may reveal the heterogeneous stratification of anxiety disorder.
Besides the abovementioned molecules, such as mGluR, BDNF, HCN, and PSD95, many other molecules may also affect anxiety disorder induced by abnormalities of synaptic transmission (). For example, overexpression of Cntn1, a subset of the neural cell adhesion protein, induced microglia activation in the hippocampus and triggered anxiety-like behavior.Citation89 The translocator protein plays a crucial role in neurosteroidogenesis and the pathophysiology of anxiety disorders, and its protein ligand promotes neurosteroidogenesism by regulating GABAergic neurotransmission, meaning translocator proteins could be as a therapeutic target for anxiety and neurological disorders.Citation90 Upregulated TNFR1 in prelimbic cortex induced the insertion of GluA1 receptor into BLA neurons, resulting in anxiety disorder in mice.Citation91 Interleukin 4 improves the loss of neurons and relieves anxiety-like behavior in a microglial PPARγ-dependent manner after traumatic brain injury.Citation92 Despite some new progress, there is still a paucity of related studies of specific mechanisms of anxiety disorder. In the nervous system, there are large differences between neurons in different locations and even between synapses of the same neuron, and many known and unknown structures and molecular mechanisms are involved, with further exploratory studies awaiting.
Table 1 Summary of the effects of synaptic plasticity-related proteins on anxiety disorder
Conclusion
Anxiety disorder is characterized by synaptic pathological changes mediated by synaptic plasticity-related molecules. mGluRs, BDNF, HCN channels, and PSD95 are pivotal for the development of the central nervous system and the key regulators of synaptic plasticity. Based on this, the regulation mechanisms of mGluRs, BDNF, HCN channels, and PSD95 in the pathogenesis of anxiety disorder were summarized in this review. These molecules, through complex molecular networks, induce changes in the morphology of key brain regions (eg, hippocampus, amygdala) involved in emotional regulation and cognitive processing, leading to changes in neuroplasticity and functioning in the brain and further affecting anxiety disorder. Synaptic plasticity studies based on protein regulation will lead to new ideas and directions for the prevention, treatment, and design of new drugs for clinical anxiety disorders.
Abbreviations
mGluRs, metabotropic glutamate receptors; BDNF, brain-derived neurotrophic factor; HCN, hyperpolarization-activated cyclic nucleotide–gated; PSD95, postsynaptic density; LTP, long-term potentiation; LTD, long-term depression; NMDAR, N-methyl-D-aspartic acid receptor; BLA, basolateral amygdala; MCPG, α-methyl-4-carboxyphenylglycine; CeA, central amygdala; MeA, medial amygdala; CNBD, cyclic nucleotide–binding domain; ACC, anterior cingulate cortex; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionicacid receptor.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by Shanghai Sailing Program (grant 20YF1446100); Shanghai Municipal Health Commission (grant shslczdzk04901); and Future program (WL-QNRC-2022002K).
References
- Craske MG, Rauch SL, Ursano R, Prenoveau J, Zinbarg RE. What is an anxiety disorder? Depress Anxiety. 2010;26(12):1066–1085. doi:10.1002/da.20633
- Proskurnina E, Liaukovich K, Bychkovskaya L, et al. Salivary antioxidant capacity and magnesium in generalized anxiety disorder. Metabolites. 2023;13(1):73. doi:10.3390/metabo13010073
- Piccirillo ML, Rodebaugh TL. Personalized networks of social anxiety disorder and depression and implications for treatment. J Affect Disord. 2022;298:262–276. doi:10.1016/j.jad.2021.10.034
- Zhu Y, Jha S, Shutta K, et al. Psychological distress and metabolomic markers: a systematic review of posttraumatic stress disorder, anxiety, and subclinical distress. Neurosci Biobehav Rev. 2022;143:104954. doi:10.1016/j.neubiorev.2022.104954
- Danielle G. Review of unnerved: anxiety, social change, and the transformation of modern mental health. Soc Forces. 2022;4:4.
- Dell’Orco M, Weisend J, Perrone-Bizzozero N, et al. Repetitive spreading depolarization induces the activation of cell differentiation, synaptic plasticity and neuroprotective pathways. bioRxiv. 2023. doi:10.1101/2023.02.27.530317
- Smirnova T, Laroche S, Errington M, Hicks A, Bliss T, Mallet J. Transsynaptic expression of a presynaptic glutamate receptor during hippocampal long-term potentiation. Science. 1993;262(5132):433–436. doi:10.1126/science.8105538
- Ito M. Long-term depression. Ann Rev Neurosci. 1989;12:85–102.
- Chen Q, Li X, Zhuo M. NMDA receptors and synaptic plasticity in the anterior cingulate cortex. Neuro Pharmacol. 2021;197:108749. doi:10.1016/j.neuropharm.2021.108749
- Lüscher C, Malenka R. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 2012;4(6):a005710. doi:10.1101/cshperspect.a005710
- Tomar A, McHugh T. The impact of stress on the hippocampal spatial code. Trends Neurosci. 2022;45(2):120–132. doi:10.1016/j.tins.2021.11.005
- Konakanchi S, Raavi V, Ml H, Shankar V. Effect of chronic sleep deprivation and sleep recovery on hippocampal CA3 neurons, spatial memory and anxiety-like behavior in rats. Neurobiol Learn Mem. 2022;187:107559. doi:10.1016/j.nlm.2021.107559
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50(1):295–322. doi:10.1146/annurev.pharmtox.011008.145533
- Chen L, Chetkovich D, Petralia R, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–943. doi:10.1038/35050030
- Zhang X, Shen C, Wang X, et al. Increased anxiety-like behaviors in Adgra1-/- Male but not female mice are attributable to elevated neuron dendrite density, upregulated PSD95 expression, and abnormal activation of the PI3K/AKT/GSK-3β and MEK/ERK pathways. Neuroscience. 2022;503:131–145. doi:10.1016/j.neuroscience.2022.09.003
- Duman R, Monteggia L. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi:10.1016/j.biopsych.2006.02.013
- Iamjan S, Thanoi S, Watiktinkorn P, et al. Changes of BDNF exon IV DNA methylation are associated with methamphetamine dependence. Epigenomics. 2021;13(12):953–965. doi:10.2217/epi-2020-0463
- Poon C, Heng B, Lim W. New insights on brain-derived neurotrophic factor epigenetics: from depression to memory extinction. Ann N Y Acad Sci. 2021;1484(1):9–31. doi:10.1111/nyas.14458
- Larsen M, Mikkelsen J, Hay-Schmidt A, et al. Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J Psychiatr Res. 2010;44(13):808–816. doi:10.1016/j.jpsychires.2010.01.005
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70(5):289–297. doi:10.1002/dneu.20758
- Jung S, Choe S, Woo H, et al. Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy. 2020;16(3):512–530. doi:10.1080/15548627.2019.1630222
- Zheng Z, Tu J, Li X, et al. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav Immun. 2021;91:505–518. doi:10.1016/j.bbi.2020.11.007
- Alinaghipour A, Salami M, Nabavizadeh F. Nanocurcumin substantially alleviates noise stress-induced anxiety-like behavior: the roles of tight junctions and NMDA receptors in the hippocampus. Behav Brain Res. 2022;432:113975. doi:10.1016/j.bbr.2022.113975
- Alkadhi K. NMDA receptor-independent LTP in mammalian nervous system. Prog Neurobiol. 2021;200:101986. doi:10.1016/j.pneurobio.2020.101986
- Privitera L, Hogg E, Lopes M, et al. The MK2 cascade mediates transient alteration in mGluR-LTD and spatial learning in a murine model of Alzheimer’s disease. Aging Cell. 2022;21(10):e13717. doi:10.1111/acel.13717
- Yadav P, Podia M, Kumari S, Mani I. Glutamate receptor endocytosis and signaling in neurological conditions. Prog Mol Biol Transl Sci. 2023;196:167–207.
- Collingridge G, Abraham W. Glutamate receptors and synaptic plasticity: the impact of Evans and Watkins. Neuropharmacology. 2022;206:108922. doi:10.1016/j.neuropharm.2021.108922
- Selig D, Lee H, Bear M, Malenka R. Reexamination of the effects of MCPG on hippocampal LTP, LTD, and depotentiation. J Neurophysiol. 1995;74(3):1075–1082. doi:10.1152/jn.1995.74.3.1075
- Luo L, Yang L, Zhang K, et al. Caveolin-1-mediated cholesterol accumulation contributes to exaggerated mGluR-dependent long-term depression and impaired cognition in Fmr1 knockout mice. Mol Neurobiol. 2023;60(6):3379–3395. doi:10.1007/s12035-023-03269-z
- Saha S, González-Maeso J. The crosstalk between 5-HTR and mGluR2 in schizophrenia. Neuropharmacology. 2023;230:109489. doi:10.1016/j.neuropharm.2023.109489
- Piszczek L, Constantinescu A, Kargl D, et al. Dissociation of impulsive traits by subthalamic metabotropic glutamate receptor 4. eLife. 2022;11:e62123.
- Khodagholi F, Maleki A, Motamedi F, Mousavi M, Rafiei S, Moslemi M. Oxytocin prevents the development of 3-NP-induced anxiety and depression in male and female rats: possible interaction of OXTR and mGluR2. Cell Mol Neurobiol. 2022;42(4):1105–1123. doi:10.1007/s10571-020-01003-0
- Singh DR, Pandey K, Mishra AK, Pandey P, Vivcharuk V. Glutamate binding triggers monomerization of unliganded mGluR2 dimers. Arch Biochem Biophys. 2021;697:697. doi:10.1016/j.abb.2020.108632
- Davis MJ, Haley T, Duvoisin RM, Raber J. Measures of anxiety, sensorimotor function, and memory in male and female mGluR4/ mice. Behav Brain Res. 2012;229(1):21–28. doi:10.1016/j.bbr.2011.12.037
- Duvoisin RM, Jeffrey BG, Haley TL, Gayet-Primo J. Effects of mGLUR compounds on the electroretinogram. Neuropharmacology. 2008;55:594.
- Duvoisin RM, Villasana L, Davis MJ, Winder DG, Raber J. Opposing roles of mGluR8 in measures of anxiety involving non-social and social challenges. Behav Brain Res. 2011;221(1):50–54. doi:10.1016/j.bbr.2011.02.049
- Keifer J. Regulation of AMPAR trafficking in synaptic plasticity by BDNF and the impact of neurodegenerative disease. J Neurosci Res. 2022;4:100.
- Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250.
- Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101. doi:10.1016/j.brainres.2014.10.019
- Annemarie D, Xu J, Louis-Tienne L, et al. Sexual dimorphism in a neuronal mechanism of spinal hyperexcitability across rodent and human models of pathological pain. Brain. 2022;3:3.
- Xu H, Qing H, Lu W, et al. Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 2002;321(1–2):65–68. doi:10.1016/S0304-3940(02)00034-4
- Chiba S, Numakawa T, Ninomiya M, Richards M, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):112–119. doi:10.1016/j.pnpbp.2012.05.018
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73(8):763–773. doi:10.1016/j.biopsych.2013.01.012
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13(1):22–37. doi:10.1038/nrn3138
- Zhu GX, Sun X, Yang Y. Reduction of BDNF results in GABAergic neuroplasticity dysfunction and contributes to late-life anxiety disorder. Behav Neurosci. 2019;133(2):212–224. doi:10.1037/bne0000301
- Casagrande BP, Ribeiro AM, Pisani LP, Estadella D. Hippocampal BDNF mediated anxiety-like behaviours induced by obesogenic diet withdrawal. Behav Brain Res. 2023;436:114077. doi:10.1016/j.bbr.2022.114077
- Sleigh JN, Villarroel-Campos D, Surana S, et al. Boosting peripheral BDNF rescues impaired in vivo axonal transport in CMT2D mice. JCI Insight. 2023;8(9). doi:10.1172/jci.insight.157191
- Heurteaux C, Lucas G, Guy N, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9(9):1134–1141. doi:10.1038/nn1749
- Li J, Wang X, Wang H, et al. The BDNF-TrkB signaling pathway in the rostral anterior cingulate cortex is involved in the development of pain aversion in rats with bone cancer via NR2B and ERK-CREB signaling. Brain Res Bull. 2022;185:185. doi:10.1016/j.brainresbull.2022.04.001
- Infantino R, Schiano C, Luongo L, et al. MED1/BDNF/TrkB pathway is involved in thalamic hemorrhage-induced pain and depression by regulating microglia. Neurobiol Dis. 2022;164:10561. doi:10.1016/j.nbd.2022.105611
- Song X, Liu B, Cui L, et al. Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol Behav. 2017;179:487–493. doi:10.1016/j.physbeh.2017.07.023
- Buttenschøn HN, Nielsen M, Glerup S, Mors O. Investigation of serum levels of sortilin in response to antidepressant treatment. Acta Neuropsychiatr. 2018;30(2):111–116. doi:10.1017/neu.2017.13
- Eggert S, Brigadski T, Kins S, Endres K. Brothers in arms: proBDNF/BDNF and sAPPα/Aβ-signaling and their common interplay with ADAM10, TrkB, p75NTR, sortilin, and sorLA in the progression of Alzheimer’s disease. Biol Chem. 2022;403(1):43–71. doi:10.1515/hsz-2021-0330
- Hashimoto K. BDNF variant linked to anxiety-related behaviors. BioEssays. 2007;29(2):116–119. doi:10.1002/bies.20534
- Rong J, Babyak MA, Brummett BH, Siegler IC, Kuhn CM, Williams RB. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism interacts with gender to influence cortisol responses to mental stress. Psychoneuroendocrinology. 2017;79:13–19. doi:10.1016/j.psyneuen.2017.02.005
- Loewenstern J, You X, Merchant J, et al. Interactive effect of 5-HTTLPR and BDNF polymorphisms on amygdala intrinsic functional connectivity and anxiety. Psychiatry Res Neuroimaging. 2019;285:1–8. doi:10.1016/j.pscychresns.2019.01.010
- Gujral S, Manuck SB, Ferrell RE, Flory JD, Erickson KI. The BDNF Val66Met polymorphism does not moderate the effect of self-reported physical activity on depressive symptoms in midlife. Psychiatry Res. 2014;218(1–2):93–97. doi:10.1016/j.psychres.2014.03.028
- Mansell T, Vuillermin P, Ponsonby AL, Collier F, Saffery R, Ryan J. Maternal mental well-being during pregnancy and glucocorticoid receptor gene promoter methylation in the neonate. Dev Psychopathol. 2016;28(4pt2):1421–1430. doi:10.1017/S0954579416000183
- Galbally M, Watson SJ, Ijzendoorn M, Saffery R, Ryan AJ. The role of glucocorticoid and mineralocorticoid receptor DNA methylation in antenatal depression and infant stress regulation. Psychoneuroendocrinology. 2020;115:104611. doi:10.1016/j.psyneuen.2020.104611
- Nazzari S, Grumi S, Mambretti F, et al. BDNFSex-dimorphic pathways in the associations between maternal trait anxiety, infant methylation, and negative emotionality. Dev Psychopathol. 2023:1–11. doi:10.1017/S0954579423000172
- Thomas M, Ranjith G, Radhakrishnan A, et al. Effects of HCN2 mutations on dendritic excitability and synaptic plasticity: a computational study. Neuroscience. 2019;2019:423.
- Mooney ER The Role of HCN Ion Channels in Pain [Doctoral dissertation]. University of Cambridge; 2014:22.
- Hou L, Guo Y, Bo L, et al. Synaptic ultrastructure might be involved in HCN1-related BDNF mRNA in withdrawal-anxiety after ethanol dependence. Front Psychiatry. 2018;9:215. doi:10.3389/fpsyt.2018.00215
- Woodman R, Student J, Miller C, Lockette W. Ivabradine-induced bradycardia is accompanied by reduced stress-related anxiety. Am J Hypertens. 2023;36(6):316–323. doi:10.1093/ajh/hpad019
- Zhou M, Li Y, Lin K, Luo P, Liu W. Chronic cerebral hypoperfusion-induced dysregulations of hyperpolarization-activated cyclic nucleotide-gated, KCNQ and G protein-coupled inwardly rectifying potassium channels correlated with susceptibility and unsusceptibility to anxiety behaviors. Curr Neurovasc Res. 2022. doi:10.2174/1567202620666221025152325
- Santoro B, Shah M. Hyperpolarization-activated cyclic nucleotide-gated channels as drug targets for neurological disorders. Annu Rev Pharmacol Toxicol. 2020;60:109–131. doi:10.1146/annurev-pharmtox-010919-023356
- He JT, Li XY, Zhao X, Liu X. Hyperpolarization-activated and cyclic nucleotide-gated channel molecules as emerging new targets in neuropathic pain. Rev Neurosci. 2019;30(6):639–649.
- Pfleger C, Kusch J, Kondapuram M, et al. Allosteric signaling in C-linker and cyclic nucleotide-binding domain of HCN2 channels. Biophys J. 2021;5:120.
- Hou L, Qi Y, Sun H, et al. Applying ketamine to alleviate the PTSD-like effects by regulating the HCN1-related BDNF. Prog Neuropsychopharmacol Biol Psychiatry. 2018;2018:S0278584618300198.
- Ni L, Xu Y, Dong S, et al. Correction: the potential role of the HCN1 ion channel and BDNF-mTOR signaling pathways and synaptic transmission in the alleviation of PTSD. Transl Psychiatry. 2020;10(1):105. doi:10.1038/s41398-020-0795-9
- Min Z. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells. 2007;23(3):259–271.
- Bie B, Brown DL, Naguib BM. Increased synaptic GluR1 subunits in the anterior cingulate cortex of rats with peripheral inflammation. Eur J Pharmacol. 2011;653(1–3):26–31.
- Moon HC, Park CA, Jeon YJ, et al. 7 tesla magnetic resonance imaging of caudal anterior cingulate and posterior cingulate cortex atrophy in patients with trigeminal neuralgia. Magn Reson Imaging. 2018;51:144–150. doi:10.1016/j.mri.2018.05.005
- Brackenbury WJ, Isom LL. Voltage-gated Na+ channels: potential for beta subunits as therapeutic targets. Expert Opin Ther Targets. 2008;12(9):1191–1203. doi:10.1517/14728222.12.9.1191
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Tye KM, Tye K. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79(4):658–664. doi:10.1016/j.neuron.2013.06.016
- Park K, Yi JH, Kim H, Choi K, Kang SJ, Shin KS. HCN channel activity-dependent modulation of inhibitory synaptic transmission in the rat basolateral amygdala. Biochem Biophys Res Commun. 2011;404(4):952–957. doi:10.1016/j.bbrc.2010.12.087
- Shao LX, Jiang Q, Liu XX, et al. Functional coupling of Tmem74 and HCN1 channels regulates anxiety-like behavior in BLA neurons. Mol Psychiatry. 2019;24(10):1461–1477. doi:10.1038/s41380-019-0402-8
- Gold MG. A frontier in the understanding of synaptic plasticity: solving the structure of the postsynaptic density. Bioessays. 2012;34(7):599–608. doi:10.1002/bies.201200009
- Sheng M. Synapse Loss, Synaptic Plasticity and the Postsynaptic Density. Berlin Heidelberg: Springer; 2008.
- Stachowicz K. Is PSD-95 entangled in the side effects of antidepressants? Neurochem Int. 2022;159:105391. doi:10.1016/j.neuint.2022.105391
- Zhang J, Xu TX, Hallett PJ, et al. PSD-95 uncouples dopamine-glutamate interaction in the D1/PSD-95/NMDA receptor complex. J Neurosci. 2009;29(9):2948–2960. doi:10.1523/JNEUROSCI.4424-08.2009
- Ma TM, Paul BD, Fu C, Hu S, Snyder SH. Serine racemase regulated by binding to stargazin and PSD-95: potential NMDA-AMPA glutamate neurotransmission cross-talk. J Biol Chem. 2014;289(43):29631–29641. doi:10.1074/jbc.M114.571604
- Karadayian AG, Bustamante J, Lores-Arnaiz S. Alcohol hangover induces nitric oxide metabolism changes by impairing NMDA receptor-PSD95-nNOS pathway. Nitric Oxide. 2021;113:113–114.
- Wang B, Wu Q, Lei L, et al. Long-term social isolation inhibits autophagy activation, induces postsynaptic dysfunctions and impairs spatial memory. Exp Neurol. 2018;311:213–224. doi:10.1016/j.expneurol.2018.09.009
- Li S, Xu W, Gong L, et al. Subchronic nonylphenol exposure induced anxiety-like behavior and decreased expressions of regulators of synaptic plasticity in rats. Chemosphere. 2021;282:130994. doi:10.1016/j.chemosphere.2021.130994
- Campelo T, Augusto E, Chenouard N, et al. AMPAR Trafficking Dependent LTP Initiates Cortical Remapping and Adaptive Behaviors During Sensory Experience. Cold Spring Harbor Laboratory; 2020.
- Chen ZJ, Su CW, Xiong S, et al. Enhanced AMPAR-dependent synaptic transmission by S-nitrosylation in the vmPFC contributes to chronic inflammatory pain-induced persistent anxiety in mice. Acta Pharmacol Sin. 2023;44(5):954–968.
- Magee JC, Grienberger C. Synaptic plasticity forms and functions. Annu Rev Neurosci. 2020;43:95–117. doi:10.1146/annurev-neuro-090919-022842
- Li S, Cao W, Zhou S, et al. Expression of Cntn1 is regulated by stress and associated with anxiety and depression phenotypes. Brain Behav Immun. 2021;95:142–153. doi:10.1016/j.bbi.2021.03.012
- Nothdurfter C, Baghai TC, Schüle C, Rupprecht R. Translocator protein (18 kDa) (TSPO) as a therapeutic target for anxiety and neurologic disorders. Eur Arch Psychiatry Clin Neurosci. 2012;262(S2):S107–S112. doi:10.1007/s00406-012-0352-5
- Gao F, Huang J, Huang GB, et al. Elevated prelimbic cortex-to-basolateral amygdala circuit activity mediates comorbid anxiety-like behaviors in chronic pain. J Clin Invest. 2023;133(9). doi:10.1172/JCI166356
- Pu H, Wang Y, Yang T, et al. Interleukin-4 mitigates anxiety-like behavior and loss of neurons and fiber tracts in limbic structures in a microglial PPARγ-dependent manner after traumatic brain injury. Neurobiol Dis. 2023;2023:10607.