Abstract
Objective
To investigate the serum soluble thrombomodulin (sTM) concentration in patients with sepsis-associated acute kidney injury (AKI) and to determine the value of sTM in predicting AKI and mortality in sepsis patients.
Methods
This prospective observational study was conducted on 71 patients diagnosed with sepsis according to Sepsis 3 at the Intensive Care Unit, Hue Central Hospital, Vietnam, from September 2021 to February 2023.
Results
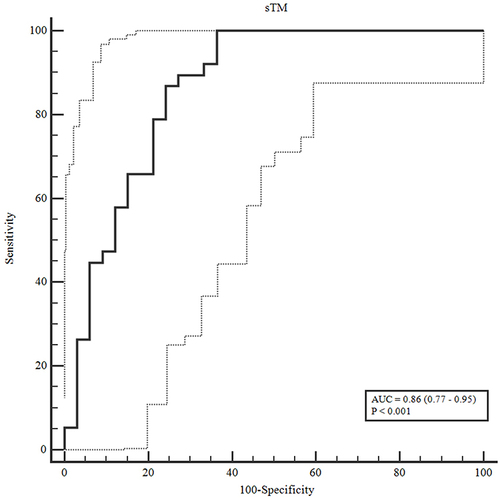
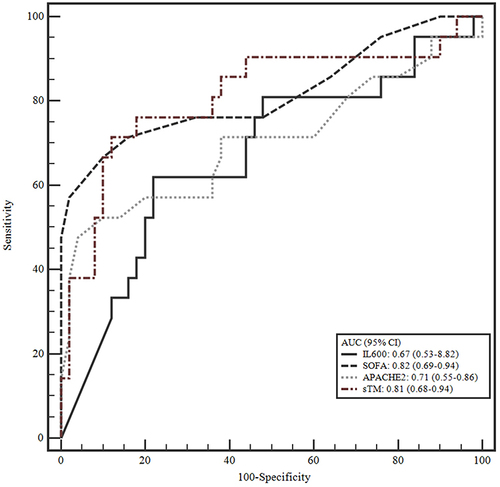
Among 71 sepsis patients, there were 38 (53.5%) AKI cases, including 16 (22.5%) cases of stage 1 AKI, 14 (19.7%) cases of stage 2 AKI, 8 (11.3%) cases of stage 3 AKI, 16 (22.5%) cases of renal replacement therapy, 28 (39.4%) cases of septic shock, and 21 (29.6%) cases of mortality within 28 days. The concentrations of lactate and IL-6 in the AKI and mortality groups were statistically significantly greater than those in the non-AKI and survival groups (p < 0.05). The serum sTM concentration was 4.33 ng/mL, the serum sTM level in the AKI group was statistically significantly higher than that in the non-AKI group (sTM [4.71 vs 2.54 ng/mL, p < 0.001]), and the serum sTM level in the mortality group was statistically significantly higher than the survival group (sTM [4.78 vs 3.87 ng/mL, p < 0.001]). The AUC of sTM for predicting AKI was 0.864; the AUCs of sTM, IL-6, SOFA, and APACHE II for predicting mortality were 0.811, 0.671, 0.816, and 0.705, respectively.
Conclusion
AKI was a prevalent complication among sepsis patients at the ICU. In the AKI and mortality groups, sTM concentration was statistically significantly higher than that in the non-AKI and survival groups. sTM was the predictor of acute kidney injury and mortality in patients with sepsis.
Keywords:
Introduction
The World Health Organization has identified sepsis as a top priority for global health. There have been significant efforts to figure out the pathogenesis of organ failure and death and to develop new treatment therapies for patients with sepsis.Citation1 Sepsis is a life-threatening organ failure induced by a dysregulated host response to infection.Citation2 It is estimated that sepsis affected approximately 48.9 million people worldwide in 2017, resulting in approximately 11 million deaths.Citation3 This rate doubled compared to 2016.Citation4 In the UK, about 250,000 sepsis cases and 44,000 sepsis-related deaths are estimated each year.Citation5 Acute kidney injury (AKI) is a prevalent complication of sepsis, contributing to increased morbidity and mortality associated with infection. The incidence of AKI in patients with sepsis was reported to be 51% in a multicenter study conducted in 24 European countries and 47.1% in China.Citation6,Citation7 Thrombomodulin (TM) is a type 1 transmembrane protein predominantly expressed on endothelial cells and plays a crucial role in various biological processes. TM circulates in various forms in biological fluids, such as blood and urine.Citation8,Citation9 TM activates protein C through binding to thrombin, which has anti-inflammatory, anticoagulant, and cytoprotective functions.Citation10,Citation11 TM levels are used as a biomarker for diagnosis, prognosis, and mortality in patients with sepsis.Citation12 It is capable of discriminating the severity of the disease and has a significant predictor of multi-organ dysfunction.Citation13 TM was significantly altered in sepsis-related AKI patients, in particular, and was an independent predictor of AKI superior to other prothrombotic and inflammatory biomarkers as well as organ function in sepsis patients.Citation14 TM was elevated in patients with AKI and in patients who died within 28 days of admission to the ICU.Citation15 Therefore, we conducted this study to determine the serum thrombomodulin concentration in patients with AKI associated with sepsis, the predictive value of AKI, and the mortality of serum sTM in patients with sepsis.
Materials and Methods
Study Population
A prospective observational study was conducted on the patients admitted to the ICU at Hue Central Hospital, Vietnam, from September 2021 to February 2023. The patients were diagnosed with sepsis according to the Sepsis32016criteria.Citation2
The patient stayed in the ICU for a minimum of 48 hours. Exclusion criteria were patients who did not consent to participate in the study, took anticoagulants, had chronic kidney disease, organ transplant, autoimmune diseases (lupus, psoriasis, rheumatoid arthritis, type I diabetes, scleroderma), cancer, and infectious diseases (HIV, Covid-19); patients who were under 18 years of age, pregnant or lactating.
Follow-up time in the study design was from patient admission to 28 days (4 weeks).
Patients were diagnosed with AKI according to KDIGO 2012.Citation16
Data Collection
Age, sex, weight, vital signs, and hourly urine output were all recorded since admission. Patients were classified according to their underlying medical history, site of infection, and vasopressors use. Glasgow, SOFA, and APACHE II scores were used to assess patient admission. We evaluated septic shock, mechanical ventilation, renal replacement therapy, length of ICU stay, and mortality within 28 days in all patients. Tests such as complete blood count, ALT, AST, total bilirubin, albumin, IL-6, procalcitonin, lactate, mineral panel, blood gas, baseline creatinine, and thrombomodulin were tested immediately upon admission. For the thrombomodulin test (Abcam brand), the blood sample was taken into a serum separator tube. After the formation of a clot, the blood sample was centrifuged at 2000 rpm for 10 minutes, then stored at −20 degrees Celsius or lower, avoiding repeating freezing and thawing for patient samples, which were tested by ELISA technique. We used Cobas® 8000 modular analyzer series - Roche Diagnostic and DxH 900 hematology analyzer - Beckman Coulter. The tests were conducted at the Department of Biochemistry and Hematology and Blood Transfusion Center of Hue Central Hospital, Vietnam.
Statistical Analysis
We processed the data using SPSS 22.0 statistical software. The study presented the results using descriptive statistics to summarize the data. Categorical data were presented as numbers and percentages, while continuous data were presented as mean and standard deviation (SD) if normally distributed, and median, minimum, and maximum if non-normally distributed. The normality of the continuous data was assessed using the Shapiro–Wilk test. The differences between categorical variables were evaluated using either the Chi-square test or Fisher’s Exact test, and the differences between continuous data were determined using either a t-test or Mann–Whitney test based on their distribution. The receiver operating characteristic (ROC) analysis was conducted to determine cut-off points for predicting AKI and death in patients, and the DeLong test was used to compare the areas under the ROC curves. Cox regression and the Log rank test were used to evaluate the predicting value of sTM for mortality, considering the effect of time and potential confounding factors in the study population. We considered AKI as a binary outcome (yes, no) and conducted multiple logistic regression models to adjust for other potential confounding factors. The results were considered statistically significant if the p-value was less than 0.05.
Results
The baseline characteristics of study population are shown in . The mean age of the study group was 61.86 ± 17.82 years old. There was no difference in age between the AKI and non-AKI groups. Comorbidities in our study group were mainly type 2 diabetes, hypertension, and coronary artery disease. The infection sources were mainly the respiratory, gastrointestinal, and urinary tracts.
Table 1 Characteristics of Study Population
Regarding clinical characteristics (), systolic blood pressure, diastolic blood pressure, mean blood pressure, CVP, and SpO2 had statistically significant differences between the two groups of AKI and non-AKI. Glasgow score, SOFA, and APACHE II at admission, rate of mechanical ventilation, rate of septic shock, rate of renal replacement therapy, and mortality within 28 days had statistically significant differences between the two groups of AKI and non-AKI.
Table 2 Clinical Characteristics
The laboratory findings are listed in . The platelet count in the AKI group was statistically significantly lower than in the non-AKI group. The difference in PCT concentration between the two groups, AKI and non-AKI, was not statistically significant. Total bilirubin, IL-6, lactate, creatinine, and sTM concentrations were significantly higher in the AKI group than in the non-AKI group.
Table 3 Laboratory Findings
shows the characteristics of survival and mortality within 28 days. AKI stages were significantly different between the survival and death groups. Factors such as age, white blood cells, albumin, and PCT were not statistically significant between the two groups of survival and death. The levels of creatinine, lactate, IL-6, sTM, SOFA score, and APACHE II score in the mortality group were statistically significantly higher than in the surviving group.
Table 4 Characteristics of Survival and Mortality Within 28 Days
The predictive value of sTM for the occurrence of acute kidney injury and mortality was analyzed. The AUC of sTM was 0.864, which has a relatively high accuracy for predicting AKI (). The markers demonstrated moderate to good accuracy in predicting patient mortality, with AUC values ranging from 0.671 to 0.816 ().
Figure 2 Receiver operating characteristic (ROC) curve of sTM, IL600, SOFA, and APACHE 2 in mortality prediction.
Odds ratio of sTM for AKI prediction after we adjusted for confounding factors by logistic regression model was 3.20 (95% CI: 1.33–7.70). The hazard ratio of sTM for mortality prediction after we adjusted for confounding factors using Cox regression model was 2.85 (95% CI: 1.22–6.62) (shown in ).
Table 5 Predictive Value of Serum Thrombomodulin for AKI and Mortality
Discussion
In our study, the majority of patients were elderly, mainly male patients. Age in the AKI group was older than in the non-AKI group, but this difference was not statistically significant. Comorbidities in our study were mainly hypertension, type 2 diabetes, and coronary artery diseases. These are common diseases among the elderly. Katayama’s study showed that the prevalence of comorbidities was: hypertension at 48.8%, ischemic heart disease (IHD) at 8.8%, congestive heart failure (CHF) at 8.8%, COPD at 5.1%, cerebrovascular accident (CVA) at 11.1%, diabetes at 25.7%, and immunodeficiency at 29.2%.Citation14 While the study by Inkinen et al showed that the comorbidities in the study population included hypertension, coronary artery disease or ASO, chronic heart failure, COPD, chronic kidney disease (GFR < 60 mL/min/1.73 m2), and diabetes, which accounted for 53.2%, 14.4%, 11.5%, 12.8%, 6.8%, and 25.5%, respectively.Citation17
The focus of infection in our study was largely the respiratory, digestive, and urinary systems. These are common infections in Southeast Asian countries. In the study of Inkinen et al, sepsis accounted for 2.9% of the central nervous system, 47.8% of the lungs, 25.2% of the gastrointestinal tract, 7.4% of the urinary tract, 8.9% of the skin and soft tissues, and 3.1% from other.Citation17
Septic shock is the most severe phase of a continuum that begins with a systemic inflammatory response to infection, severe sepsis, septic shock, and multiple organ failure. Shock comes on very quickly and is extremely dangerous. If diagnosed late and not promptly treated, it can be life-threatening. The rate of septic shock in our study was lower than that of Katayama et al, but higher than that of Vucelic et al. In the study of Katayama et al, the rate of septic shock in patients with sepsis was 45.7%, and there was a statistically significant difference between the AKI and non-AKI groups with p < 0.0001.Citation14 Research by Vucelic et al showed that the rate of patients with septic shock in patients with sepsis was 35.3%.Citation18
The AKI group had a higher mortality rate than the non-AKI group. In a study by Gameiro et al on 256 patients with AKI associated with sepsis, the 30-day mortality rate was 24.5%.Citation19 A meta-analysis of studies from 2002–2016 also found a mortality rate of patients with sepsis greater than 30%.Citation20 In the study of Kellum et al, with three groups of sepsis, no infection, and infection no sepsis, in the combined analysis of all groups, the 30-day mortality rate was 23% for patients with stage 2–3 AKI compared with 14% without stage 2–3 AKI within the first 3 days.Citation21
Regarding laboratory tests, in our study, the concentrations of lactate, IL-6, and platelets in the AKI group were different from those in the non-AKI group. This difference was statistically significant with p < 0.05. The same was true for the survival and mortality groups. Meanwhile, PCT concentration showed no difference between AKI and non-AKI groups as well as survival and death groups. The study by Inkinen et al showed that the IL-6 concentration in sepsis patients was 0.57 ng/mL, the IL-6 concentration in the AKI group was higher than the non-AKI group with p = 0.011, and the lactate level in the patients with sepsis was 1.8 mmol/L. The lactate level was higher in the mortality group than in the surviving group, with p < 0.001.Citation17 The study by Katayama et al showed that the platelets in sepsis patients were 144 x 109/L, and the AKI group had lower platelets than the non-AKI group, with p < 0.0001.Citation14 Research by Liu et al showed that the concentrations of lactate (mmol/L) and IL-6 (pg/mL) in patients with sepsis and septic shock were 2.5 (1.9–4.1) and 313.7 (121.3–2565.3), respectively, the difference between the two groups of survival and death was statistically significant with p < 0.05, while the concentrations of procalcitonin (ng/mL) and the mean amount of platelets (x 109/L) were 28.5 (6.3–97.1) and 169.7 (108.0), respectively, the difference between the two groups of survival and death was not statistically significant with p > 0.05.Citation22
In our study, the rate of AKI was 53.5%, of which AKI stage 1, stage 2, and stage 3 accounted for 22.5%, 19.7%, and 11.3%, respectively. The rate of renal replacement therapy was 22.5%. The AKI group had a significantly higher rate of renal replacement therapy than the non-AKI group. In a study by Katayama et al on 514 sepsis patients, the AKI rate was 68.3%.Citation14 According to Jiang’s study on 3107 patients admitted to the ICU, the rate of AKI was 51.0%, in which AKI stage 1, stage 2, and stage 3 were 23.1%, 11.8%, and 15.7%, respectively; the rate of renal replacement therapy was 9%.Citation23 In another study on 619 sepsis patients, the rate of AKI was 51.1%, of which AKI stage 1 accounted for 19.7%, AKI stage 2 accounted for 8.7%, and AKI stage 3 accounted for 22.6%. The rate of renal replacement therapy was 16.3%.Citation17
In this study, soluble thrombomodulin (sTM) concentration in patients with sepsis was 4.33 ng/mL. sTM concentration in the AKI group was statistically significantly higher than that in the non-AKI group with p < 0.001, and the AKI predictive serum sTM curve was 0.864 (p < 0.001).
The study by Bouchard et al showed that in comparison to serum and urine neutrophil gelatinase-associated lipocalin (NGAL), cystatin C, urine renal injury molecule-1, and liver-fatty acid-binding protein, the AUC of sTM concentration to predict AKI was 0.77 (95% CI 0.62–0.89).Citation24 Another study showed that sTM was an independent predictor of AKI with an AUC of 0.758 (p < 0.0001).Citation14 The study by Inkinen et al showed that, after adjusting for confounding factors, sTM was linked to the occurrence of AKI 12 hours after admission to the ICU.Citation17 In a study of critically ill patients admitted to the ICU, Zhou et al showed that the AUC for the combination of urine sTREM-1, serum sTREM-1, and serum sTM together provided a better clinical reference value for predicting the occurrence of AKI at 0.879 and the mortality rate at 0.84. The optimal critical value of sTM in diagnosing the development of AKI in the AKI and non-AKI group was 9.18 ng/mL; that value in predicting mortality in AKI patients was 11.98 ng/mL.Citation15 Research by Rodrigues et al showed that the group of patients with a SOFA score greater than or equal to 12 points had a statistically significantly higher level of sTM than the group with a SOFA score less than 12 points.Citation25
According to this study, the levels of sTM, IL-6, SOFA score, and APACHE II score in the group that died within 28 days were statistically significantly higher than those in the survival group (p < 0.05). Using ROC analysis, our study showed that the AUC of sTM, IL-6, SOFA score, and APACHE II score in predicting mortality was 0.811, 0.671, 0.816, and 0.705, respectively. As we know, dysregulated inflammatory responses that damage endothelial cells play an important role in the pathogenesis of sepsis, which is the cause of increased sTM in the blood. The plasma sTM concentration is significantly elevated in patients with sepsis.Citation26 sTM is used for diagnosis, prognosis, and mortality in patients with sepsis.Citation8 Many studies have shown a positive correlation between sTM concentration and the severity of sepsis in both adults and children.Citation13,Citation27,Citation28 It has been shown that sTM is better at predicting serious complications such as MODS (Multiple Organ Dysfunction Syndrome), surpassing accepted risk and prognosis assessment methods such as SOFA and APACHE II.Citation13 In addition, patients with sepsis who died had higher plasma sTM concentrations than the non-fatal group.Citation28,Citation29
Conclusion
Acute kidney injury is a common complication of sepsis, contributing to increased mortality. Early detection and prompt treatment lead to better long-term outcomes. Several biomarkers will help detect acute kidney injury early before serum creatinine rises. In our study, the serum sTM level in AKI patients associated with sepsis was significantly higher than in non-AKI patients, and the serum sTM level was elevated in the mortality group of patients with sepsis compared with the surviving group. Serum sTM concentration is a very good predictor of AKI and mortality in patients with sepsis.
Ethics Approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by The Ethics Committee of Hue Central Hospital. All patients involved in the present study provided written informed consent.
Disclosure
The authors declare no conflicts of interest regarding the contents of this article.
References
- Cavaillon JM, Singer M, Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med. 2020;12(4):e10128. doi:10.15252/emmm.201810128
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
- Fleischmann C, Thomas–Rueddel DO, Hartmann M, et al. Hospital incidence and mortality rates of sepsis: an analysis of hospital episode (DRG) statistics in Germany from 2007 to 2013. Deutsches Ärzteblatt Int. 2016;113(10):159. doi:10.3238/arztebl.2016.0159
- Teggert A, Datta H, Ali Z. Biomarkers for point-of-care diagnosis of sepsis. Micromachines. 2020;11(3):286. doi:10.3390/mi11030286
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi:10.1097/01.CCM.0000194725.48928.3A
- X. X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10(9):1510–1518. doi:10.2215/CJN.02140215
- Boron M, Hauzer-Martin T, Keil J, Sun X-L. Circulating thrombomodulin: release mechanisms, measurements, and levels in diseases and medical procedures. TH Open. 2022;6(03):e194–e212. doi:10.1055/a-1801-2055
- Califano F, Giovanniello T, Pantone P, et al. Clinical importance of thrombomodulin serum levels. Eur Rev Med Pharmacol Sci. 2000;4(3):59–66.
- Khan KA, McMurray JL, Mohammed F, Bicknell R. C‐type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019;286(17):3299–3332. doi:10.1111/febs.14985
- Watanabe-Kusunoki K, Nakazawa D, Ishizu A, Atsumi T. Thrombomodulin as a physiological modulator of intravascular injury. Front Immunol. 2020;11:575890. doi:10.3389/fimmu.2020.575890
- Khattab AA, Dawood AAER, Saleh NY. Value of thrombomodulin as a marker for sepsis in critically ill children. Indian J Pediatr. 2021;88:864–871. doi:10.1007/s12098-020-03564-w
- Mihajlovic DM, Lendak DF, Draskovic BG, et al. Thrombomodulin is a strong predictor of multiorgan dysfunction syndrome in patients with sepsis. Clin Appl Thromb Hemost. 2015;21(5):469–474. doi:10.1177/1076029613508600
- Katayama S, Nunomiya S, Koyama K, et al. Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit Care. 2017;21(1):1–9. doi:10.1186/s13054-017-1815-x
- Zhou J, Wang L, Guo J, et al. Correlation between sTREM-1 and serum sTM in patients with AKI and the predictive value of their joint evalution in AKI occurrence and patients’ death. Int J Clin Exp Med. 2020;13(4):2798–2806.
- Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
- Inkinen N, Pettilä V, Lakkisto P, et al. Association of endothelial and glycocalyx injury biomarkers with fluid administration, development of acute kidney injury, and 90-day mortality: data from the FINNAKI observational study. Ann Intensive Care. 2019;9(1):1–11. doi:10.1186/s13613-019-0575-y
- Vucelić V, Klobučar I, Đuras Cuculić B, et al. Sepsis and septic shock–an observational study of the incidence, management, and mortality predictors in a medical intensive care unit. Croat Med J. 2020;61(5):429–439. doi:10.3325/cmj.2020.61.429
- Gameiro J, Carreiro C, Fonseca JA, et al. Acute kidney disease and long-term outcomes in critically ill acute kidney injury patients with sepsis: a cohort analysis. Clin Kidney J. 2021;14(5):1379–1387. doi:10.1093/ckj/sfaa130
- Luhr R, Cao Y, Soederquist B, Cajander S. Trends in sepsis mortality over time in randomised sepsis trials: a systematic literature review and meta-analysis of mortality in the control arm, 2002–2016. Crit Care. 2019;23(1):1–9. doi:10.1186/s13054-019-2528-0
- Kellum JA, Artigas A, Gunnerson KJ, et al. Use of biomarkers to identify acute kidney injury to help detect sepsis in patients with infection. Crit Care Med. 2021;49(4):e360. doi:10.1097/CCM.0000000000004845
- Liu J, Bai C, B. L, et al. Mortality prediction using a novel combination of biomarkers in the first day of sepsis in intensive care units. Sci Rep. 2021;11(1):1275. doi:10.1038/s41598-020-79843-5
- Jiang L, Zhu Y, Luo X, et al. Epidemiology of acute kidney injury in intensive care units in Beijing: the multi-center BAKIT study. BMC Nephrol. 2019;20(1):1–10. doi:10.1186/s12882-019-1660-z
- Bouchard J, Malhotra R, Shah S, et al. Levels of protein C and soluble thrombomodulin in critically ill patients with acute kidney injury: a multicenter prospective observational study. PLoS One. 2015;10(3):e0120770. doi:10.1371/journal.pone.0120770
- Rodrigues AT, Rodrigues JT, Rodrigues CT, et al. Association between thrombomodulin and high mobility group box 1 in sepsis patients. World J Crit Care Med. 2020;9(4):63–73. doi:10.5492/wjccm.v9.i4.63
- Nozaki Y, Ri J, Sakai K, Niki K, Funauchi M, Matsumura I. Protective effects of recombinant human soluble thrombomodulin on lipopolysaccharide-induced acute kidney injury. Int J Mol Sci. 2020;21(7):2519. doi:10.3390/ijms21072519
- Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi:10.1016/S2352-3026(20)30216-7
- Lin JJ, Hsiao HJ, Chan OW, Wang Y, Hsia S-H, Chiu C-H. Increased serum thrombomodulin level is associated with disease severity and mortality in pediatric sepsis. PLoS One. 2017;12(8):e0182324. doi:10.1371/journal.pone.0182324
- Iba T, Yagi Y, Kidokoro A, Fukunaga M, Fukunaga T. Increased plasma levels of soluble thrombomodulin in patients with sepsis and organ failure. Surg Today. 1995;25(7):585–590. doi:10.1007/BF00311430