Abstract
Background
Recurrent metastasis after radical resection in patients of colorectal cancer (CRC) is a great challenge for the world, in which genomic alterations play a major role in tumorigenesis. MUC4 plays a significant role in recurrence and metastasis in tumor. This study is aimed at exploring the association between MUC4 variants and metastatic recurrence of CRC.
Methods
Forty-seven patients relapsing with metastasis and 37 patients remaining disease‐free postoperatively were enrolled. Next-generation sequencing (NGS) detected mutations. Mutation and mRNA expression data were downloaded from TCGA and cBioPortal databases. We analyzed the relationship between MUC4 variants and clinical parameters, as well as possible molecular mechanisms.
Results
MUC4 variants rs56359992 and rs781124621 were associated with survival in patients with CRC. Rs56359992 was more common in patients with metastatic recurrence. MAPK pathway, PI3K-Akt pathway, JAK-STAT pathway, cell cycle, WNT pathway and mTOR pathway were found to correlate with MUC4 mutation by GO/KEGG analysis, as well as resting and activated mast cell related to MUC4 mutation by CIBERSORT analysis.
Conclusion
Genetic variants of MUC4 with CRC may constitute a molecular signature of metastatic recurrence. MUC4 may become a new target for the treatment of CRC recurrence.
Introduction
Colorectal cancer (CRC) is a common malignant tumor that seriously threatens human life. Colorectal cancer is the third most common malignancy in cancer according to existing studies.Citation1 In 2020, CRC accounted for 10% of global cancer incidence and 9.4% of cancer deaths, just less than breast cancer and lung cancer. Based on population aging, population growth and human development, new CRC cases worldwide are expected to reach 3,200,000 in 2040.Citation2 According to data evaluation, in the next 20 years, the number of new CRC patients in China will increase from 560,000 in 2020 to 910,000 in 2040.Citation3
Mucins are high molecular weight glycoproteins responsible for protecting, repairing, and surviving epithelial tissue in the gut.Citation4,Citation5 Mucins have a surface protective effect on epithelial cells by trapping pathogens and are also involved in cell signaling pathways.Citation6,Citation7 Several mucins have been found to be involved in the tumorigenesis of various solid tumors. In addition, limited studies are published about MUCs in CRC.Citation8
MUC4 ordinarily lines the goblet cells and epithelial cells of the normal human small and large intestine.Citation5 MUC4 has an effect on tumor proliferation, recurrence and metastasis. MUC4 promoted aggressive activity in triple negative breast cancer cells by altering the expression of EGFR, ErbB2, and ErbB3 molecules and their downstream signaling.Citation9 In two pancreatic cancer cell lines, knocking down MUC4 downregulated lysosomal degradation of E-cadherin, resulting in downregulation of Wnt/β-catenin signaling pathway. The above process eventually led to the proliferation and metastasis of the tumor.Citation10 In stage I non-small cell lung cancer, MUC4 mutation was related to early recurrence.Citation11 Conflicting results associated with MUC4’s role in colon tumor have been reported.Citation12 It was reported that overexpression of MUC4 in tissues obtained from colon cancer was related to a poorer prognosis.Citation12 However, others reported a loss of MUC4 in tissue samples from patients diagnosed with CRC.Citation13
Recurrent metastasis after radical resection in CRC patients is a great challenge.Citation14 About 60–80% of recurrences of CRC occurred 2 years after radical resection and 90% in the first 4 years.Citation15 Metastasis is the main driver of CRC-related mortality, with liver, lung and peritoneal metastasis being the most commonly affected.Citation16–18 CRC with peritoneal metastasis had a poor prognosis and was generally considered a terminal illness.Citation19 A comparison of isolated site metastases among peritoneal, liver and lung metastasis reported median overall survival (OS):16.3 months in peritoneal metastasis, 19.1 months in liver metastasis, and 24.6 months in lung metastasis.Citation20
Although many studies have shown abnormal expression of MUC4 in CRC,Citation21 the relationship between genetic variants of MUC4 and metastatic recurrence in CRC is not clear. We analyzed genetic mutations of MUC4 in Chinese based on next-generation sequencing (NGS). Our study assessed the association of MUC4 variants with metastatic recurrence in CRC.
Materials and Methods
Study Population
This study included patients diagnosed with CRC who underwent radical surgery at Cangzhou Central Hospital from January 2018 to March 2021. A total of 84 patients with CRC stage I–III were enrolled: 47 patients in case group subsequently relapsed with metastasis; 37 age‐matched patients in control group remained disease‐free postoperatively. Clinicopathological data and follow-up data were collected from the hospital’s case database and follow-up office respectively. We obtained information on registered patients including age, gender, time to diagnosis, TNM stage, nodules, lymphovascular invasion, perineural invasion, tumour localization, and their follow-up data on recurrence, metastasis, and survival. The study’s inclusion criteria included: (1) Patients were not less than 18 years old; (2) Patients were pathologically diagnosed with CRC; (3) Patients had radical surgery. Patients would be excluded if they: (1) had metastasis; (2) comorbid other malignancies; (3) received neoadjuvant chemotherapy or targeted therapy or radiation therapy; (4) had no follow-up. This retrospective study was approved by the ethics committee of the Chinese PLA General Hospital and Cangzhou Central Hospital (S2022-305-01) and conformed to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Genomic Data Analysis
DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) of tumor tissue specimens for NGS. We used noncancer Genome Aggregation Database (gnomAD) as representative of normal population controls. Genetic alterations were subsequently analyzed according to standard principles. Clinical and genetic data were downloaded from cBioPortal for Cancer Genomics (https://www.cbioportal.org).
mRNA Expression Profile Analysis
Corresponding mRNA expressions were downloaded from TCGA database. The expression data were divided into two groups including MUC4 mutation group and MUC4 wild type (MUC4 WT) group based on mutation data. R package was used for GO, KEGG, GSEA, ESTIMATE and CIBERSORT analysis and visualization.
Statistical Analysis
Data analyses were performed by using R software version 4.2.2 for Windows and SPSS 23.0. The Kaplan–Meier method and Log rank test were applied to determine survival function. Chi-squared test or Fisher’s exact test was used to evaluate statistical heterogeneity. Differences between two groups were determined by Student’s t test when data were normally distributed. The p-value obtained was considered statistically significant at a threshold of p <0.05.
Results
Comparison of Baseline Characteristics
We consulted NCBI database and selected three genetic variants of MUC4 with mutation frequency ≥10% in our study, including rs56359992, rs1560322790, and rs781124621. We assessed baseline characteristics of 84 patients (). Colon cancer, N stage (N1-N2), lymphovascular invasion and rs56359992 were more in the case group than control group, with P=0.013, P<0.001, P=0.039, P=0.019 respectively (). Other demographic and clinicopathological characteristics were not statistically significant in the comparison between the two groups.
Table 1 Baseline Characteristics of Case and Control Group (n,%)
Relationship Between MUC4 Mutations and CRC Metastasis Recurrence
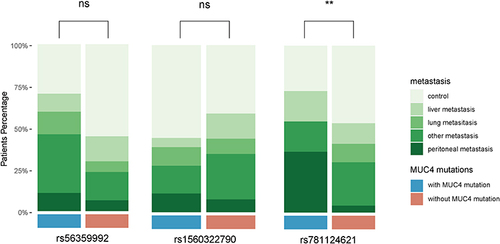
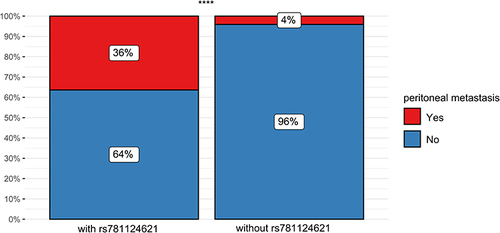
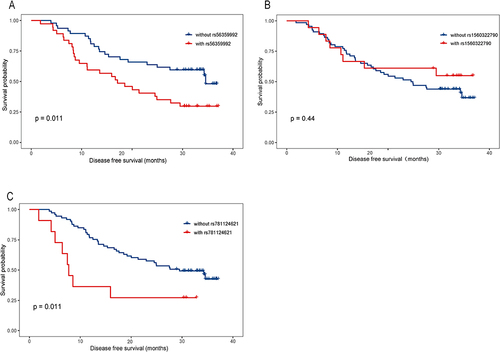
Kaplan–Meier analysis was used for survival analyses (). We found potential relations between genetic variants of MUC4 and patient survival. Variants rs56359992 () and rs781124621 () were related to prognosis. Variants rs1560322790 () were not related to prognosis. We further analyzed the association of genetic mutations with specific metastasis of CRC, including liver, lung, peritoneal and other organs. 70.3% of rs56359992, 44.4% of rs1560322790 and 72.7% of rs781124621 were metastasis (). The analysis showed that different metastases were distributed differently in rs781124621, while no statistical significance in rs56359992 and rs1560322790 (). We analyzed that patients with rs781124621 were more likely to have recurrent peritoneal metastasis ().
Figure 1 Correlation between MUC4 variants and prognosis in CRC patients.(A) Kaplan–Meier DFS curves for CRC patients with rs56359992 and without rs56359992. (B) Kaplan–Meier DFS curves for CRC patients with rs1560322790 and without rs1560322790. (C) Kaplan–Meier DFS curves for CRC patients with rs781124621 and without rs781124621.
Functional Enrichment Analysis
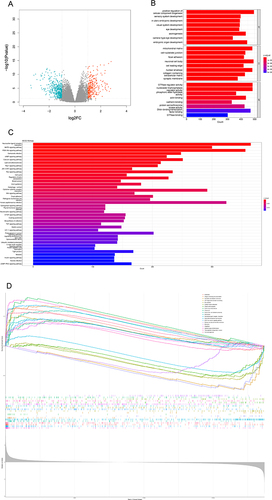
We downloaded data from public databases to further analyze possible biological processes involved in MUC4 mutation in CRC. We divided data into MUC4-mutated and MUC4-WT groups and performed GO, KEGG and GESA analysis (). The volcano map showed that 268 of differentially expressed genes (DEGs) were upregulated and 451 were downregulated (). Enrichment analysis showed that DEGs were mainly involved in some biological functions () like cell−substrate junction, focal adhesion, and signaling pathways () such as MAPK pathway, PI3K-Akt pathway, JAK-STAT pathway, cell cycle, WNT pathway and mTOR pathway. GSEA analysis also found immune activity like natural killer cell-mediated cytotoxicity ().
Immunological Correlation of MUC4 Mutation
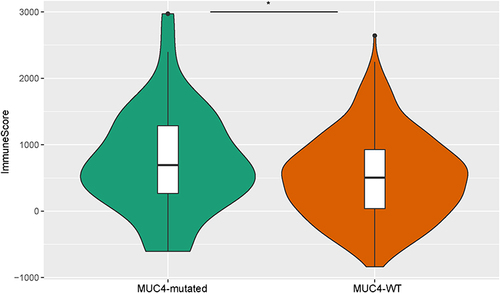
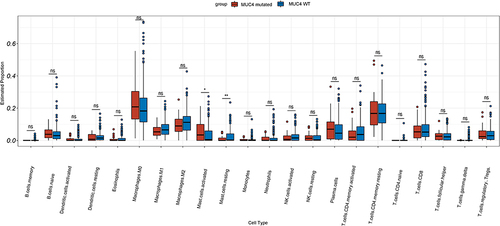
MUC4 mutations were found to be associated with immune-related biological activity in our study. To obtain more specific results, we performed ESTIMATE and CIBERSORT analyses. The immunescore of the MUC4 mutated group was significantly higher than that of the MUC4 WT group (). Analysis of infiltrated immune cells of two groups showed that activated mast cell was higher in MUC4-mutated group, while mast cell resting was higher in MUC4-WT group ().
Discussion
CRC is a worldwide problem, and surgery is still the first-line treatment for early-stage CRC.Citation22 Despite the improved initial cure rates for CRC, 20%–30% of stage I–III patients still experienced recurrences, including liver metastasis, lung metastasis, peritoneal metastasis and other types of recurrence.Citation23 Metastatic recurrence of tumors involved a series of complex processes. For example, epithelial–mesenchymal transition (EMT) can promote tumor recurrence and metastasis.Citation24 Early detection and treatment of disease recurrence were proved beneficial.Citation23 Patients were detected metastases by regular surveillance to improve prognosis,Citation25 but the results were not satisfactory. Therefore, scholars tried to find suitable predictors for CRC recurrence and prognosis.Citation26–28 For this purpose, we investigated the relationship between CRC recurrence and MUC4 mutations.
First, we compared the baseline characteristics of patients and found that people with metastatic recurrences had more colon cancer, N stage (N1-N2) and lymphovascular invasion. Researchers found that anatomical location of primary tumor and N stage were related to tumor recurrence.Citation29,Citation30 Lymphovascular invasion was also detected to be associated with a high recurrence rate.Citation25 Therefore, recurrence of CRC was associated with clinicopathological features, such as tumor localization, N stage and lymphovascular invasion.
In addition, we analyzed the relationship between MUC4 mutations and CRC prognosis and found that MUC4 variants rs56359992 and rs781124621 were associated with CRC prognosis. Meanwhile, MUC4 mutation rs56359992 was found associated with CRC metastatic recurrence.
Scholars looked for molecular markers to predict CRC recurrence and mucins had great exploration value. Mucins contain a large amount of clustered oligosaccharides O-glycosidically.Citation31 Mucins play a role in cell adhesion, metastasis, and signal transduction.Citation32 In the early stages of metastasis, mucins reduced cell adhesion, and changed matrix interaction, which gave cancer cells access to cell motility and invasion.Citation33 MUC4 was found to be involved in both tumor metastasis and progression.Citation34 Loss of MUC4 expression in gastric adenocarcinoma was significantly related to recurrence.Citation35 Previous studies showed that MUC4 was related to proliferation, invasion and distant metastasis of pancreatic cancer cells.Citation36 Mutations were found to be associated with recurrent metastasis of CRC.Citation37 MUC4 genetic variants were associated with mortality and susceptibility to CRC.Citation38 These findings showed that MUC4 mutations were closely related to CRC metastasis and prognosis and could be novel biomarkers for the prediction of CRC patients’ prognosis.
Moreover, we further analyzed the relationship between MUC4 variants and CRC metastasis, and found that CRC patients with rs781124621 were more likely to have peritoneal metastasis. Recurrent peritoneal metastasis in CRC patients was tricky. The first three sites of recurrent metastasis after radical CRC surgery were liver, lung and peritoneum.Citation25 The median overall survival of patients with peritoneal metastasis was found to be not optimistic and related to mucus histology.Citation17,Citation39 People were still not very clear about the genetic changes and mutations in peritoneal metastasis. Researchers found that mutations in KRAS and BRAF were associated with overall survival in peritoneal metastasis patients.Citation40 Peritoneal metastases were presumed to begin with the shedding of single cells or tumor masses by transmitting tumor cells through the adhesion molecule E-cadherin.Citation41 Downregulation of E-cadherin was also involved in the EMT process, which could promote tumor metastasis.Citation42 At the same time, high levels of E-cadherin were required for adhesion connections.Citation43 MUC4 loss in pancreatic cancer cells resulted in downregulation of lysosomal degradation of E-cadherin.Citation10 MUC4 may promote peritoneal metastasis of CRC via E-cadherin. Thus, MUC4 mutation may play an important role in peritoneal metastasis in CRC patients.
Furthermore, we analyzed the biological activity associated with MUC4 mutations in CRC through GO, KEGG and GSEA analyses. We obtained some biological functions like cell−substrate junction and focal adhesion through GO analysis, and MAPK and PI3K−Akt signaling pathways through KEGG analysis, and natural killer cell-mediated cytotoxicity through GSEA analysis. Focal adhesion played a significant role in tumor cell survival, proliferation, differentiation and invasion.Citation44 MUC4 deletion led to a decrease in focal adhesion kinase which promoted tumor progression.Citation45,Citation46 MAPK and PI3K−Akt signaling pathways highly depended on calcium ion to activate key component proteins.Citation47 Regulating E-cadherin and β-Chain protein signaling promoted the progression of CRC through MAPK pathway.Citation48 Autophagy activated through PI3K/Akt/mTOR pathway could suppress cancer cell migration and invasion.Citation49 Enhancing the EMT process through PI3K/AKT/mTOR pathway can promote migration and invasion of CRC cells.Citation50 MUC4 was demonstrated to regulate the MAPK signaling pathway.Citation51 These results suggested MUC4 may be involved in CRC recurrent metastasis by regulating MAPK pathway and PI3K-AKT pathway, but more experiments were needed to prove these conjectures.
Finally, we analyzed the relationship between MUC4 mutations and immune activity, and found immune score of MUC4 mutation group was higher than that of MUC4 WT group, and found that activated mast cells appeared higher in MUC4 mutation group. In our study, MUC4 mutations were also involved in inflammatory pathways in KEGG analysis, such as JAK-STAT signaling pathway. Meanwhile GSEA also found natural killer cell-mediated cytotoxicity. MUC4 protects disseminated tumor cells from immune recognition by masking various immunogenic cell surface antigens through its large glycosylated extracellular domain.Citation45 The physical interaction between MUC4 and platelets and immune cells enhanced the survival and extravasation of disseminated tumor cells.Citation52 In CRC, mast cell was correlated with angiogenesis, migration and prognosis.Citation53 The relationship between MUC4 and mast cells was not clear. MUC4 and mast cells may influence CRC through interactions, which needed further verification. Our research suggested that MUC4 mutations were related to immune activity in CRC.
Several limitations in this study may affect the accuracy of experiment and further research is needed. Firstly, the DNA required for NGS was extracted from FFPE, resulting in more pseudo mutations than fresh or frozen tissue. Secondly, not enough patients were recruited to the point which may occur inaccurate results. Thirdly, no matching other omics data accompanied the NGS data for in-depth analysis. Finally, the potential targets found in this study required further biological validation.
Conclusion
Understanding molecular characteristics of metastatic recurrence of CRC was crucial to evolve future therapeutic strategies. NGS was performed on FFPE from CRC patients to show that MUC4 may play a key role in CRC metastatic recurrence especially peritoneal metastasis, and maybe a potential therapeutic target. This study investigated the molecular mechanism of CRC metastatic recurrence and provided some new insights for further genomic research.
Ethics Approval and Informed Consent
This retrospective study was approved by the ethics committee of the Chinese PLA General Hospital and Cangzhou Central Hospital (S2022-305-01) and conformed to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Confidentiality and anonymity were assured as no personal identifiers were used, and the requirement for informed consent was therefore waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors wish to thank Lai Song for help of data analysis and the Beijing MyGenostics Co. Ltd. for technical support.
References
- Yue X, Pengfei X. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. doi:10.1016/j.tranon.2021.101174
- Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi:10.1038/s41575-019-0189-8
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765(2):189–222. doi:10.1016/j.bbcan.2006.01.002
- Zeljkovic A, Vekic J, Mihajlovic M, et al. Revealing the Role of High-Density Lipoprotein in Colorectal Cancer. Int J Mol Sci. 2021;22(7):3352. doi:10.3390/ijms22073352
- Kaur S, Kumar S, Momi N, et al. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10(10):607–620. doi:10.1038/nrgastro.2013.120
- Pai P, Rachagani S, Dhawan P, et al. MUC4 is negatively regulated through the Wnt/beta-catenin pathway via the Notch effector Hath1 in colorectal cancer. Genes Cancer. 2016;7(5–6):154–168. doi:10.18632/genesandcancer.108
- Almasmoum H. The Roles of Transmembrane Mucins Located on Chromosome 7q22.1 in Colorectal Cancer. Cancer Manag Res. 2021;13:3271–3280. doi:10.2147/CMAR.S299089
- Mukhopadhyay P, Lakshmanan I, Ponnusamy MP, et al. MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS One. 2013;8(2):e54455. doi:10.1371/journal.pone.0054455
- Zhi X, Tao J, Xie K, et al. MUC4-induced nuclear translocation of beta-catenin: a novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014;346(1):104–113. doi:10.1016/j.canlet.2013.12.021
- Hu C, Shu L, Chen C, et al. A prediction model integrated genomic alterations and immune signatures of tumor immune microenvironment for early recurrence of stage I NSCLC after curative resection. Translational Lung Cancer Res. 2022;11(1):24–42. doi:10.21037/tlcr-21-751
- Shanmugam C, Jhala NC, Katkoori VR, et al. Prognostic value of mucin 4 expression in colorectal adenocarcinomas. Cancer. 2010;116(15):3577–3586. doi:10.1002/cncr.25095
- Biemer-Hüttmann AE, Walsh MD, McGuckin MA, et al. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47(8):1039–1048. doi:10.1177/002215549904700808
- Kahlenberg MS, Sullivan JM, Witmer DD, et al. Molecular prognostics in colorectal cancer. Surg Oncol. 2003;12(3):173–186. doi:10.1016/S0960-7404(03)00006-9
- Ergun Y, Bal O, Dogan M, et al. Does primary tumor resection contribute to overall survival in unresectable synchronous metastatic colorectal cancer? J Res Med Sci. 2020;25:14. doi:10.4103/jrms.JRMS_1056_18
- Chandra R, Karalis JD, Liu C, et al. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers. 2021;13(24):6206. doi:10.3390/cancers13246206
- Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. doi:10.1038/srep29765
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
- Ceelen W, Ramsay RG, Narasimhan V, et al. Targeting the Tumor Microenvironment in Colorectal Peritoneal Metastases. Trends Cancer. 2020;6(3):236–246. doi:10.1016/j.trecan.2019.12.008
- Franko J. Therapeutic efficacy of systemic therapy for colorectal peritoneal carcinomatosis: surgeon’s perspective. Pleura peritoneum. 2018;3(1):20180102. doi:10.1515/pp-2018-0102
- Lu S, Catalano C, Huhn S, et al. Single nucleotide polymorphisms within MUC4 are associated with colorectal cancer survival. PLoS One. 2019;14(5):e0216666. doi:10.1371/journal.pone.0216666
- Shinji S, Yamada T, Matsuda A, et al. Recent Advances in the Treatment of Colorectal Cancer: a Review. J Nippon Med School. 2022;89(3):246–254. doi:10.1272/jnms.JNMS.2022_89-310
- van der Stok EP, Spaander MCW, Grünhagen DJ, et al. Surveillance after curative treatment for colorectal cancer. Nat Rev Clin Oncol. 2017;14(5):297–315. doi:10.1038/nrclinonc.2016.199
- Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016–1026. doi:10.1084/jem.20181827
- Miyake H, Kawai K, Nozawa H, et al. Less intensive surveillance after radical surgery for stage I-III colorectal cancer by focusing on the doubling time of recurrence. Surg Today. 2021;51(4):550–560. doi:10.1007/s00595-020-02135-y
- Mo S, Ye L, Wang D, et al. Early Detection of Molecular Residual Disease and Risk Stratification for Stage I to III Colorectal Cancer via Circulating Tumor DNA Methylation. JAMA oncol. 2023;9(6):770–778. doi:10.1001/jamaoncol.2023.0425
- Chen G, Peng J, Xiao Q, et al. Postoperative circulating tumor DNA as markers of recurrence risk in stages II to III colorectal cancer. J Hematol Oncol. 2021;14(1):80. doi:10.1186/s13045-021-01089-z
- Turgunov Y, Ogizbayeva A, Shakeyev K, et al. The dynamics of the lipopolysaccharide-binding protein (LBP) level in assessing the risk of adverse outcomes in operated colorectal cancer patients. Asian J Surg;2023. S1015-9584(23)01268–X. doi:10.1016/j.asjsur.2023.08.101
- Qaderi SM, Galjart B, Verhoef C, et al. Disease recurrence after colorectal cancer surgery in the modern era: a population-based study. Int J Colorectal Dis. 2021;36(11):2399–2410. doi:10.1007/s00384-021-03914-w
- Yamano T, Yamauchi S, Tsukamoto K, et al. Evaluation of appropriate follow-up after curative surgery for patients with colorectal cancer using time to recurrence and survival after recurrence: a retrospective multicenter study. Oncotarget. 2018;9(39):25474–25490. doi:10.18632/oncotarget.25312
- Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi:10.1023/A:1025815113599
- Mizejewski GJ. The adenocarcinoma cell surface mucin receptor for alpha-fetoprotein: is the same receptor present on circulating monocytes and macrophages? A commentary. Tumour Biol. 2014;35(8):7397–7402. doi:10.1007/s13277-014-2183-7
- Ganguly K, Rauth S, Marimuthu S, et al. Unraveling mucin domains in cancer and metastasis: when protectors become predators. Cancer Metastasis Rev. 2020;39(3):647–659. doi:10.1007/s10555-020-09896-5
- Dhanisha SS, Guruvayoorappan C. Pathological Implications of Mucin Signaling in Metastasis. Curr Cancer Drug Targets. 2023;23(8):585–602. doi:10.2174/1568009623666230320121332
- Hwang I, Kang YN, Kim JY, et al. Prognostic significance of membrane-associated mucins 1 and 4 in gastric adenocarcinoma. Exp Ther Med. 2012;4(2):311–316. doi:10.3892/etm.2012.598
- Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5(4):309–320. doi:10.1158/1541-7786.MCR-06-0353
- Lan YT, Chang SC, Lin PC, et al. Clinicopathological and Molecular Features of Patients with Early and Late Recurrence after Curative Surgery for Colorectal Cancer. Cancers. 2021;13(8). doi:10.3390/cancers13081883
- Kwon MJ, Lee JY, Kim EJ, et al. Genetic variants of MUC4 are associated with susceptibility to and mortality of colorectal cancer and exhibit synergistic effects with LDL-C levels. PLoS One. 2023;18(6):e0287768. doi:10.1371/journal.pone.0287768
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. doi:10.1002/(SICI)1097-0142(20000115)88:2<358::AID-CNCR16>3.0.CO;2-O
- Schneider MA, Eden J, Pache B, et al. Mutations of RAS/RAF Proto-oncogenes Impair Survival After Cytoreductive Surgery and HIPEC for Peritoneal Metastasis of Colorectal Origin. Ann Surg. 2018;268(5):845–853. doi:10.1097/SLA.0000000000002899
- Roth L, Russo L, Ulugoel S, et al. Peritoneal Metastasis: current Status and Treatment Options. Cancers. 2021;14(1):60. doi:10.3390/cancers14010060
- Loh CY, Chai JY, Tang TF, et al. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: signaling, Therapeutic Implications, and Challenges. Cells. 2019;8(10):1118. doi:10.3390/cells8101118
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, et al. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163(4):847–857. doi:10.1083/jcb.200308162
- Duperret EK, Ridky TW. Focal adhesion complex proteins in epidermis and squamous cell carcinoma. Cell Cycle. 2013;12(20):3272–3285. doi:10.4161/cc.26385
- Chaturvedi P, Singh AP, Chakraborty S, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68(7):2065–2070. doi:10.1158/0008-5472.CAN-07-6041
- Zhang P, Cao X, Guan M, et al. CPNE8 Promotes Gastric Cancer Metastasis by Modulating Focal Adhesion Pathway and Tumor Microenvironment. Int J Biol Sci. 2022;18(13):4932–4949. doi:10.7150/ijbs.76425
- Liu L, Wu N, Wang Y, et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K / AKT oncogenic signaling. J Exp Clin Cancer Res. 2019;38(1):106. doi:10.1186/s13046-019-1061-y
- Shen L, Zhang J, Xu M, et al. DDX3 acts as a tumor suppressor in colorectal cancer as loss of DDX3 in advanced cancer promotes tumor progression by activating the MAPK pathway. Int J Biol Sci. 2022;18(10):3918–3933. doi:10.7150/ijbs.73491
- Zhang M, Liu S, Chua MS, et al. SOCS5 inhibition induces autophagy to impair metastasis in hepatocellular carcinoma cells via the PI3K/Akt/mTOR pathway. Cell Death Dis. 2019;10(8):612. doi:10.1038/s41419-019-1856-y
- Liao H, Zhang L, Lu S, et al. viaKIFC3 Promotes Proliferation, Migration, and Invasion in Colorectal Cancer PI3K/AKT/mTOR Signaling Pathway. Front Genet. 2022;13:848926. doi:10.3389/fgene.2022.848926
- Jonckheere N, Skrypek N, Merlin J, et al. The mucin MUC4 and its membrane partner ErbB2 regulate biological properties of human CAPAN-2 pancreatic cancer cells via different signalling pathways. PLoS One. 2012;7(2):e32232. doi:10.1371/journal.pone.0032232
- Rowson-Hodel AR, Wald JH, Hatakeyama J, et al. Membrane Mucin Muc4 promotes blood cell association with tumor cells and mediates efficient metastasis in a mouse model of breast cancer. Oncogene. 2018;37(2):197–207. doi:10.1038/onc.2017.327
- Liu X, Li X, Wei H, et al. Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front Immunol. 2023;14:1209056. doi:10.3389/fimmu.2023.1209056