Abstract
Background
This investigation evaluated the prognostic significance of the neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), and introduced a combined NLR-PLR score to evaluate the correlation between NLR-PLR score and hepatocellular carcinoma (HCC) recurrence.
Material/Methods
We enrolled 110 patients who underwent orthotopic liver transplantation (LT) for HCC. The neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) were assessed, and appropriate cut-off values were established. The NLR-PLR score ranged from 0 to 2 as follows: score of 2, high NLR (≥3.37) and high PLR (≥105.96); score of 1, either high NLR or high PLR; score of 0, neither high NLR nor high PLR.
Results
The median overall survival (OS) of patients with NLR-PLR score of 0, 1 and 2 was 27, 26.5, and 6 months, respectively. The median OS of patients with NLR-PLR score of 2 was shorter than those with 0 (P < 0.001) and 1 (P < 0.001). The median disease-free survival (DFS) time of patients with NLR-PLR score of 0, 1 and 2 was 24.5, 24, and 6 months, The median DFS of patients with NLR-PLR score of 2 was shorter than those with 0 (P = 0.001) and 1 (P = 0.015). Multivariate analysis showed that NLR-PLR score was an independent risk factor for prognosis and survival.
Conclusion
NLR, PLR and NLR-PLR score can predict the long-term survival of patients, and NLR-PLR score, having more predictive value than NLR and PLR alone is an independent risk factor for patient survival. more predictive value than NLR and PLR alone.
Background
HCC accounts for 85% to 90% of primary liver cancer, its the sixth most common cancer and the third leading cause of cancer-related deaths worldwide.Citation1 The treatment of HCC mainly includes radical resection, liver transplantation, radiofrequency ablation, transcatheter arterial chemoembolization (TACE), radiotherapy, and systemic anti-tumor therapy. Studies have shown that in unresectable HCC patients who have not previously received systemic therapy, compared with the standard regimen of sorafenib, PD-L1 immune checkpoint inhibitor Attilizumab (Tecentech) combined with the antiangiogenic drug bevacizumab (Avitine) immunotherapy (“ T+A ”regimen) improved OS and progression-free survival (PFS).Citation2–5 In China, many patients with hepatitis B viral (HBV) infection and often combined liver cirrhosis.Citation6 Liver transplantation is the best treatment plan since it can remove not only the tumor but also the hardening liver. Despite the current use of Milan criteria (MC) and the University of California, San Francisco criteria (UCSF) for patient selection, tumor recurrence is still a major factor limiting the long-term survival of patients,Citation7 therefore it is urgent to establish other simple and effective selection criteria. At present, studies have pointed out that inflammation is closely related to tumor, and inflammation indicators have been applied to predict the long-term survival of patients after liver transplantation. In this paper, in order to establish a more reasonable and effective prediction method for patients with HCC who need liver transplantation, we calculated NLR and PLR by inflammatory indicators. We combined them to obtain the NLR-PLR score, which was applied to select receptors. There were previous studies on the correlation between inflammatory factors and the prognosis of hepatocellular carcinoma,Citation8 but they relied on markers that are challenging to obtain and often required invasive examination procedures like pathological biopsy. However, the NLR-PLR score discussed in this study is easy to obtain, non-invasive and highly accepted by patients. In addition, As far as we know, although there have been previous studies on the application of NLR or PLR in liver transplantation for HCC, there is no research on the combination of NLR and PLR to predict the prognosis of liver transplantation patients with HCC.
Material and Methods
General Information
A total of 110 patients who underwent LT due to HCC in the Third Affiliated Hospital of Hebei Medical University from July 2015 to October 2021 were collected. Inclusion criteria: 1. Classic liver transplantation; 2. HCC confirmed by postoperative pathology; 3. The clinical data were complete. Exclusion criteria: 1. The operation was other liver transplantation methods, such as living-donor liver transplantation (LDLT) and split liver transplantation (SLT). 2. Postoperative pathology showed other types of liver malignant tumor, such as intrahepatic cholangiocarcinoma. 3. Clinical data were incomplete. This study has been reviewed by the Ethics Committee of the Third Hospital of Hebei Medical University (W2022-008-1). All organs were donated voluntarily by brain-dead patients. All patients signed informed consent forms. All the donated organs were donated voluntarily with written informed consent from the Third Hospital of Hebei Medical University. This was conducted in accordance with the Declaration of Istanbul. This study was conducted in accordance with the Declaration of Istanbul and Helsinki and the samples obtained were approved by the ethics committee of the Third Hospital of Hebei Medical University (W2022-008-1). The written informed consent was obtained from each patient and their family for clinical data and publication.
Data Collection
Collect clinical and follow-up data of the patients, including blood routine, biochemical test results, postoperative tumor pathological data, alpha-fetoprotein (AFP), MLED score, Child-Pugh grade, OS, and DFS. According to the results of the last preoperative blood routine, calculate NLR and PLR. The NLR was determined as the neutrophil count divided by the lymphocyte count, while the PLR was determined as the platelet count divided by the lymphocyte count.
Follow-Up
Including telephone and outpatient follow-up. The end of follow-up was May 1, 2022. OS was defined as the period after liver transplantation surgery until death, or loss of follow-up, and DFS was defined as the period after liver transplantation until the confirmed recurrence, death, or loss of follow-up.
Statistical Analysis
SPSS25.0 software was used for statistical analysis. The receiver operating characteristic (ROC) curve of NLR and PLR was constructed, and the cut-off value of NLR and PLR were determined according to the ROC curve. According to the cut-off value, the patients were divided into low NLR group (NLR<3.37), low PLR group (<105.96), high NLR group (NLR≥3.37) and high PLR group (≥ 105.96). The NLR-PLR score was calculated according to the NLR and PLR values. OS and DFS were calculated using Kaplan–Meier method with the Log rank test. Multivariate analysis was performed using Cox proportional hazards regression model. All variables selected on univariate analysis (P < 0.05) were included in the multivariate analysis, P <0.05 was considered statistically significant.
Result
Baseline Clinical and Pathological Characteristics
Among the 110 patients, 99 (90%) were male and 11 (10%) were female. There were 90 (81.8%) of Child-Pugh A/B and 20 (18.2%) of Child-Pugh C; There were 52 (47.3%) comply the UCSF criteria, and 58 (52.7%) did not comply the UCSF criteria. There were 42 (38.2%) with Microvascular invasion (MVI) and 68 (61.8%) without MVI. AFP < 400ug/L in 83 cases (75.5%), AFP≥400ug/L in 27 cases (24.5%); The age ranged from 28 to 67 years, with a median age of 54 years. MELD scores range from 6 to 38, with a median MELD score of 12 ().
Table 1 Clinical Characteristics of Patients
NLR and PLR Cut-off Values
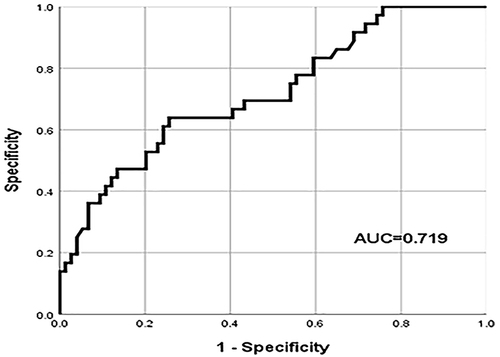
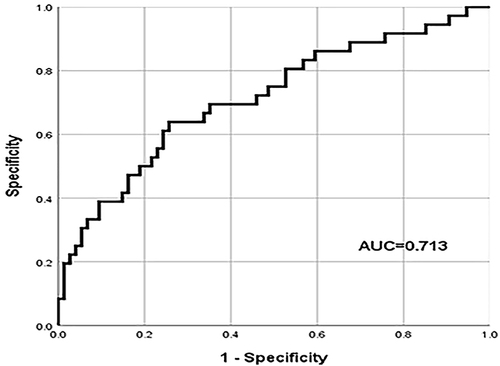
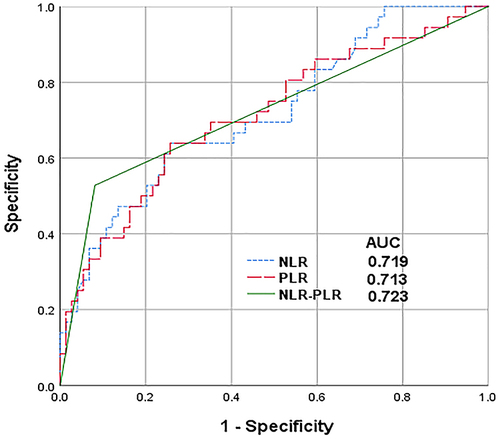
The results of the latest preoperative blood routine were analyzed to calculated NLR and PLR, and drawn the ROC curve. According to the ROC curve calculated Youden index, and the calculation method was sensitivity + specificity −1. The NLR and PLR values corresponding to the maximum Youden index were the cut-off values. The cut-off value of NLR was 3.37 (AUROC curve: 0.719; 95% CI: 0.617–0.822; P < 0.001) () and the cut-off value of PLR was 105.96 (AUROC curve: 0.713; 95% CI: 0.607–0.819; P < 0.001) (). Patients were divided into high NLR group (NLR≥3.37) and low NLR group (NLR < 3.37), high PLR group (PLR≥105.96) and low PLR group (PLR < 105.96) according to the cut-off value.
Effect of NLR, PLR and NLR-PLR Score on Survival of HCC Patients After LT
Survival analysis of NLR < 3.37 and NLR≥3.37 groups was performed by K-M curve. The results showed that the median OS with high NLR was significantly lower compared to patients with low NLR (14 months VS 27 months, P< 0.001;). The median DFS with high NLR was significantly lower compared to patients with low NLR (8 months VS 23.5 months, P= 0.009;).
Survival analysis of PLR < 105.96 and PLR≥105.96 groups was performed by K-M curve. The results showed that the median OS of low PLR group was 23 months, while that of high PLR group was 14.5 months, and the difference was statistically significant (P< 0.001). The median DFS was 22 months in the low PLR group and 9 months in the high PLR group, and the difference was not statistically significant (P= 0.057).
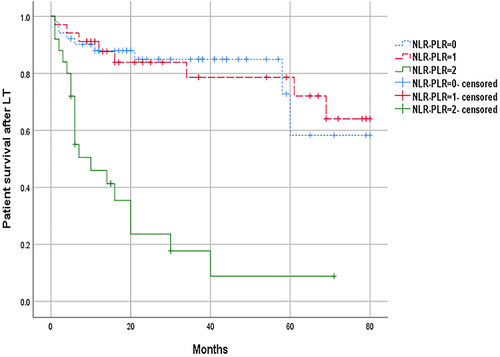
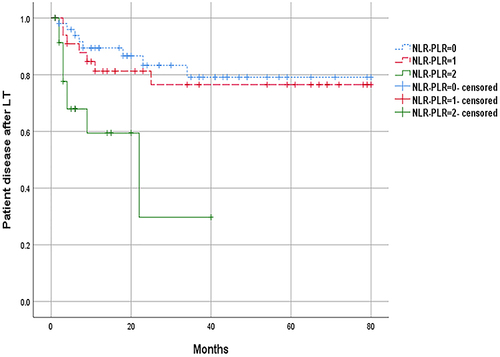
The NLR-PLR score ranged from 0 to 2 as follows: score of 2, high NLR (≥3.37) and high PLR (≥ 105.96); score of 1, either high NLR or high PLR; score of 0, neither high NLR nor high PLR. The results showed that the median OS of patients with NLR-PLR score of 0 was 27 months, those with 1 was 26.5 months, and those with 2 was 6 months (). The median OS of patients with NLR-PLR score of 2 was significantly shorter than those with 0 (6 months VS 27 months P<0.001) and 1 (6 months VS 26.5 months P<0.001), and the difference was statistically significant. However, there was no significant difference between 0 group and 1 group (27 months VS 26.5 months P=0.953). The median DFS of the NLR-PLR 0 group was 24.5 months, the NLR-PLR 1 group was 24 months, and the NLR-PLR 2 group was 6 months (). The median DFS of the NLR-PLR 2 group was significantly shorter than those of the 0 (6 months VS 24.5 months P=0.001) and 1 group (6 months VS 24 months P=0.015). The difference was statistically significant, but there was no significant difference between 0 group and 1 group (P=0.628) ().
Table 2 Comparison of Median DFS and OS After Transplantation in Patients with a NLR-PLR Score of 0 or 1 or 2
Comparison of the Predictive Value of NLR, PLR and NLR-PLR Score in HCC Patients After LT
The areas under the ROC curve (AUC) was used to compare the predictive value of NLR, PLR and NLR-PLR score for HCC patients after LT. The results showed that (): The AUCs for NLR, PLR and NLR-PLR were 0.719, 0.713 and 0.723 (P< 0.001). Compared with NLR and PLR alone, the AUCs for NLR-PLR was the largest, which proved that NLR-PLR had better predictive value.
Univariate and Multivariate Analysis of HCC Patients After LT
Gender, age, Child-Pugh score, MELD score, AFP, NLR, PLR, NLR-PLR score, UCSF criteria, MVI were included in the multivariate univariate analysis. Through univariate analysis, it was found that Child-Pugh grade, MELD score, NLR, PLR, NLR-PLR score, UCSF criteria, MVI were correlated with HCC after LT postoperative survival. The indicators associated with HCC after LT postoperative survival in these univariate analyses were included in the multivariate analysis. Further multivariate analysis showed that NLR-PLR score, UCSF criteria, MELD score were independent risk factors affecting the prognosis of patients ().
Table 3 COX Regression Analysis
Discussion
Liver transplantation (LT) is considered to be the best choice for HCC, especially for the patients with HBV infection and liver cirrhosis. LT effectively removes both the tumor and the underlying liver disease. However, organ shortages present a significant challenge that cannot be ignored. Therefore, accurately predicting the prognosis of HCC patients after LT and making reasonable selections of transplant recipients are critical issues that deserve our attention. In previous studies, Milan and UCSF criteria were applied. However, their stringent nature often resulted in many patients losing the opportunity for treatment. For a long time, researchers have been trying to find more accurate prognostic indicators to ensure the most rational utilization of hard-won organs.
At present, many studies have found that inflammation is closely related to the occurrence and progression of tumors. Neutrophils, platelets, and lymphocytes are all involved in the process of inflammation and immunity in the body, previous papers have proved that NLR and PLR calculated on the basis of the three are associated with the prognosis of a variety of malignant tumors.Citation9–12
NLR is a systemic indicator of inflammation, which is calculated by the ratio of neutrophil count to lymphocyte count. Neutrophils can enhance the biological behavior of tumors, promoting tumor growth and metastasis, and the decrease of the absolute or relative value of lymphocytes will reduce the anti-tumor effect of the body. A number of studies have proved the role of NLR in the prognosis of liver transplantation for HCC. Harimoto et alCitation13 compared 213 HCC LDLT patients with and without tumor recurrence and found that des-γ-carboxy-prothrombin (DCP)≥300 mAU/mL and NLR≥2.66 were independent predictors of DFS. Wang et alCitation14 also obtained the same result through a study on 101 liver transplant recipients from deceased donors. In our study, the cut-off value was determined as 3.37 by drawing the ROC curve. According to the cut-off value, the patients were divided into the high NLR group and the low NLR group for survival analysis. The results showed that the median OS and DFS of the low NLR group were significantly prolonged. The results were statistically significant, so it is believed that the NLR level has a certain predictive value for HCC after LT. The patients with increased NLR have a poor prognosis, which is consistent with the results of previous studies.
PLR is the ratio of platelet count to lymphocyte count. Platelet is also involved in the process of tumor metastasis, and the specific mechanism may be as follows: (1) Platelets are proposed to facilitate epithelial-mesenchymal transition (EMT), a complex series of molecular changes where neoplastic epithelial cells lose apico-basal polarity, overcome anoikis and assume a migratory, invasive phenotype. On the other hand, upregulation of MMPs that facilitate degradation of the basement membrane and underlying extracellular matrix aid tumor cell movement through the tissue.Citation15 (2) Platelets release various cytokines, such as platelet-derived growth factor and transforming growth factor (TGF) -β, and transport them to specific sites to participate in angiogenesis, tissue repair and other processes.Citation16 Ismael et alCitation17 found PLR>150 was associated with poor OS and recurrence-free survival (RFS), suggesting that PLR was an independent predictor of long-term survival in liver transplantation recipients with HCC. In our study, after grouping the patients according to the ROC curve, it was found that the OS of patients in the low PLR group was significantly higher than that in the high PLR group, and the results were statistically significant, which proved the role of PLR in predicting the outcome of liver transplantation patients, namely, PLR level was negatively correlated with survival after LT.
There have been previous studies on the impact of NLR and PLR on the prognosis of liver transplantation patients with liver cancer, but the combination of the two indexes was not applied. In this study, we established a combined scoring system based on the cutoff value determined by the ROC curve and survival analysis results. The results showed that the OS and DFS of patients with NLR-PLR 2 score were significantly lower than those with 0 and 1 score, but there was no significant difference between those with 0 and 1 scores. The results indicate that patients with preoperative NLR-PLR score of 2 have the worst prognosis, which is an effective predictor after liver transplantation. Further compared the AUC of NLR, PLR and NLR-PLR by ROC curve, and the AUC of NLR-PLR was the largest, which proved that the combined scoring system was more accurate than the previous single index. Traditional liver transplant recipient selection criteria such as Milan criteria and UCSF criteria are only related to tumor size, but this study combined biological indicators to achieve more accurate results. However, there are also some problems, for example, the cut-off value of NLR and PLR in this study are only obtained from the data of our center, and the different cut-off value of different centers lead to different grouping situations. In addition, studies have been conducted to predict the prognosis of melanoma patients with anti-PD-1 drugs by observing the bioenergetics of peripheral blood mononuclear cells (PBMC).Citation18 This study inspires us, and the next research direction can further start from the level of cell metabolism to elaborate the relationship between NRL-PLR and the prognosis of liver transplantation patients with hepatocellular carcinoma. Additionally, this study is retrospective, and further multicenter prospective studies are required to validate the efficacy of this scoring system.
Conclusion
In conclusion, NLR-PLR score is an effective predictor of hepatocellular carcinoma after liver transplantation and can be used as another reference criterion for selecting liver transplantation recipients in addition to UCSF criteria.
Disclosure
The authors report no conflicts of interest in this work.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Santoni M, Rizzo A, Mollica V, et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596. doi:10.1016/j.critrevonc.2022.103596
- Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol. 2020;16(32):2587–2589. doi:10.2217/fon-2020-0669
- Rizzo A, Ricci AD, Brandi G. Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy. Future Oncol. 2021;17(7):755–757. doi:10.2217/fon-2020-0986
- Rizzo A, Ricci AD, Brandi G. Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy. 2021;13(8):637–644. doi:10.2217/imt-2021-0026
- Ren A, Li Z, Zhang X, et al. Inflammation-based prognostic scores in patients with hepatitis B virus-related hepatocellular carcinoma after liver transplantation. J Hepatocell Carcinoma. 2020;7:101–106. doi:10.2147/jhc.S259992
- Citores MJ, Lucena JL, de la Fuente S, et al. Serum biomarkers and risk of hepatocellular carcinoma recurrence after liver transplantation. World J Hepatol. 2019;11(1):50–64. doi:10.4254/wjh.v11.i1.50
- Bian S, Ni W, Zhu M, et al. Flap endonuclease 1 facilitated hepatocellular carcinoma progression by enhancing USP7/MDM2-mediated P53 inactivation. Int J Biol Sci. 2022;18(3):1022–1038. doi:10.7150/ijbs.68179
- Najjar M, Agrawal S, Emond JC, et al. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17–28. doi:10.2147/jhc.S86792
- Hong YM, Cho M, Yoon KT, et al. Neutrophil-lymphocyte ratio predicts the therapeutic benefit of neoadjuvant transarterial chemoembolization in patients with resectable hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2020;32(9):1186–1191. doi:10.1097/meg.0000000000001629
- Lee BM, Chung SY, Chang JS, et al. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342–352. doi:10.5009/gnl17216
- Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672. doi:10.1186/s12885-019-5903-y
- Harimoto N, Yoshizumi T, Shimagaki T, et al. Inflammation-based prognostic score in patients with living donor liver transplantation for hepatocellular carcinoma. Anticancer Res. 2016;36(10):5537–5542. doi:10.21873/anticanres.11137
- Wang GY, Yang Y, Li H, et al. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 2011;6(9):e25295. doi:10.1371/journal.pone.0025295
- Miyashita T, Tajima H, Makino I, et al. Metastasis-promoting role of extravasated platelet activation in tumor. J Surg Res. 2015;193(1):289–294. doi:10.1016/j.jss.2014.07.037
- Li R, Ren M, Chen N, et al. Presence of intratumoral platelets is associated with tumor vessel structure and metastasis. BMC Cancer. 2014;14:167. doi:10.1186/1471-2407-14-167
- Ismael MN, Forde J, Milla E, et al. Utility of inflammatory markers in predicting hepatocellular carcinoma survival after liver transplantation. Biomed Res Int. 2019;2019:7284040. doi:10.1155/2019/7284040
- Triozzi PL, Stirling ER, Song Q, et al. Circulating immune bioenergetic, metabolic, and genetic signatures predict melanoma patients’ response to anti-PD-1 immune checkpoint blockade. Clin Cancer Res. 2022;28(6):1192–1202. doi:10.1158/1078-0432.Ccr-21-3114