Abstract
Purpose
Delayed onset of lactogenesis is a significant barrier to achieving the WHO-recommended 50% exclusive breastfeeding rate in the first six months. This study maps the main factors influencing this condition, addressing gaps in the current research landscape.
Methods
Following Arksey and O’Malley’s scoping review framework, databases such as PubMed, Web of Science (WOS), Embase, Cochrane Library, CINAHL plus with full text, China National Knowledge Infrastructure (CNIK), Weipu Chinese Journal Service Platform (VIP), Wanfang Data Knowledge Service Platform, and China Biomedical Literature Database (CBM) were searched on February 1, 2023. Studies in Chinese and English involving pregnant and postpartum women, focusing on delayed onset of lactogenesis, were included without restrictions on publication date or geography.
Results
Forty-six studies published between 2002 and 2022 met the inclusion criteria, revealing variable incidences of delayed lactogenesis among different groups. Thirty-four influencing factors were identified and organized into five themes: maternal-infant characteristics, perinatal mental state, physical activity participation during pregnancy, breastfeeding behaviors, and medical staff interventions. Within eighteen major factors highlighted, factors such as age, pre-pregnancy BMI, gestational weight gain, average LATCH score within 24 hours postpartum, labor analgesia, sleep, frequency of postpartum breastfeeding, and timing of initial breast suckling/pumping showed inconsistent or conflicting conclusions.
Conclusion
High and variable incidences of delayed lactogenesis underline its multifactorial nature. Effective interventions require strong advocacy from healthcare professionals and adherence by pregnant women. Further research using standardized methods is essential to clarify inconsistent or conflicting findings on the influencing factors.
Introduction
Breastfeeding is widely recognized for its unique benefits in enhancing the health of both mothers and infants. It can help mothers prevent breast cancer, ovarian cancer, and type 2 diabetes.Citation1 Additionally, breastfeeding plays a vital role in infants’ immune regulation and tissue development. It provides a rich source of essential nutrients necessary for growth and development, along with active cells, probiotics, immune-active components, triglycerides, oligosaccharides, etc.Citation2 Beyond these direct benefits, research has suggested that various nutrients in breast milk may regulate genetic inheritance characteristics through DNA damage repair, stable methylation modification, and real-time gene transcription regulation.Citation3,Citation4
Given these numerous benefits, the World Health Organization recommends an exclusive breastfeeding rate of over 50% for the first six months.Citation5 However, global rates of exclusive breastfeeding within this period are suboptimal.Citation1,Citation6 Delayed onset of lactogenesis (DOL), a critical factor affecting exclusive breastfeeding, has a significant global occurrence rate. Studies indicate that DOL rates vary from 10.1% to 58.0% in different countries,Citation7 with a global average rate of 26%,Citation8 meriting serious attention.
Lactogenesis typically occurs in two stages: Stage I during pregnancy and Stage II, characterized by the onset of copious milk production, usually between 24 and 72 hours post-childbirth.Citation9 Delayed onset of lactogenesis or delayed Stage II involves delayed breast fullness and engorgement experienced by mothers after 72 hours postpartum.Citation10 This delay can affect breastfeeding duration,Citation10,Citation11 leading to early termination and resulting in complications such as jaundice, hypoglycemia, dehydration, hypernatremia, and pathological weight loss in newborns.Citation12 Therefore, reducing the incidence of DOL is crucial for long-term health benefits.
Several factors influence DOL, including parity,Citation13–15delivery mode,Citation15–17 maternal age,Citation18–20 pre-pregnancy BMI,Citation19,Citation21,Citation22 gestational weight gain,Citation13,Citation19,Citation23 gestational diabetes,Citation24–26 gestational hypertension disorders,Citation24,Citation26,Citation27 average LATCH score within 24 hours postpartum,Citation18,Citation28,Citation29 preterm birth,Citation30,Citation31 depression,Citation14,Citation20,Citation32 anxiety,Citation24,Citation31,Citation33 labor analgesia,Citation17,Citation19,Citation34 sleep,Citation35–37 prenatal physical activity and sedentary time,Citation38 postpartum breastfeeding frequency,Citation23,Citation39,Citation40 initial breast suckling/pumping time,Citation15,Citation24,Citation41 breastfeeding education and knowledge,Citation33,Citation42 among others.
However, due to variations in research methods, such as inconsistent age categorization and different tools for measuring sleep quality, research findings have been diverse. Additionally, existing reviews tend to focus primarily on specific core factors and lack a comprehensive examination.Citation8,Citation9,Citation43 Therefore, there is a need for a thorough and universally applicable summary, encompassing the main influencing factors, diverse conclusions, and key reasons for discrepancies, to facilitate early identification and support for women at potential risk and to enhance the quality of relevant research.
Methods
A scoping review, a method designed to rapidly map research progress in a specific area and identify its limitations,Citation44 is suitable for systematically reviewing the main influencing factors of delayed onset of lactogenesis, summarizing various conclusions, and explaining the reasons for their variations.
Adopting the methodological framework for scoping reviews proposed by Arksey and O Malley,Citation45 we addressed the following research question: What are the current status and main influencing factors of delayed onset of lactogenesis?
Inclusion/Exclusion Criteria
Based on the “PCC: Participants, Concept, Context” principle,Citation46 the inclusion criteria were as follows: ① Participants: Pregnant and postpartum women, aged ≥18 years. ② Concept: Delayed onset of lactogenesis. ③ Context: Current situation and influencing factors. ④Types of evidence sources: Primary research published in English or Chinese.
The exclusion criteria included: ①Studies not defining delayed onset of lactogenesis as delayed maternal perception. ②Studies directly using the mean and standard deviation of lactogenesis time from the sample for regression analysis. ③Editorials, reviews, study protocols, studies without full texts, incomplete data, and duplicates.
Search Strategy
A comprehensive search strategy was employed across multiple databases, including PubMed, Web of Science (WOS), Embase, Cochrane Library, CINAHL plus with full text, China National Knowledge Infrastructure (CNKI), VIP, Wanfang Data Knowledge Service Platform, and China Biomedical Literature Database (CBM). The research utilized subject headings and relevant keywords to identify pertinent peer-reviewed literature. Keywords included: ①Pregnant Women or expectant mother(s); ②Lactation; ③Risk factors, protective factors, causality, root cause analysis, or social determinants of health (Appendix Table 1 for the complete PubMed search strategy). The references of the included studies were manually tracked for additional relevant studies.
Study Selection
All retrieved records were imported into EndNote X9 for deduplication. To enhance the reliability of the screening process, two reviewers, including the first author and co-first author, independently performed an initial screening based on the inclusion and exclusion criteria by reviewing titles and abstracts, followed by a secondary screening of full texts. Any disagreements were resolved through consultation with the corresponding author. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)Citation47 chart documented document decisions related to inclusion/exclusion, ensuring transparency and rigor.
Review and Extraction
Upon finalizing the set of studies for inclusion, the following information was extracted using a custom template in Microsoft Office Word 2019:
Author(s), publication year
Study location, study design
Description of sample (eg, first-time mothers, maternal separation mothers), participant numbers
Occurrence rate of delayed onset of lactogenesis
Assessment tools, influencing factors, data analysis results
The initial extraction was conducted by Sen Li and Tajiguli Wupuer. Rui Hou then reviewed the extraction to ensure completeness and accuracy. The initial analysis was prepared by Tajiguli Wupuer, and all authors reviewed and agreed upon the descriptive and narrative summary of findings.
Results
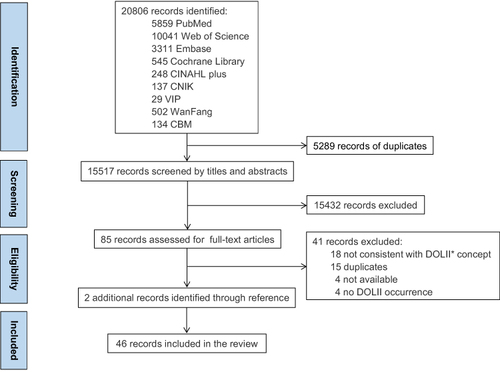
A total of 20,806 records were identified from nine electronic databases. After removing 5289 duplicates, 15,517 records were screened by titles and abstracts. Since the literature search did not restrict the field of study, studies involving animal experiments were excluded, resulting in 15,432 records being labeled as “exclude”. The full texts of the remaining 85 records were retrieved for eligibility assessment. Following full-text screening, 41 studies were excluded. Two additional records were identified through hand-searching reference lists, culminating in 46 unique studies ( for the PRISMA diagram).
As indicated in , the review encompasses 28 Chinese and 18 English studies, comprising 43 journal articles, 2 conference papers, and 1 dissertation. It includes 2 cross-sectional studies and 44 prospective cohort studies, published between 2002 and 2022. A majority (73.91%) were published from 2017 to 2022. Of these studies, 95.65% were prospective cohort studies. Among the English studies, the most publications originated from the United States (44.44%), followed by China (33.33%), with lesser representation from other countries. Within China, the 34 articles (English: n = 6; Chinese: n = 28) spanned 11 provincial-level administrative regions, with Anhui and Jiangsu provinces being the most represented (14.29% each).
Table 1 Characteristics of Studies
The incidence rate of delayed onset of lactogenesis in postpartum women ranged from 8.70% to 57.90%. Among the 46 included studies, only fiveCitation25,Citation27,Citation36,Citation37,Citation49 focused on maternal-infant separation in postpartum women. Out of eight categories of research subjects, only one study each investigated high-risk pregnant women in maternal intensive care unit (MICU)Citation49 and pregnant women with hypothyroidismCitation48 ( and Appendix Table 2 for details).
Table 2 DOLII* Study Status
Based on an analysis of narrative findings from this review, 34 influencing factors extracted from 46 records were categorized into five themes: ①maternal-infant characteristics. ②perinatal mental state. ③physical activity participation during pregnancy. ④breastfeeding behaviors. ⑤medical staff interventions. These are discussed below.
Maternal-Infant Characteristics
This including parity (41.30%, 19/46), maternal age (34.78%, 16/46), perinatal complications (34.78%, 16/46), pre-pregnancy BMI (30.43%, 14/46), delivery mode (30.43%, 14/46), gestational weight gain (26.09%, 12/46), average LATCH score within 24 hours postpartum (6.52%, 3/46), gestational age (4.35%, 2/46), labor duration (4.35%, 2/46), baby birth weight (4.35%, 2/46), social support for breastfeeding (4.35%, 2/46), alcohol consumption during pregnancy (2.17%, 1/46), nipple morphology (2.17%, 1/46), and family economic status (2.17%, 1/46).
There was unanimous agreement that primiparity is an independent risk factor for delayed onset of lactogenesis (100%, 19/19).Citation13–15,Citation24,Citation26,Citation28,Citation30,Citation39,Citation51–58,Citation60–62
Regarding maternal age, one studyCitation40 identified age ≥30 years as a risk factor (6.25%, 1/16), while nine studiesCitation24–26,Citation30–32,Citation49,Citation54,Citation56 proposed age ≥35 years (56.25%, 9/16). Additionally, six studiesCitation18–20,Citation53,Citation55,Citation62 did not specify a specific age threshold (3.75%, 6/16).
Research on perinatal complications primarily focused on gestational hypertension (37.50%, 6/16),Citation24,Citation26,Citation27,Citation36,Citation37,Citation49 gestational diabetes mellitus (31.25%, 5/16),Citation24–26,Citation28,Citation51 insulin therapy for gestational diabetes (12.50%, 2/16),Citation18,Citation55 and irregular blood glucose monitoring (6.25%, 1/16).Citation56 Other complications investigated included perineal lacerations or episiotomy during delivery (6.25%, 1/16),Citation30 hypothyroidism in pregnancy (6.25%, 1/16),Citation48 serum albumin concentration <35g/L (6.25%, 1/16),Citation14 and postpartum hemorrhage ≥300mL (6.25%, 1/16).Citation29
Concerning pre-pregnancy BMI, studies since 2003 have not reached consensus. Five studiesCitation18,Citation19,Citation31,Citation40,Citation55 found that pre-pregnancy BMI ≥30kg/m2 could be a risk factor (37.71%, 5/14), while two studiesCitation21,Citation42 reported that pre-pregnancy BMI ≥24kg/m2 could also predict delayed onset of lactogenesis (14.29%, 2/14). Other viewpoints include BMI ≥29kg/m2 (7.14%, 1/14),Citation61 BMI ≥28kg/m2 (7.14%, 1/14),Citation22 BMI >27kg/m2 (7.14%, 1/14),Citation16 BMI ≥25kg/m2 (14.29%, 2/14),Citation17,Citation39 besides the studiesCitation25,Citation52 that did not suggest a specific BMI threshold (14.29%, 2/14).
Similarly, for gestational weight gain, the same situation arises. Two studiesCitation23,Citation59 identified a gestational BMI gain of ≥7.6kg/m2 as a risk factor for delayed onset of lactogenesis (16.67%, 2/12), while another studyCitation27 suggested that a gain of ≥7.2kg/m2 could also be a predictor (8.33%, 1/12). Furthermore, nine studiesCitation10,Citation13,Citation17,Citation19,Citation26,Citation28–30,Citation52 proposed excessive gestational weight gain as an indicator (75.00%, 9/12). Additionally, one studyCitation42 posited that a pre-pregnancy BMI of ≥24kg/m2, combined with appropriate gestational weight gain, may contribute to the occurrence of delayed onset of lactogenesis.
Regarding delivery mode, thirteen studiesCitation15–17,Citation23,Citation24,Citation26,Citation41,Citation48,Citation57–60,Citation62 unanimously identified cesarean section as a risk factor for delayed onset of lactogenesis (100%, 13/13). Additionally, one studyCitation30 focused on women who underwent vaginal delivery, suggesting that the use of single or combined induction methods during delivery can contribute to delayed onset of lactogenesis.
Moreover, three studiesCitation18,Citation28,Citation29 found that a low average LATCH score within 24 hours postpartum could be a contributor for delayed onset of lactogenesis. Among them, one studyCitation18 considered average LATCH score of ≤7.5 points within 24 hours postpartum as an indicator (33.33%, 1/3), while another studyCitation29 proposed a LATCH score of ≤8 points (33.33%, 1/3). Furthermore, one studyCitation28 directly described specific items from the LATCH scoring system, stating that weak newborn suckling, unclear swallowing movements, and the need for assistance during breastfeeding on the first day postpartum are risk factors for delayed onset of lactogenesis (33.33%, 1/3).
Additionally, two studies each reported that preterm birth,Citation30,Citation31 longer labor durationCitation16,Citation30 and higher baby birth weightCitation29,Citation40 are associated with a higher incidence of delayed onset of lactogenesis. Two studiesCitation10,Citation22 found that social support for breastfeeding is a protective factor. Also, one study each reported alcohol consumption during pregnancy,Citation20 a poverty income ratio of ≥3.5,Citation17 and flat or inverted nipplesCitation16 as predictors of delayed onset of lactogenesis.
Perinatal Mental State
This includes depression (17.39%, 8/46), anxiety (15.22%, 7/46), pain (10.87%, 5/46), sleep (6.52%, 3/46), childbirth stress (4.35%, 2/46), stressful life events (4.35%, 2/46), childbirth experience (2.17%, 1/46), and coping strategies for the mother–infant relationship (2.17%, 1/46).
The conclusions regarding the correlation between antenatal depression, anxiety, and delayed onset of lactogenesis were relatively consistent, with studies indicating that antenatal depressionCitation14,Citation20,Citation24,Citation31–33,Citation53,Citation54 (100%, 8/8) and anxietyCitation10,Citation24,Citation31–33,Citation53,Citation54 (100%, 7/7) are risk factors for delayed onset of lactogenesis.
Studies on pain were categorized mainly into two main types: breastfeeding-related nipple pain and labor analgesia. One studyCitation40 found that experiencing no nipple discomfort during the first 3 days postpartum could predict the occurrence of delayed onset of lactogenesis. Regarding labor analgesia, one studyCitation30 considered it a protective factor, while three othersCitation17,Citation19,Citation34 suggested the opposite.
Regarding the impact of sleep on delayed onset of lactogenesis, two studiesCitation36,Citation37 identified shorter sleep duration as a risk factor in mothers experiencing mother-infant separation, while another studyCitation35 found higher sleep efficiency and more consistent nocturnal sleep duration as protective factors in primiparous mothers.
Additionally, two studies each reported that higher labor stressCitation25,Citation62 and greater exposure to stressful life events during early pregnancyCitation23,Citation59 are linked to a higher incidence of delayed onset of lactogenesis. Furthermore, one study each found a correlation between mothers who had negative experiences with vaginal delivery,Citation30 employed negative coping strategies for mother–infant relationshipCitation25 and experienced delayed onset of lactogenesis.
Physical Activity Participation During Pregnancy
This includes physical activity in late pregnancy (2.17%, 1/46) and sedentary time (2.17%, 1/46). One studyCitation38 indicated that an adequate level of physical activity (moderate or higher) acts as a protective factor, while sedentary time of ≥6.5 hours/day is a risk factor.
Breastfeeding Behaviors
These include postpartum breastfeeding frequency (15.22%, 7/46), initial breast suckling/pumping time (6.52%, 4/46), breastfeeding knowledge consultation during pregnancy (6.52%, 3/46), early mother-infant contact (6.52%, 2/46), maternal breast massage/lactation stimulation (4.35%, 2/46), and the use of pacifiers or formula supplements (4.35%, 2/46).
Seven studiesCitation10,Citation23,Citation39–42,Citation58 noted a correlation between a lower frequency of postpartum breastfeeding and a higher incidence of delayed onset of lactogenesis. Among them, two studiesCitation23,Citation40 specifically reported breastfeeding less than 2 times within 24 hoursCitation40 or less than 3 timesCitation23 (28.57%, 2/7) as contributing factors. Two other studiesCitation10,Citation58 suggested breastfeeding ≤2 times within 24–48 hours as a risk factor (28.57%, 2/7). Three studiesCitation39,Citation41,Citation42 did not specify a threshold for postpartum breastfeeding frequency (42.86%, 3/7).
Moreover, four studiesCitation15,Citation24,Citation41,Citation49 reported an association between delayed initial breast suckling/pumping time and delayed onset of lactogenesis. Among them, one studyCitation49 did not provide a specific time threshold (25.00%, 1/4), two studiesCitation24,Citation41 concluded that ≥30 minutes is a risk factor (50.00%, 2/4), while another studyCitation15 identified ≥2 hours as a risk factor (25.00%, 1/4).
Besides, three studiesCitation39,Citation41,Citation42 indicated that breastfeeding knowledge consultation during pregnancy can effectively reduce the occurrence of delayed onset of lactogenesis. Two studies each identified maternal breast massage/nipple stimulation during pregnancyCitation10,Citation41 and early mother-infant contactCitation33,Citation41 as protective factors. Finally, two studiesCitation10,Citation21 reported predominant formula feeding as a contributing factor to delayed onset of lactogenesis.
Medical Staff Interventions
This includes antenatal breastfeeding education (4.35%, 2/46), direct rooming-in (2.17%, 1/46), breast stimulation using a low-frequency pulse rehabilitation therapy instrument (2.17%, 1/46), and psychological intervention (2.17%, 1/46).
Regarding medical staff interventions, two studiesCitation33,Citation41 indicated that incorporating antenatal breastfeeding education into routine care effectively reduces the incidence of delayed onset of lactogenesis. Additionally, one study each reported that direct rooming-in post-childbirth,Citation41 breast stimulation with a low-frequency pulse rehabilitation therapy instrument, and psychological interventionCitation33 act as protective factors against delayed onset of lactogenesis.
Discussion
The incidence of delayed onset of lactogenesis varied significantly across different populations with higher incidence among particular groups.
The incidence rate of delayed onset of lactogenesis in postpartum women from 2002 to 2022 ranged between 8.70% and 57.90%. Women who experienced mother-infant separation(35.67%-42.40%),Citation25,Citation27,Citation36,Citation37,Citation49 had advanced maternal age(38.30%-42.50%),Citation10,Citation53,Citation54 and underwent cesarean section(31.50%-38.30%),Citation10,Citation14,Citation32,Citation39 were in high-risk pregnancies in intensive care unit(42.40%)Citation49 or had comorbid hypothyroidism(45.30%)Citation48 showed higher incidence rates than the global average(26%).Citation8 These results highlight substantial variations in the occurrence of delayed onset of lactogenesis among different populations, with particular groups experiencing higher rates, underscoring the need for heightened focus. Recent studies suggest future research trends and propose more targeted clinical interventions for delayed onset lactogenesis.
There are numerous influencing factors of delayed onset of lactogenesis, but some of them are still waiting to be verified.
Maternal-Infant Characteristics
Factors such as parity, delivery mode, maternal age, pre-pregnancy BMI, gestational weight gain, perinatal complications, average LATCH score within 24 hours postpartum, and preterm birth have been identified as primary influencers in maternal-infant characteristics.
From 2002 to 2022, numerous studies have confirmed that primiparity,Citation13–15,Citation24,Citation26,Citation28,Citation30,Citation39,Citation51–58,Citation60–62 cesarean delivery,Citation15–17,Citation23,Citation24,Citation26,Citation41,Citation48,Citation57–60,Citation62 advanced maternal age,Citation18–20,Citation24–26,Citation30–32,Citation40,Citation49,Citation53–56,Citation62 high pre-pregnancy BMI,Citation16–19,Citation21,Citation22,Citation25,Citation31,Citation39,Citation40,Citation42,Citation52,Citation55 excessive gestational weight gain,Citation10,Citation13,Citation17,Citation19,Citation23,Citation26–30,Citation52,Citation59 gestational diabetes,Citation24–26,Citation28,Citation51 pregnancy-induced hypertension,Citation24,Citation26,Citation27,Citation36,Citation37,Citation49 and low LATCH scoresCitation18,Citation28,Citation29 are risk factors for delayed onset of lactogenesis. The physiological mechanism linking primiparity, cesarean delivery, and advanced maternal age involves stress-induced stimulation of the vagus nerve, affecting lactogenesis initiation by increasing blood catecholamine levels.Citation14,Citation26,Citation52 Research on cesarean delivery has focused more on timingCitation63 and urgency,Citation15,Citation62 suggesting that emergency and nighttime cesarean deliveries are more predictive. Regarding advanced maternal age, three different conclusions have been drawn: age ≥30 years,Citation40 possibly related to the sample’s age distribution and criteria (primiparous women), age ≥35 years,Citation24–26,Citation30–32,Citation49,Citation56,Citation64 in line with the domestic definition of advanced maternal age for high-risk pregnancies, and studies that do not specify an age threshold, likely due to inclusion of analyses treating age as a continuous variable.Citation18–20,Citation53,Citation55,Citation62
Primiparity, cesarean delivery, and advanced maternal age are uncontrollable risk factors. Therefore, clinical and primary healthcare professionals should inform women with these conditions about the potential for delayed onset of lactogenesis.Citation19,Citation60 Additionally, they should enhance women’s breastfeeding knowledge and skills through videos, live demonstrations, simulations, and experience sharing before delivery.Citation56 During labor, providing supportive and comforting companionship can help improve positive experiences, reducing childbirth fear and negative emotions.Citation51,Citation52 Post-delivery, continuous breastfeeding support and encouragement are crucial to ensure successful initiation of lactogenesis.Citation14,Citation15,Citation26,Citation52,Citation60 Researchers should also meticulously select appropriate age classification standards to align with the specific population.
Although there is a consensus that high pre-pregnancy BMI and excessive gestational weight gain are risk factors for delayed onset of lactogenesis, details surrounding these factors remain controversial and require further exploration.
Research on the pre-pregnancy BMI threshold presents multiple conclusions, attributable to:
①Variations in the BMI classification guidelines referenced by researchers. Three studiesCitation21,Citation22,Citation42 used the Blue Book of Obesity Prevention and Control in China, while nine studiesCitation16–19,Citation31,Citation39,Citation40,Citation52,Citation55 followed the World Health Organization BMI Classification Guidelines. One studyCitation16 adjusted the overweight threshold to BMI > 27 kg/m2 based on actual circumstances. Additionally, one studyCitation61 utilized the Institute of Medicine BMI Classification Guidelines (pre-2009 version), and another studyCitation25 did not use any specific BMI classification.
②Differences in statistical analysis methods. Twelve studiesCitation16–19,Citation21,Citation22,Citation31,Citation39,Citation40,Citation42,Citation55,Citation61 conducted categorical regression analysis on pre-pregnancy BMI, while one studyCitation52 only presented the trend of BMI changes in logistic regression, and another studyCitation25 performed regression analysis on the continuous variable. Consequently, the latter two studiesCitation25,Citation52 did not include BMI classification thresholds.
Furthermore, while theories such as insulin resistanceCitation55 decreased sensitivity of breast peripheral nerves,Citation65 and hormonal imbalanceCitation42 have been proposed to explain why overweight or obese mothers experience a higher incidence of delayed onset of lactogenesis, the exact risk factor—whether obesity or overweight—and the underlying reasons remain uncertain.
The physiological mechanism underlying excessive gestational weight gain is similar to that of high pre-pregnancy BMI; specifically, progesterone in adipose tissue competes with and inhibits the effects of prolactin, consequently delaying lactogenesis.Citation13,Citation17,Citation26,Citation27 Three different perspectives on gestational weight gain merit discussion. The first perspective suggests that a greater increase in gestational BMI correlates with a higher incidence of delayed onset of lactogenesis.Citation23,Citation27,Citation59 The second indicates that an increased body weight during pregnancy raises the incidence of delayed onset of lactogenesis.Citation10,Citation13,Citation17,Citation19,Citation26,Citation28–30,Citation52 The third suggests that it may be necessary to perform a combined analysis of pre-pregnancy BMI and gestational weight gain.Citation42 Concerning the first viewpoint, although Zhu et al argued that gestational BMI increase has a stronger correlation with delayed onset of lactogenesis compared to relative gestational weight gain,Citation23 setting a gestational BMI increase threshold based on quartiles alone cannot eliminate the impact of sample gestational BMI increase on statistical analysis results. Regarding the second viewpoint, six studiesCitation13,Citation17,Citation19,Citation26,Citation30,Citation52 referenced the gestational weight gain recommendations by the Institute of Medicine, while four studiesCitation10,Citation13,Citation28,Citation29 utilized quartiles to categorize gestational weight gain. Huang et al, in their 2019 study, observed significant data when categorizing gestational weight gain by quartiles, but not when using the Institute of Medicine’s standards.Citation13 This discrepancy might be attributed to the study’s Chinese population, highlighting the importance of selecting suitable reference indicators.
Weak awareness, inadequate knowledge, lack of skills, and insufficient support from healthcare institutions are key influencing factors for high pre-pregnancy BMI and excessive gestational weight gain.Citation66 Therefore, weight management-related education, motivational interviews,Citation27 and other methods can effectively enhance pregnant women’s awareness of weight management and correct misconceptions about prenatal care, promoting lactogenesis.
Gestational hypertension and diabetes can disrupt lactogenesis through mechanisms such as ischemic necrosis of trophoblast cellsCitation19,Citation24,Citation26,Citation27,Citation36,Citation37,Citation49,Citation67–69 and disturbances in glucose metabolism.Citation17,Citation24,Citation70,Citation71 Women with these conditions can reduce the risk of delayed onset of lactogenesis through regular prenatal check-ups, daily monitoring, and therapeutic medications. Furthermore, although only one included studyCitation48 discusses the association between gestational hypothyroidism and delayed onset of lactogenesis, research has confirmed that elevated thyroid-stimulating hormone levels can inhibit protein synthesis.Citation72 Hence, clinical attention to pregnant women with hypothyroidism remains crucial.
Although studiesCitation18,Citation28,Citation29 have demonstrated that the LATCH score can identify mothers who may need additional breastfeeding guidance, covering five aspects: newborns’ latching and swallowing, mothers’ breastfeeding experience and comfort, and the need for external assistance, there is still debate over the specific score thresholds. Matias et al considered a LATCH score ≤7.5 as a risk factor for delayed onset of lactogenesis,Citation18 while Huang et al in their 2015 study suggested a LATCH score ≤8 as the threshold,Citation29 and later in 2017, they proposed a LATCH score ≤8.5.Citation28 The main reason for this discrepancy lies in the statistical analysis method used, where researchers set the median LATCH score of the sample as the threshold.
Despite these differences, healthcare professionals, in both clinical and primary care settings, can offer personalized health education on early breastfeeding initiation and exclusive breastfeeding based on the LATCH score. However, caution is needed when interpreting varying LATCH score results.
Research indicates that mothers of preterm infants are more prone to experience delayed onset of lactogenesis than mothers of full-term infants, due to lactation system immaturity and post-delivery mother-infant separation.Citation30,Citation31 Therefore, clinical practice should encourage such mothers to engage in breast massage and lactation stimulationCitation10,Citation41 to promote the timely establishment of the prolactin axis.
Perinatal Mental State
Depression, anxiety, labor pain, and sleep are significant factors influencing delayed onset of lactogenesis in the perinatal mental state.
Although depression and anxiety have gained prominence only in the last five years, multiple studies confirm their impact on delayed onset of lactogenesis through effects on sleep quality and nutritional intake of pregnant and postpartum women.Citation10,Citation24,Citation32,Citation54 Their physiological mechanismsCitation14,Citation20,Citation31,Citation33,Citation54 are akin to those associated with primiparity, cesarean section, and advanced maternal age. Additionally, depression and anxiety often stem from childbirth stress in women who are primiparous,Citation20 of advanced maternal age,Citation10,Citation53,Citation54 have undergone cesarean sections,Citation10,Citation14,Citation32 or have gestational diabetes.Citation31 Therefore, targeted psychological counseling before delivery can mitigate the impact of negative emotions on lactogenesis.
Regarding labor analgesia’s effect on lactogenesis, scholars hold varying opinions on whether it acts as a protective or risk factor for delayed onset. Dong et al believe that analgesics can lessen the impact of negative emotions such as pain and anxiety on prolactin secretion.Citation30 Conversely, Lind et al argue that analgesics used during labor can affect early effective breastfeeding by entering the fetal bloodstream and influencing lactation reflex by reducing maternal plasma oxytocin levels.Citation72 Another study indicates that the short half-life and high clearance rate of labor analgesics are unlikely to delay lactogenesis,Citation64 but due to the small sample size, further validation is needed.
High-quality sleep is identified as a protective factor against delayed onset of lactogenesis. However, slight differences exist in the discussion and analysis of sleep components due to the use of varied survey tools across studies. Some studies,Citation36,Citation37 like those by Lu et al and Yu et al, mainly focus on sleep duration, while Casey et al also consider sleep efficiency and stability in addition to duration.Citation35 The main mechanisms influencing this are high levels of prolactin secretion during sleepCitation35,Citation37,Citation54 and the interaction between negative emotions and sleep.Citation10 Thus, healthcare professionals can aid lactogenesis by enhancing the sleep environment for pregnant and postpartum women and providing psychological support.
Physical Activity Participation During Pregnancy
Among the 46 studies included, only oneCitation38 explored the correlation between physical activity during pregnancy and delayed onset of lactogenesis. However, the benefits of physical activity during pregnancy in controlling gestational weight gain and improving muscle tissue insulin sensitivity,Citation73 as well as reducing late pregnancy and early postpartum anxiety and depression, have already been proved in other studies.Citation74,Citation75 Therefore, sufficient physical activity during pregnancy is a critical protective factor against delayed onset of lactogenesis. It is essential to develop tailored exercise prescriptions for pregnant women at different pregnancy stages in obstetrics and gynecology clinics. These prescriptions should aim to mitigate the effects of excessive gestational weight gain, gestational diabetes, and negative emotions on lactogenesis. Additionally, increasing physical activity during pregnancy cannot offset the adverse effects of prolonged sitting on lactogenesis initiation.Citation38 Thus, reducing sedentary time while increasing physical activity is important.
Breastfeeding Behaviors
Postpartum breastfeeding frequency, initial breast suckling/pumping time, and breastfeeding knowledge consultation during pregnancy have been identified as key influencing factors in breastfeeding behaviors.
Regarding the research on postpartum breastfeeding frequency, although the association between higher breastfeeding frequency and lower incidence of delayed onset of lactogenesis,Citation10,Citation23,Citation39–42,Citation58 as well as the physiological mechanism of early postpartum breastfeeding in facilitating the timely formation of the lactation hormone axis via sensory signals from the nipple,Citation24,Citation39,Citation41 have been demonstrated, inconsistency remains in the specific definition of breastfeeding frequency. The reasons for this inconsistency include:
①Different thresholds for defining enough breastfeeding frequency. Two studiesCitation39,Citation41 used mean ± standard deviation or confidence intervals, three studiesCitation10,Citation23,Citation58 utilized binary classification, one studyCitation41 adhered to the on-demand breastfeeding requirement recommended by Breastfeeding Promotion Strategy Guide (2018), while anotherCitation40 did not specify a defining basis.
②Variations in time frames for predictive indicators. Two studies each used the first 24 hours,Citation23,Citation40 24–48 hours,Citation10,Citation58 or the average frequency of daily breastfeedingCitation41,Citation42 as predictors of delayed onset of lactogenesis, while one studyCitation39 employed 48 hours postpartum as the indicator.
③Different statistical analysis methods. Four studiesCitation10,Citation23,Citation40,Citation58 conducted categorical regression analysis for postpartum breastfeeding frequency, whereas three studiesCitation39,Citation41,Citation42 presented trends in logistic regression without specifying categories.
Regarding the threshold of postpartum breastfeeding frequency, it is necessary to clarify whether the data refer to “breast suckling frequency” or “effective breastfeeding frequency”. Until specific thresholds are established, it is advisable for mothers to at least adhere to on-demand breastfeeding requirements.
The initial breast suckling/pumping time is believed to have a physiological mechanism similar to that of postpartum breastfeeding frequency, with three distinct conclusions. The first conclusion identifies an initial breast suckling/pumping time of ≥30 minutes as a risk factor;Citation24,Citation41 the second suggests ≥2 hours as a risk factor;Citation15 and the third pertains to late initiation of pumping time.Citation49 Concerning the first viewpoint, while researchers have justified it as “early suckling stimulates the timely formation of the lactation hormone axis”Citation24,Citation41 and the Breastfeeding Promotion Strategy Guide (2018) also explicitly recommends newborns should suckle within 30 minutes post-delivery, this advice is considered a strong recommendation with very low-quality evidence.Citation76 Moreover, the emergence of the second viewpoint indicates the need for further research to validate its scientific basis. The third viewpoint, presented by Xie Xiaoxing et al in their 2021 study involving high-risk pregnant women in the intensive care unit,Citation49 highlights the importance of pumping for separated mother-infant pairs but necessitates additional research to establish a threshold for initiation pumping time, as they directly conducted regression analysis on continuous variables.
Jiang Yanli,Citation41 Ding Juan,Citation39 Zhang Yingying,Citation42 and others, in 2022, proposed that prenatal counseling on breastfeeding knowledge is an effective preventive measure against delayed onset of lactogenesis. TheyCitation39,Citation41 explained its rationale as “changing the breastfeeding perception of pregnant women to make them more actively cope with postpartum breastfeeding”. This aligns with the intervention behaviors of healthcare professionals, discussed in detail in Medical Staff Interventions section.
Medical Staff Interventions
Breastfeeding knowledge education, as the primary intervention behavior of healthcare professionals, plays a vital role in promoting lactogenesis and is an important measure to prevent delayed onset of lactogenesis. Specifically, scientific, rigorous, and personalized health education not only enables pregnant women to anticipate the possibility of delayed onset of lactogenesis in advance but also intervenes timely to mitigate the impact of controllable factors such as adverse mental states and exercise habits during the perinatal period on lactogenesis. Furthermore, it encourages pregnant women to adopt scientifically informed breastfeeding behaviors postpartum. Given that existing breastfeeding knowledge education primarily focuses on the benefits of breastfeeding, incorporating knowledge about preventing delayed onset of lactogenesis is necessary in clinical practice.
Additionally, there is an overlap between certain breastfeeding behaviors of pregnant women, such as breastfeeding knowledge consultation during pregnancy,Citation39,Citation41,Citation42 breast massage/lactation stimulation,Citation10,Citation41 early mother-infant contact,Citation33,Citation41 and the intervention behaviors of healthcare professionals, including prenatal breastfeeding education,Citation33,Citation41 breast stimulation with a low-frequency pulse rehabilitation therapy instrument,Citation33 direct rooming-in.Citation41 This underscores the necessity of providing pregnant women with education and nursing interventions like breast stimulation, while also emphasizing the importance of effective communication and enhancing pregnant women’s compliance to improve the efficacy of interventions for delayed onset of lactogenesis.
Conclusion
A comprehensive review of the current status of delayed onset of lactogenesis reveals significant variability in its incidence across different populations, with certain groups exhibiting higher rates, necessitating considerable attention. The review of influencing factors identified several key elements, including maternal-infant characteristics, perinatal mental status, physical activity during pregnancy, breastfeeding behaviors, and medical staff interventions. Uncontrollable factors are mainly associated with maternal-infant characteristics. Among the controllable and uncontrollable factors, inconsistencies were found in maternal age, pre-pregnancy BMI, gestational weight gain, average LATCH scores within 24 hours postpartum, sleep, postpartum breastfeeding frequency, and initial breast suckling/pumping time. Labor analgesia presented conflicting conclusions. Furthermore, this study underscored the critical role of breastfeeding knowledge education in preventing delayed onset of lactogenesis. Based on these findings, it is recommended that clinical and primary healthcare professionals focus more on controllable factors when providing knowledge education and related interventions. Researchers should conduct more targeted studies on special populations and further investigate and validate inconsistent or conflicting influencing factors by using standardized data classification criteria and recognized research methods.
Abbreviations
WOS, Web of Science; CNIK, China National Knowledge Infrastructure; VIP, Weipu Chinese Journal Service Platform; CBM, China Biomedical Literature Database; DOLII, Delayed onset of Lactogenesis II.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Disclosure
The authors declare no competing interests in this work.
Acknowledgments
We would like to express our gratitude to Professors Zhiwen Wang and Hong Lu for their valuable suggestions, which have helped improve the logical structure of our paper. Additionally, we would like to thank Senior Student Yuxuan Li for her assistance in addressing the issues we encountered during the literature screening and data extraction processes.
References
- Victora CG, Bahl R, Barros AJD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi:10.1016/s0140-6736(15)01024-7
- Ren YW, Gao HF. Theory and Practice of Breastfeeding. Beijing: People’s Medical Publishing House; 2018:19–26.
- He BZ, Wang DH. Epigenetic mechanisms of early life nutrition impacting long-term health. Chin J Neonatol. 2013;28(5):346–348. doi:10.3969/j.issn.1673-6710.2013.05.018
- Ma JR, Wang DH. Epigenetic effects of human breastfeeding. Chin J Contemp Pediatr. 2016;18(10):926–930. doi:10.7499/j.issn.1008-8830.2016.10.002
- World Health Organization. Infant and young child feeding. Available from: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding. Accessed May 2, 2024.
- Yang Z, Lai J, Yu D, et al. Breastfeeding rates in China: a cross-sectional survey and estimate of benefits of improvement. Lancet. 2016;388:47. doi:10.1016/s0140-6736(16)31974-2
- Wu ZF, Xu Q, Yang NN, Shi M. Analysis of the current status and intervention measures for delayed initiation of postpartum lactation. J Nurs. 2018;25(1):34–38.
- Hu SS, Liu J, Jiang PH, Sun ZN, Zhu QX, Fu JY. A systematic review of the incidence and influencing factors of delayed onset of lactogenesis II. Chin Gene Pract. 2021;24(24):3110–3115. doi:10.12114/j.issn.1007-9572.2021.00.460
- Zheng XL, Zhang Y, Xu XF. Research progress on factors influencing delayed lactation initiation and its interventions. Chin J Nurs. 2014;49(3):340–344. doi:10.3761/j.issn.0254-1769.2014.03.022
- Ding QY. Analysis of factors influencing delayed onset of lactogenesis and construction of a predictive model in advanced cesarean section mothers. Guangxi University of Chinese Medicine; 2022. Available from: https://d.wanfangdata.com.cn/thesis/ChJUaGVzaXNOZXdTMjAyMzAxMTISCUQwMjg0ODcxNhoIdDY0bmN0ejE%3D. Accessed May 2, 2024.
- Chapman DJ, Pérez-Escamilla R. Does delayed perception of the onset of lactation shorten breastfeeding duration? J Hum Lactation. 1999;15(2):107–111. doi:10.1177/089033449901500207
- The Fed Is Best Foundation. Know your risks for delayed onset of full breast milk supply. Available from: https://fedisbest.org/resources-for-parents/know-risks-delayed-onset-full-breast-milk-supply/. Accessed May 2, 2024.
- Huang L, Chen X, Zhang Y, et al. Gestational weight gain is associated with delayed onset of lactogenesis in the TMCHC study: a prospective cohort study. Clin Nutr. 2019;38(5):2436–2441. doi:10.1016/j.clnu.2018.11.001
- Lian WN, Ding J, Xiong TT, Liuding JD, Nie L. Determinants of delayed onset of lactogenesis II among women who delivered via Cesarean section at a tertiary hospital in China: a prospective cohort study. Int Breastfeed J. 2022;17(1):81. doi:10.1186/s13006-022-00523-3
- Mullen AJ, O’Connor DL, Hanley AJ, Piedimonte G, Wallace M, Ley SH. Associations of metabolic and obstetric risk parameters with timing of lactogenesis II. Nutrients. 2022;14(4):876. doi:10.3390/nu14040876
- Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3):607–619. doi:10.1542/peds.112.3.607
- Haile ZT, Chavan BB, Teweldeberhan A, Chertok IR. Association between gestational weight gain and delayed onset of lactation: the moderating effects of race/ethnicity. Breastfeed Med. 2017;12(2):79–85. doi:10.1089/bfm.2016.0134
- Matias SL, Dewey KG. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am J Clin Nutr. 2014;99(1):115–121. doi:10.3945/ajcn.113.073049
- Preusting I, Brumley J, Odibo L, Spatz DL, Louis JM. Obesity as a Predictor of Delayed Lactogenesis II. J Hum Lactation. 2017;33(4):684–691. doi:10.1177/0890334417727716
- Rocha BO, Machado MP, Bastos LL, et al. Risk Factors for delayed onset of lactogenesis II among primiparous mothers from a Brazilian baby-friendly hospital. J Hum Lactation. 2020;36(1):146–156. doi:10.1177/0890334419835174
- Ren ZQ, Zhang AX, Zhang JJ, Wang R, Xia HO. Role of perinatal biological factors in delayed lactogenesis II among women with pre-pregnancy overweight and obesity. Biol. Res. Nurs. 2022;24(4):459–471. doi:10.1177/10998004221097085
- Tao XY, Huang K, Yan SQ, et al. Pre-pregnancy BMI, gestational weight gain and breast-feeding: a cohort study in China. Public Health Nutrition. 2017;20(6):1001–1008. doi:10.1017/s1368980016003165
- Zhu P, Hao J, Jiang X, Huang K, Tao F. New insight into onset of lactation: mediating the negative effect of multiple perinatal biopsychosocial stress on breastfeeding duration. Breastfeed Med. 2013;8:151–158. doi:10.1089/bfm.2012.0010
- Huang HL, Gu XQ, Feng HW. Status and influencing factors of delayed initiation of lactation Phase II in parturients in Baoshan district, shanghai. South China J Prevent Med. 2021;47(12):1493–1496.
- Lu Y, Yin Y, Jiang H. Influence of pregnancy stress and coping style on delayed onset of lactogenesis in maternal separation. Chin Nurs Res. 2022;36(23):4148–4153. doi:10.12102/j.issn.1009-6493.2022.23.003
- Wei ZC, Zhao ZL, Meng T, Ye Q, Wan Y, Liu YF. Occurrence and related factors of delayed lactation initiation of parturients in Tongzhou District, Beijing. South China J Prevent Med. 2022;48(7):817–821. doi:10.12183/j.scjpm.2022.0817
- Li JJ, Yu XR, Wang YF, Liu WJ, Kong HF. The influencing factors of delayed onset of lactogenesis II in preterm parturient women separated from their infants. Chin J Nurs Educ. 2022;19(4):368–372. doi:10.3761/j.issn.1672-9234.2022.04.015
- Huang L, Zhang Y, Chen X, et al. Delayed Initiation of postpartum lactation and its influencing factors: a prospective cohort study. presented at: The 13th National Congress of the Chinese Nutrition Society and the Global Chinese Nutrition Scientists Conference. Beijing, China; 2017.
- Huang L, Zhang Y, Wang WM, et al. Excessive gestational weight gain and feeding conditions impact lactation initiation. presented at: Danone Nutrition Center 18th Annual Academic Conference. Ningxia, China; 2015.
- Dong FX, Li L, Zhu KH, et al. Analysis of current status and influencing factors of lactation initiation delay in women with vaginal delivery. Chin J Pract Nurs. 2022;38(19):1496–1502. doi:10.3760/cma.j.cn211501-20211228-03548
- Luo FJ, Bao NZ, Li SH. Establishment and validation of a predictive model for the risk of delayed lactation initiation in pregnant women with gestational diabetes mellitus. J Clin Pathol Res. 2020;40(6):1394–1400. doi:10.3978/j.issn.2095-6959.2020.06.009
- Wang YF. Study on the influencing factors of delayed lactation initiation after cesarean section and evaluation of the effect of targeted nursing intervention. Fertil Health. 2021;29(24):35–38.
- Quan Y, Liu SY, Zhou HL. First lactation time and delayed lactation of parturient in Zhumadian area. South China J Prevent Med. 2022;48(9):1037–1040. doi:10.12183/j.scjpm.2022.1037
- Lind JN, Perrine CG, Li R. Relationship between use of labor pain medications and delayed onset of lactation. J Hum Lact. 2014;30(2):167–173. doi:10.1177/0890334413520189
- Casey T, Sun H, Burgess HJ, et al. Delayed lactogenesis II is associated with lower sleep efficiency and greater variation in nightly sleep duration in the third trimester. J Hum Lactation. 2019;35(4):713–724. doi:10.1177/0890334419830991
- Luan DD, Yu XR, Lin XY. Correlation between the onset time of lactation period II and lactation yield of the early stage after delivery in preterm’s mothers. Chin J Modern Nurs. 2018;24(8):874–878. doi:10.3760/cma.j.issn.1674-2907.2018.08.002
- Yu XR, Li JH, Lin XY, Luan DD. Association between delayed lactogenesis II and early milk volume among mothers of preterm infants. Asian Nurs Res. 2019;13(2):93–98. doi:10.1016/j.anr.2019.02.001
- Zhao JJ, Li YH, Yu M, et al. Maternal physical activity in the third trimester and delayed onset of lactogenesis. Chin Gene Pract. 2022;25(18):2268–2274. doi:10.12114/j.issn.1007-9572.2022.0070
- Ding J, Lian WN, Ma X. Construction and validation of risk prediction model for delayed onset of lactogenesis stage II following cesarean section. Chin J Perinl Med. 2022;25(9):661–669. doi:10.3760/cma.j.cn113903-20220427-00421
- Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92(3):574–584. doi:10.3945/ajcn.2010.29192
- Jiang YL, Gao RL, Chen JP, Zhou FJ, Chen YR. Incidence of delayed initiation of stage II lactation and related behavioral factors of mothers and infants. South China J Prevent Med. 2022;48(9):1058–1061.
- Zhang YY, Zhou H, Wang J, Zhang JH, Cai QM. Effects of pre-pregnancy body mass index, gestational weight gain and early feeding behavior on lactogenesis stage II: a prospective study. Chin J Perinl Med. 2022;25(7):504–512. doi:10.3760/cma.j.cn113903-20220301-00192
- Farah E, Barger M, Klima C, Rossman B, Hershberger P. Impaired lactation: review of delayed lactogenesis and insufficient lactation. J Midwifery Women’s Health. 2021;66(5):631–640. doi:10.1111/jmwh.13274
- Davis K, Drey N, Gould D. What are scoping studies? A review of the nursing literature. Int J Nurs Stud. 2009;46(10):1386–1400. doi:10.1016/j.ijnurstu.2009.02.010
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
- Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Implement. 2021;19(1):3–10. doi:10.1097/xeb.0000000000000277
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/m18-0850
- Zhang ZY, Liu J, Hu SS, Jiang PH, Liu M, Zhou Y. Correlation between pregnancy women with hypothyroidism and delayed lactation initiation. J Mod Med Health. 2022;38(15):2536–2539. doi:10.3969/j.issn.1009-5519.2022.15.004
- Xie XX, Zhao MH. Current status of delayed onset of lactogenesis II of high-risk pregnant women in maternal intensive care unit and its influence. J Nurs. 2021;28(7):49–52. doi:10.16460/j.issn1008-9969.2021.07.049
- Zhang MJ, Zhang Y, Bian YM, Wang LS. Survey and countermeasure research on the perinatal period and lactation status. Mater Child Health Car China. 2021;36(1):152–156. doi:10.19829/j.zgfybj.issn.1001-4411.2021.01.050
- Ding PP, Zhao M, Zhang FY, et al. Nutrients intake in the third trimester and associated factors of delayed onset of lactogenesis II in maternal women. Chin Gene Pract. 2020;23(5):534–539. doi:10.12114/j.issn.1007-9572.2020.00.105
- Wang SW, Guo NF, Jiang H. Correlations of pre-pregnancy body mass index and gestational weight gain with delayed onset of lactogenesis. Modern Clin Nurs. 2020;19(9):1–6. doi:10.3969/j.issn.1671-8283.2020.09.001
- Lin SH, Xu CY. Factors influencing delayed onset of lactation in elderly parturients. Pract Clin Med. 2019;20(9):92–94. doi:10.13764/j.cnki.lcsy.2019.09.034
- Teng ZM, Tan XX, Zhou YZ, Chen LF, Jin DE. Status of delayed onset of lactogenesis and its related factors among women of advanced reproductive age. J Nurs Sci. 2018;33(10):33–35. doi:10.3870/j.issn.1001-4152.2018.10.033
- Liao Y, Xu MY. Factors analysis of delayed lactation onset in gestational diabetes patients. Chin Nurs Res. 2022;25(18):3785–3788. doi:10.12102/j.issn.1009-6493.2018.23.036
- Si ML, Gu P, Zhang AX, Shi ZY. Risk factors of delayed onset of lactogenesis among puerpera with gestational diabetes mellitus. J Nurs Sci. 2017;32(8):19–21. doi:10.3969/j.issn.2096-2479.2018.25.150
- Su XY, You LM, Su YY. Investigation on postpartum lactating situation of women in the community and follow-up visit and guidance to nursing in the countryside. Nurs Pract Res. 2016;13(24):130–132. doi:10.3969/j.issn.1672-9676.2016.24.054
- Xue YC, Xu Q, Liu L, Liu ZH, Huang XM, Ma J. Dietary intake and factors influencing delayed onset of lactation among postpartum women in Guangzhou. South China J Prevent Med. 2015;41(7):218–223. doi:10.13217/j.scjpm.2015.0218
- Zhu P, Tao FB, Jiang XM, et al. Impact of stressful life event, weight gain during pregnancy and mode of delivery on the delayed onset of lactation in primiparas. J Hyg Res. 2010;39(4):478–482. doi:10.19813/j.cnki.weishengyanjiu.2010.04.023
- Scott JA, Binns CW, Oddy WH. Predictors of delayed onset of lactation. Mater Child Nutr. 2007;3(3):186–193. doi:10.1111/j.1740-8709.2007.00096.x
- Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. J Hum Lact. 2004;20(1):18–29. doi:10.1177/0890334403261345
- Grajeda R, rez-Escamilla RP. Stress during labor and delivery is associated with delayed onset of lactation among urban Guatemalan women. J Nutr. 2002;132(10):3055–3060. doi:10.1093/jn/131.10.3055
- Ilhan G, Atmaca FV, Çümen A, Zebitay AG, Güngör ES, Karasu AFG. Effects of daytime versus night-time cesarean deliveries on Stage II lactogenesis. J Obstet Gynaecol Res. 2018;44(4):717–722. doi:10.1111/jog.13562
- Xu Y, Teng P, Zhou Y, et al. Effect of epidural labor analgesia on neonatal lactation behavior and breastfeeding. Chin Med Herald. 2022;19(10):111–118.
- Yang XH, Yan SH, Wu H, Zhang F, Xie J. Analysis of influencing factors of newborn suction negative pressure and its clinical significance. Chongqing Med. 2019;48(16):2750–2754. doi:10.3969/j.issn.1671-8348.2019.16.012
- Fang T, Li JZ. Research progress on gestational weight management and its influencing factors. J Qiqihar UnivMed. 2018;39(16):1939–1942. doi:10.3969/j.issn.1002-1256.2018.16.035
- Bonifacino E, Schwartz EB, Jun H, Wessel CB, Corbelli JA. Effect of lactation on maternal hypertension: a systematic review. Breastfeed Med. 2018;13(9):578–588. doi:10.1089/bfm.2018.0108
- Demirci J, Schmella M, Glasser M, Bodnar L, Himes KP. Delayed Lactogenesis II and potential utility of antenatal milk expression in women developing late-onset preeclampsia: a case series. BMC Pregnancy Childbirth. 2018;18(1):68. doi:10.1186/s12884-018-1693-5
- Garrido-Gomez T, Quiñonero A, Dominguez F, Rubert L, Perales A, Simon KAHC. Preeclampsia: a defect in decidualization is associated with deficiency of Annexin A2. Am J Obstet Gynecol. 2020;222(4):376. doi:10.1016/j.ajog.2019.11.1250
- Ley SH, Chavarro JE, Li M, et al. Lactation duration and long-term risk for incident type 2 diabetes in women with a history of gestational diabetes mellitus. Diabetes Care. 2020;43(4):793–798. doi:10.2337/dc19-2237
- Panuganti PL, Hinkle SN, Rawal S, et al. Lactation duration and long-term thyroid function: a study among women with gestational diabetes. Nutrients. 2018;10(7):938. doi:10.3390/nu10070938
- Motil KJ, Thotathuchery M, Montandon CM, et al. Insulin, cortisol and thyroid hormones modulate maternal protein status and milk production and composition in humans. J Nutr. 1994;124(8):1248–1257. doi:10.1093/jn/124.8.1248
- Wang J, Wen D, Liu X, Liu Y. Impact of exercise on maternal gestational weight gain: an updated meta-analysis of randomized controlled trials. Medicine(Baltimore). 2019;98(27):e16199. doi:10.1097/md.0000000000016199
- Hamer M, Coombs N, Stamatakis E. Associations between objectively assessed and self-reported sedentary time with mental health in adults: an analysis of data from the health survey for England. BMJ Open. 2014;4(3):e004580. doi:10.1136/bmjopen-2013-004580
- Vargas-Terrones M, Barakat R, Santacruz B, Fernandez-Buhigas I, Mottola, MF. Physical exercise programme during pregnancy decreases perinatal depression risk: a randomised controlled trial. Br J Sports Med. 2019;53(6):348–353. doi:10.1136/bjsports-2017-098926
- Editorial Department of Clinical Research and Practice. Guidelines for promoting breastfeeding (2018 Edition). Clin Res Pra. 2018;3(13):201.