Abstract
Purpose
This study aims to investigate whether dexmedetomidine could prevent postoperative cognitive dysfunction and delirium in patients with lobectomy.
Patients and Methods
Patients with lung cancer who underwent thoracoscopic lobectomy under general anesthesia were enrolled in this study and divided into dexmedetomidine group or control group. Propensity-score match (PSM) was used to reduce the bias and imbalance of confounding variables. After PSM, 87 patients in each group were included. Primary outcomes were postoperative cognitive function and delirium. Secondary outcomes include plasma TNF-α, IL-6, and S100 β protein concentrations. Adverse events were also collected.
Results
There were no significant differences in the demographic characteristics and hemodynamic parameters between the two groups. Compared with the control group, the MoCA scores were significantly higher (P<0.01), while the incidence of delirium (P<0.01) and the plasma TNF-α (P<0.01), IL-6 (P<0.01), and S100 β protein (P<0.01) concentrations were significantly lower in the dexmedetomidine group at 7 days post-operatively. The incidences of adverse events were similar between the two groups.
Conclusion
Dexmedetomidine could prevent postoperative cognitive dysfunction and delirium in patients with lobectomy by decreasing neuroinflammation.
Introduction
Lung cancer has been the leading cause of death from cancer globally.Citation1 Approximately 870,982 new cases and 766,898 cancer-related deaths are estimated to occur in China in 2022.Citation2 Surgical resection is one of the main therapies for lung cancer, and pulmonary lobectomy is considered the preferred treatment with the best curative effects and prolonged survival.Citation3,Citation4
Postoperative cognitive dysfunction (POCD) and delirium are common conditions after surgery, expressed as a decline in cognitive function, memory, and orientation.Citation5 The incidence of POCD and delirium after thoracic surgery including pulmonary lobectomy has been estimated at 31.9% and 18.8%, respectively.Citation6 Delirium is a harbinger of POCD, usually occurs within the first 3 postoperative days; while POCD occurs at the end of the first week and has no effect on consciousness, and its duration may be significantly prolonged.Citation7 Currently, the pathogenesis of POCD and delirium is still unclear, some studies argued that surgery and/or anesthesia caused systemic inflammatory response may be a reason.Citation8,Citation9 Thus, it is important to select appropriate narcotic drugs to effectively control inflammation after pulmonary lobectomy and prevent the occurrence of POCD and delirium, especially in the elderly who are vulnerable to memory disturbances.
Dexmedetomidine is an α2-adrenoceptor agonist with sedative, anxiolytic, sympatholytic, and analgesic-sparing effects, and minimal depression of respiratory function.Citation10 Its use has been linked to reductions in postoperative delirium, stress, and inflammatory responses, resulting in improved nervous system protection.Citation11 Recent findings by Glumac et al showed that preoperative administration of corticosteroids ameliorates inflammatory response induced by surgery and thereby decreased the risk of early POCD after cardiac surgery.Citation12 Moreover, some other studies reported that the incidence of POCD and delirium caused by anesthesia was reduced in patients treated with dexamethasone.Citation13,Citation14 However, the effect of dexmedetomidine on cognitive function and delirium was rarely reported in lung cancer patients with lobectomy.
This study aims to evaluate whether dexmedetomidine could provide protection from inflammation in the central nervous system (CNS) and assess its effect on the risk of cognitive function and delirium in elderly patients with lobectomy. We hypothesized that cognitive dysfunction and incidence of delirium would be reduced in patients who received dexmedetomidine compared with those in the control group.
Materials and Methods
Patients
From January 2019 to Dec. 2021, medical records of patients aged 65 years or older who underwent lobectomy for lung cancer at the First Affiliated Hospital of Jinan University were retrospectively reviewed. All the enrolled patients were diagnosed with lung cancer after the pulmonary lobectomy and anesthetized with sevoflurane or sevoflurane combined with dexmedetomidine, and it was confirmed that there was no local and distant metastasis after the operation. Besides, all of them were American Society of Anesthesiologists (ASA) I–II patients and met the conditions of pulmonary lobectomy. Exclusion criteria includeCitation1 patients with cardiac insufficiency;Citation2 patients with other primary malignant tumors;Citation3 patients with abnormal blood coagulation or bone marrow function;Citation4 patients with liver or kidney insufficiency;Citation5 patients who changed to thoracotomy from thoracoscopic surgery due to massive hemorrhage;Citation6 patients with severe hearing or vision impairment;Citation7 patients with cognitive impairment (MoCA score<26 points);Citation8 patients with incomplete clinical data.
This study was approved by the Ethics Committee of The First Affiliated Hospital of Jinan University (approval No. 2,022,025).
Study Design
The included patients were divided into two groups according to the methods of anesthetization used: control group or dexmedetomidine group. All patients were fasted for 6–8 hours and forbidden to drink for 2–4 hours before surgery. The general hemodynamic parameters including arterial blood pressure, heart rate (HR), and internal jugular central venous pressure (CVP) were monitored in the operating room and recorded every 5 min.
In the dexmedetomidine group, dexmedetomidine (Yangzijiang Pharmaceutical Group Co, Ltd, China) was injected intravenously 12 minutes before induction of anesthesia with a loading amount of 1 ug/kg, and the loading dose was followed by a continuous intravenous infusion at a rate of 0.5 μg/kg/h. In both groups, anesthesia was induced using a single slow intravenous injection of midazolam (2–3 mg), etomidate (0.3 mg/kg), and an infusion of sufentanil (0.4 μg/kg). Anesthesia was maintained by 1 minimum alveolar concentration (MAC) sevoflurane (Yangzijiang Pharmaceutical Group Co, Ltd, China) in both the dexmedetomidine group and the control group. Maintenance of anesthesia was supplemented by an intravenous infusion of remifentanil (commenced at 0.15 μg/kg/min and titrated according to clinical need). The sevoflurane was titrated based on hemodynamic change (HR and systolic arterial blood pressure), BIS (50–60), and somatic (swallowing and movement) and autonomic signs (flushing, sweating, and salivating) until symptoms were resolved. Sevoflurane was discontinued at the start of suturing. Epidural anesthesia was not used as it was reported to reduce the incidence of POCD and delirium in several studies and may cause bias to our study.Citation15,Citation16
Propensity-score match (PSM) was used to reduce the bias and imbalance of confounding variables. A 1:1 greedy match was performed based on a caliper width of 0.2 for propensity score.
Primary and Secondary Outcomes
Primary outcomes were postoperative cognitive function and delirium. Montreal cognitive assessment (MoCA) was used to assess the patient’s cognitive function on the day before the operation and the 1st, 3rd, and 7th days after the operation. MoCA consists of the executive function of visual space, language ability, attention and calculation, delayed recall, and abstract thinking, which has been reported to be suitable for assessing mild cognitive dysfunction. Delirium assessment was performed on the 1st, 3rd, and 7th days after the operation, twice a day (08:00 and 20:00), using the 3D-CAM by our trained study staff.
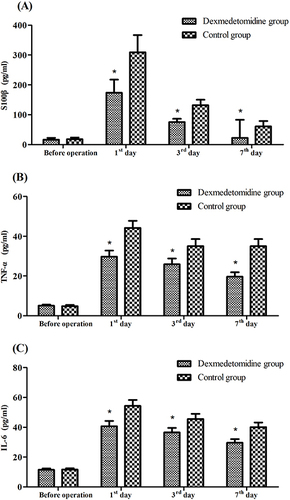
Secondary outcomes were IL-6, TNF-α, and S100 β protein concentrations. Blood was collected from the patients at different time points, 10 min before anesthesia and the 1st, 3rd, and 7th days after the operation, and plasma was prepared by centrifugation at 4000 ×g for 20 min at 4°C and stored at −80°C until use. IL-6, TNF-α, and S100 β protein concentrations were detected using an ELISA kit following the manufacturer’s instructions and calculated using the standard curve provided with the kit.
Statistical Analysis
Statistical power was determined on the primary outcome of postoperative cognitive function. It was determined that 87 patients in each group could provide a power of >90% with an alpha of 5% to determine a difference of 1 point in the MoCA score between the two groups.
Statistical analysis was performed using the SPSS 20.0 for Windows (SPSS, IBM, USA). All data were expressed as means ± SDs. Significant differences were assessed using Student’s t-tests for continuous data and Chi-square tests for categorical data. Repeated measurement data were analyzed by repeated measures one-way analysis of variance (ANOVA), followed by post-hoc analyses. P<0.05 was considered statistically significant.
Results
Participants’ Demographic Characteristics and Hemodynamic Parameters
Among 543 older patients who underwent lobectomy for lung cancer, 298 patients (211 in the control group and 87 in the dexmedetomidine group) met the inclusion and exclusion criteria. After PSM, 87 patients in each group were included.
There were no significant differences between the two groups in the baseline characteristics including age, gender, body mass index (BMI), education level, ASA grade, histology, and operation and anesthesia time (). As shown in , the differences in hemodynamic parameters including mean arterial pressure (MAP), HR, and CVP were not significant between the two groups at baseline and after regaining consciousness.
Table 1 Baseline Characteristics in Both Groups
Table 2 Comparison of Hemodynamic Parameters at Baseline and After Regaining Consciousness Between Two Groups
Comparison of Cognitive Function and Delirium
Repeated measure ANOVA showed that after controlling for baseline characteristics as covariates, both between- and within-group effects were significant (both P<0.01), and the interaction between time and treatments was also significant (P<0.01) in the MoCA (), indicating that anesthesia significantly increased the risk of POCD in elderly patients, and dexmedetomidine led to improved cognitive functions. Post-hoc analysis showed that the differences between the two groups in the MoCA were not significant before operation, and significant (P<0.01) on the 1st day, 3rd day, and 7th day.
Figure 1 Comparison of (A) Montreal Cognitive Assessment (MoCA) scores and (B) incidence of delirium between the two groups. * P<0.05 compared with control group.
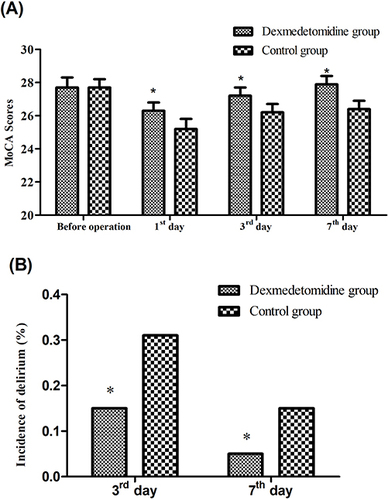
The incidences of delirium in the dexmedetomidine group were significantly lower than those in the control group on the 3rd day (P=0.02) and 7th day (P<0.01) ().
Comparison of Plasma S100β, TNF-α, and IL-6 Concentrations
Repeated measure ANOVA showed that after controlling for baseline characteristics as covariates, both between- and within-group effects were significant (both P<0.01), and the interaction between time and treatments was also significant (P<0.01) in the plasma S100β, TNF-α, and IL-6 concentrations (), indicating that anesthesia significantly increased the release of inflammatory cytokines into the systemic circulation in elderly patients, and dexmedetomidine may be an effective way to alleviate their release. Post-hoc analysis showed that the differences between the two groups in the plasma S100β, TNF-α, and IL-6 concentrations were not significant before operation, and significant (P<0.01) on the 1st day, 3rd day, and 7th day.
Comparison of Adverse Events
Although several adverse events were documented in both groups, no significant differences were detected between the two groups ().
Table 3 Comparison of Adverse Events Between Two Groups
Discussion
In the present study, we observed that sevoflurane anesthesia in the control group induced increases in the expression of neuroinflammatory factors and risk of POCD and delirium, and dexmedetomidine could alleviate neuroinflammation and prevent the risk of cognitive dysfunctions and delirium.
Anesthesia and surgery are shown to be associated with a modest acceleration in the rate of cognitive decline and delirium in older patients.Citation17 Neuroinflammation is common after surgery, which often brings secondary damages including delirium, Alzheimer’s disease, and cognitive dysfunction.Citation18,Citation19 An excessive inflammatory response could result in severe tissue damage and even death.Citation20 Inhalational anesthetics like sevoflurane and midazolam can trigger pathological inflammatory responses during surgery. Several preclinical trialsCitation14,Citation21,Citation22 have reported that sevoflurane could increase the levels of IL-6, IL-8, and TNF-α in the cortex and hippocampus of rats and cause a decline in learning and memory. Recent clinical studies have reported that sevoflurane exposure may have harmful effects on cognitive function in humans.Citation23 In addition, midazolam, the most abundantly used benzodiazepine in anesthesia, is also reported to be associated with postoperative complications such as cognitive impairment and delirium.Citation24
Dexmedetomidine is an α2-adrenoreceptor agonist that can inhibit the inflammatory and stress response, reduce neuronal toxicity and apoptosis, and promote brain protection through synapse formation and neurotrophic nutrition.Citation25 A preclinical study in miceCitation21 reported that dexmedetomidine exerted a neuroprotective effect against sevoflurane‑induced apoptosis, inflammation, oxidative stress, and neurocognitive impairment, which may be mediated by α2 adrenoceptors. Zeng et alCitation26 showed that in in diabetic rats, dexmedetomidine can affect proinflammatory factor expression and reduce the inflammatory response in blood vessels by activating the corresponding signaling pathways. Moreover, some clinical studies in pulmonary surgery and cancers reported the neuroprotective effects of dexmedetomidine. A recent study conducted by Liu et al indicated that in older patients undergoing pulmonary surgery, dexmedetomidine significantly increased the MoCA scores (27.1±0.79 vs 26.6±0.80) compared with placebo.Citation9 In addition, among 120 elderly patients with esophageal carcinoma, dexmedetomidine alleviated POCD through decreasing plasma TNF‑α and IL‑6 concentrations.Citation13 In line with these findings, the present study demonstrated that dexmedetomidine significantly improved cognitive functions and decreased the risk of delirium, and suppressed the release of plasma S100β, TNF-α, and IL-6 in elderly patients with lobectomy.
This study has some limitations. First, as it’s a retrospective study, we could only analyze data from existing patient records, other confounding factors that were not collected may also influence the outcomes. For instance, as risk factors for cognitive impairment, impairments in hearing and vision have an impact on perioperative complications in the elderly.Citation27 We excluded patients with severe impairments in hearing or vision via reviewing medical records, but the hearing and vision abilities were not assessed by screening tools for the included population, which may bring information bias. Moreover, though MoCA has been validated as a highly sensitive tool for mild cognitive impairment in hundreds of studies, a sound assessment of cognitive impairment may require a battery of neurocognitive tests (eg Wechsler Memory Scale–III Word List Learning subtest, the Wechsler Adult Intelligence Scale–III Block Design and Digit Symbol–Coding subtests) and other screening tests (eg Mini-Cog).Citation28 The effect of dexmedetomidine on cognitive functions should be further confirmed in further prospective studies with more screening tools.
Conclusion
In conclusion, Dexmedetomidine could prevent POCD and delirium in patients with lobectomy with the decrease in plasma S100β, TNF-α, and IL-6 concentrations.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of The First Affiliated Hospital of Jinan University (approval No. 2022025). All procedures involving human participants were performed by the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. As it is a retrospective study, informed consent was waived by the Ethic Committee of The First Affiliated Hospital of Jinan University. All data were fully anonymized and kept confidentially.
Author Contributions
Chaojun Tang and Yalan Li conceived the study idea and designed the study; Yong Lai acquired the data and performed the data analysis; Chaojun Tang drafted the manuscript; Yong Lai and Yalan Li substantially revised the article. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare in this work.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available as it could compromise the privacy of research participants, but are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. PubMed PMID: 33433946. doi:10.3322/caac.21654
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. PubMed PMID: 35143424; PubMed Central PMCID: PMCPMC8920425. doi:10.1097/CM9.0000000000002108
- Berfield KS, Farjah F, Mulligan MS. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer. Ann Thorac Surg. 2019;107(2):893–899. PubMed PMID: 30278164. doi:10.1007/s40262-017-0507-7
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg. 2016;5(2):76–84. PubMed PMID: 27134832; PubMed Central PMCID: PMCPMC4827401. doi:10.21037/acs.2016.03.17
- Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. PubMed PMID: 9525362. doi:10.1016/s0140-6736(97)07382-0
- Tong C, Huang C, Wu J, Xu M, Cao H. The prevalence and impact of undiagnosed mild cognitive impairment in elderly patients undergoing thoracic surgery: a prospective cohort study. J Cardiothorac Vasc Anesth. 2020;34(9):2413–2418. PubMed PMID: 32381306. doi:10.1053/j.jvca.2020.03.011
- Glumac S, Kardum G, Karanovic N. Postoperative cognitive decline after cardiac surgery: a narrative review of current knowledge in 2019. Med Sci Monit. 2019;25:3262–3270. PubMed PMID: 31048667; PubMed Central PMCID: PMCPMC6511113. doi:10.12659/MSM.914435
- Olotu C. Postoperative neurocognitive disorders. Curr Opin Anaesthes. 2020;33(1):101–108. PubMed PMID: 31764008. doi:10.1097/ACO.0000000000000812
- Liu T, Liu FC, Xia Y, et al. Effect of dexmedetomidine on the Montreal cognitive assessment in older patients undergoing pulmonary surgery. J Int Med Res. 2022;50(9):3000605221123680. PubMed PMID: 36151758; PubMed Central PMCID: PMCPMC9513575. doi:10.1177/03000605221123680
- Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. PubMed PMID: 28105598; PubMed Central PMCID: PMCPMC5511603. doi:10.1007/s40262-017-0507-7
- Yang S, Lee H. A dose-finding study of preoperative intravenous dexmedetomidine in children’s emergence delirium after epiblepharon surgery. Eur J Ophthalmol. 2014;24(3):417–423. PubMed PMID: 24338578. doi:10.5301/ejo.5000396
- Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol. 2017;34(11):776–784. PubMed PMID: 28985195. doi:10.1097/EJA.0000000000000647
- Zhang H, Wu Z, Zhao X, Qiao Y. Role of dexmedetomidine in reducing the incidence of postoperative cognitive dysfunction caused by sevoflurane inhalation anesthesia in elderly patients with esophageal carcinoma. J Cancer Res Ther. 2018;14(7):1497–1502. PubMed PMID: 30589029. doi:10.4103/jcrt.JCRT_164_18
- Wang N, Wang M. Dexmedetomidine suppresses sevoflurane anesthesia-induced neuroinflammation through activation of the PI3K/Akt/mTOR pathway. BMC Anesthesiol. 2019;19(1):134. PubMed PMID: 31351473; PubMed Central PMCID: PMCPMC6661092. doi:10.1186/s12871-019-0808-5
- Mandal S, Basu M, Kirtania J, et al. Impact of general versus epidural anesthesia on early post-operative cognitive dysfunction following Hip and knee surgery. J Emerg Trauma Shock. 2011;4(1):23–28. PubMed PMID: 21633563; PubMed Central PMCID: PMCPMC3097574. doi:10.4103/0974-2700.76829
- Anwer HM, Swelem SE, el-Sheshai A, Moustafa AA. Postoperative cognitive dysfunction in adult and elderly patients--general anesthesia vs subarachnoid or epidural analgesia. Mid East J Anaesthes. 2006;18(6):1123–1138. PubMed PMID: 17263267.
- Vacas S, Cole DJ, Cannesson M. Cognitive Decline Associated With Anesthesia and Surgery in Older Patients. JAMA. 2021. PubMed PMID: 34338712; PubMed Central PMCID: PMCPMC8807795. doi:10.1001/jama.2021.4773
- Umholtz M, Nader ND. Anesthetic Immunomodulation of the Neuroinflammation in Postoperative Cognitive Dysfunction. Immunol Invest. 2017;46(8):805–815. PubMed PMID: 29058541. doi:10.1080/08820139.2017.1373898
- Cortese GP, Burger C. Neuroinflammatory challenges compromise neuronal function in the aging brain: postoperative cognitive delirium and Alzheimer’s disease. Behav Brain Res. 2017;322(Pt B):269–279. PubMed PMID: 27544872; PubMed Central PMCID: PMCPMC5450823. doi:10.1016/j.bbr.2016.08.027
- Pol RA, Van Leeuwen BL, Izaks GJ, et al. C-reactive protein predicts postoperative delirium following vascular surgery. Ann Vasc Surg. 2014;28(8):1923–1930. PubMed PMID: 25017770. doi:10.1016/j.avsg.2014.07.004
- Zhang Y, Li M, Cui E, et al. Dexmedetomidine attenuates sevoflurane induced neurocognitive impairment through alpha adrenoceptor. Mol Med Rep. 2021;23(1). PubMed PMID: 33179100; PubMed Central PMCID: PMCPMC7684862. doi:10.3892/mmr.2020.11676
- Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. PubMed PMID: 9872743. doi:10.1126/science.283.5398.70
- Huang X, Ying J, Yang D, et al. The Mechanisms of Sevoflurane-Induced Neuroinflammation. Front Aging Neurosci. 2021;13:717745. PubMed PMID: 34421578; PubMed Central PMCID: PMCPMC8375153. doi:10.3389/fnagi.2021.717745
- Rump K, Holtkamp C, Bergmann L, et al. Midazolam impacts acetyl-And butyrylcholinesterase genes: an epigenetic explanation for postoperative delirium? PLoS One. 2022;17(7):e0271119. PubMed PMID: 35802656; PubMed Central PMCID: PMCPMC9269431. doi:10.1371/journal.pone.0271119
- Zhou M, Lyu Y, Zhu Y, et al. Effect of ulinastatin combined with dexmedetomidine on postoperative cognitive dysfunction in patients who underwent cardiac surgery. Front Neurol. 2019;10:1293. PubMed PMID: 31920917; PubMed Central PMCID: PMCPMC6930879. doi:10.3389/fneur.2019.01293
- Zeng X, Wang H, Xing X, Wang Q, Li W, Ahmad M. Dexmedetomidine protects against transient global cerebral ischemia/reperfusion induced oxidative stress and inflammation in diabetic rats. PLoS One. 2016;11(3):e0151620. PubMed PMID: 26982373; PubMed Central PMCID: PMCPMC4794239. doi:10.1371/journal.pone.0151620
- Gan S, Yu Y, Wu J, et al. Preoperative assessment of cognitive function and risk assessment of cognitive impairment in elderly patients with orthopedics: a cross-sectional study. BMC Anesthesiol. 2020;20(1):189. PubMed PMID: 32738902; PubMed Central PMCID: PMCPMC7395982. doi:10.1186/s12871-020-01096-6
- Drew DA, Tighiouart H, Rollins J, et al. Evaluation of Screening Tests for Cognitive Impairment in Patients Receiving Maintenance Hemodialysis. J Am Soc Nephrol. 2020;31(4):855–864. PubMed PMID: 32132197; PubMed Central PMCID: PMCPMC7191919. doi:10.1681/ASN.2019100988