Abstract
Background
Emerging evidence suggests that systemic inflammatory and nutritional biomarkers, along with derived indices, could serve as predictors for sarcopenia in cancer population. This study aimed to compare these predictors, focusing on the nutritional risk index (NRI) and evaluate its diagnostic value, for sarcopenic patients without cancer.
Methods
This cross-sectional retrospective study included 1674 participants. Sarcopenia is defined by skeletal muscle mass index (SMI). Laboratory data reflected the values of systemic inflammatory and nutritional biomarkers, from which the derived indices were calculated. Multiple logistic regression analysis, ROC curve analysis, and the Youden index were utilized to assess the association between these markers and sarcopenia and determine the cutoff value for predicting sarcopenia.
Results
Among all participants (1110 men and 564 women, mean age 61.97 ± 9.83 years), 398 individuals were diagnosed with sarcopenia, indicating a prevalence of 23.78% in China’s middle-aged and elderly population without cancer. Logistic regression analysis revealed significant associations between all biomarkers and derived indices with sarcopenia. Following adjustment for potential confounders, lower NRI values were significantly associated with a higher incidence of sarcopenia. For sarcopenia diagnosis, the area under the curve (AUC) for NRI was 0.769 ([95% CI, 0.742, 0.796], P < 0.001), with a cutoff value of 106.016, sensitivity of 75.6% and specificity of 66.1%. NRI demonstrated greater predictive advantage for sarcopenia incidence in men compared to women.
Conclusion
A lower NRI value was associated with a higher prevalence of sarcopenia. NRI shows promise for early, rapid, and effective sarcopenia screening, particularly in China’s middle-aged and elderly male population without cancer.
Introduction
Sarcopenia is a chronic, progressive, and systemic disease affecting skeletal muscle, primarily characterized by the loss of skeletal muscle mass (SMM), strength, and function. This condition significantly increases the risk of adverse outcomes associated with falls, functional decline, frailty, and mortality.Citation1 Individuals with sarcopenia experience a notably lower health-related quality of life (HRQoL) compared to those without the condition.Citation2 The prevalence of sarcopenia has escalated in tandem with the aging population, with pooled estimates ranging from 9.9% to 40.4%.Citation3 A previous study of ours confirmed a sarcopenia prevalence of 15.34% among patients undergoing health check-ups.Citation4 The molecular mechanisms underlying sarcopenia are multifactorial and include causes such as inflammation, mitochondrial dysfunction, and imbalances in protein metabolism.Citation5,Citation6 While the exact molecular pathways remain unclear, inflammation and inflammatory cytokines (CRP, IL-6, TNF-α) are believed to play a crucial role in the progression of sarcopenia.Citation7–9
Inflammation, a major clinical characteristic, is shared by sarcopenia and cachexia in cancer, alongside the loss of muscle mass, strength, and function.Citation10 Cancer patients are particularly susceptible to developing sarcopenia due to inflammation, leading to a higher incidence of postoperative complications and poorer overall survival. Inflammatory and nutritional biomarkers have shown predictive value in this population. For instance, a higher platelet-to lymphocyte-ratio (PLR) is associated with a 2.36 times greater risk of sarcopenia in the elderly.Citation11 PLR and neutrophil-to-lymphocyte ratio (NLR) have also been positively correlated with sarcopenia in urologic carcinoma patients.Citation12,Citation13 Additionally, the lymphocyte-to-monocyte ratio (LMR) has been linked to a poor prognosis in non-metastatic breast cancer patients.Citation14 Studies have shown that the systemic immune inflammation index (SII) is positively correlated with sarcopenia and sarcopenic obesity, serving as an independent prognostic indicator for poor prognosis in gastric cancer patients.Citation15,Citation16 Similarly, higher systemic inflammatory response index (SIRI) values are closely associated not only with sarcopenia in older adults but also associated with psoas muscle index (PMI) in patients with hepatocellular carcinoma (HCC).Citation17,Citation18 Other indices such as the pan-immune-inflammation value (PIV), advanced lung cancer inflammation index (ALI), prognostic nutritional index (PNI), and nutritional risk index (NRI) or geriatric nutritional risk index (GNRI) have shown correlations with sarcopenia and have been used to predict the overall survival or prognosis of patients with carcinoma.Citation19–23
Inflammatory and nutritional states can be influenced by sarcopenia, and alterations in the microenvironment can further exacerbate sarcopenia. Numerous studies have demonstrated an association between sarcopenia and systemic inflammatory and nutritional biomarkers. Currently, sarcopenia is typically diagnosed using dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA) to measure skeletal muscle mass index (SMI). However, blood biomarkers for predicting low SMI or sarcopenia are not yet utilized in clinical practice. Among the common risk factors associated with sarcopenia, nutritional status has been repeatedly validated in the development of sarcopenia.Citation24 The NRI has been identified as a surrogate indicator of nutritional status in older adults.Citation25 The correlation between the index for judging body nutrition and SMM and sarcopenia is continuously being explored, although studies on this topic are relatively scarce. Therefore, it is particularly important to clarify the ability of the NRI to effectively and rapidly screen for sarcopenia in the middle-aged and elderly population in China without cancer.
Materials and Methods
Study Population
In this retrospective analysis, clinical data were collected from electronic medical records at the Department of General Practice, Wuhan Union Hospital (n = 2385) covering November 2021 through September 2023. Exclusion criteria included insufficient information on clinical and laboratory tests, acute infection, acute coronary syndrome and stroke, active rheumatic immune disease, hormone therapy, and hemodialysis for renal failure. Baseline data comprised 1739 inpatients aged 50 or older.Citation1 All participants underwent sarcopenia assessment and biochemical tests. Those diagnosed with cancer (n = 65) were excluded resulting in the inclusion of all remaining participants (n = 1674) in the study.
Definition of Sarcopenia
Due to the cross-sectional study design, data are available only for SMM and other body composition parameters, including height, body weight, BMI, and SMI (SMM (kg)/Height (m2)). Body composition analysis instruments (Inbody 270, South Korea). According to the Asian Working Group for Sarcopenia’s recommended threshold values, SMI less than 7.0 kg/m2 in men and less than 5.7 kg/m2 in women are classified as sarcopenia.Citation26
Biochemical Tests
Basic demographic data, including age, gender, smoking, alcohol consumption, and history of chronic diseases, were provided by all participants. Blood samples were collected from each participant for the first time following an overnight fast during hospitalization. Biochemical tests included albumin levels and a full blood count, comprising white blood cell (WBC), neutrophil, platelet, lymphocyte, monocyte and hemoglobin counts.
Derived Inflammatory and Nutritional Biomarkers
The derived inflammatory and nutritional biomarkers were calculated, respectively, as follows: PLR = platelet count/lymphocyte count, NLR = neutrophil count/lymphocyte count, LMR = lymphocyte count/monocyte count, SII = neutrophil count × platelet count/lymphocyte count,Citation27 SIRI = neutrophil count × monocyte count/lymphocyte count,Citation28 PIV = platelet count × neutrophil count × monocyte count/lymphocyte count,Citation29 ALI = BMI × albumin (g/dl)/NLR,Citation30 PNI = 10 × albumin (g/dl) + 0.005 × lymphocyte (/μL),Citation31 NRI = 1.519 × albumin (g/l) + 0.417 × (body weight/ideal weight × 100).Citation22 For the convenience of calculating NRI in the middle-aged and elderly population, ideal weight was adjusted as height (cm) - 105.
Statistical Analysis
In this study, all analyses were conducted using IBM SPSS Statistics software (version 25.0). The baseline data of the study population were divided into two groups (sarcopenic and non-sarcopenic group) based on sarcopenia status. Since the ratio of missing data to the total data for continuous variables was less than 5%, calculations were performed using the expectation-maximization method. To compare baseline data between the two groups, t-tests were used for continuous variables (mean ± SD), and chi-square tests were used for categorical variables (number or percentage (%). Logistic regression analysis was employed to estimate the odds ratio (OR) and 95% confidence interval (CI) of sarcopenia incidence based on baseline characteristics, albumin, full blood count, and derived inflammatory and nutritional biomarkers. Model 1 was adjusted for age and sex, while Model 2 included additional adjustments for chronic diseases (hypertension, COPD, hyperuricemia, fatty liver). The cutoff value was obtained by ROC curve and calculating the Youden index (sensitivity + specificity − 1). Considering that the threshold values of SMI from the Asian Working Group for Sarcopenia (AWGS2019) were differed by gender, the separate ROC curves were generated for the middle-aged and elderly male and female populations without cancer, respectively. All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Characteristics of the Study Population
As shown in and , our study included 1674 participants (1110 men and 564 women), and the prevalence of sarcopenia was 23.78% (398 participants) in the middle-aged and elderly population without cancer. A comparison of baseline data and biochemical test results between the sarcopenic and non-sarcopenic groups revealed several significant differences. The sarcopenic group, in contrast to the non-sarcopenic group, was characterized by older age (66.29 vs 60.62 years) and was with significant differences in smoking, alcohol consumption, and various chronic diseases. Additionally, the sarcopenic group exhibited significantly lower body weight, BMI, and the values of albumin, hemoglobin, lymphocytes, LMR, ALI, PNI, and NRI (all P < 0.01), along with significantly higher age, PLR, NLR, SII, SIRI, and PIV (all P < 0.01). However, there were no significant differences in WBC, neutrophil, platelet, or monocyte counts between the two groups.
Table 1 Characteristics of the Study Population for Different Groups (n = 1674)
Associations Between Derived Inflammatory and Nutritional Biomarkers and Sarcopenia Based on Logistic Regression Analysis
To investigate the association between derived inflammatory and nutritional biomarkers and sarcopenia, logistic regression models unadjusted, adjusted Model 1 (age and sex) and adjusted Model 2 (model 1 + chronic diseases: hypertension, COPD, hyperuricemia, and fatty liver) were utilized. In the unadjusted logistic regression model (), it was observed that the middle-aged and elderly population with higher values of PLR, NLR, SII, SIRI and PIV had a higher incidence of sarcopenia (P < 0.01). Conversely, individuals with lower values of LMR, ALI, PNI and NRI also had a higher incidence of sarcopenia (P < 0.01). Furthermore, these participants also exhibited lower values of lymphocytes compared to the non-sarcopenic group (P < 0.001). After adjusting for confounders in Model 1 and Model 2, higher values of PLR (P < 0.01), NLR (P < 0.01), SII (P < 0.01), SIRI (P < 0.01), PIV (P < 0.01), as well as lower values of LMR (P < 0.01), ALI (P < 0.001), and NRI (P < 0.001), remained correlated with the odds ratios (ORs) for the prevalence of sarcopenia. Notably, only in Model 2, the result of PNI was not statistically significant between the sarcopenia and non-sarcopenia groups. Across all models, the results indicated that the middle-aged and elderly population with lower values of hemoglobin had a higher prevalence of sarcopenia.
Table 2 Associations Between Derived Inflammatory and Nutritional Biomarkers and Sarcopenia According to Unadjusted and Adjusted Logistic Regression Models (n = 1674)
ROC Curves for Derived Inflammatory and Nutritional Biomarkers and Sarcopenia
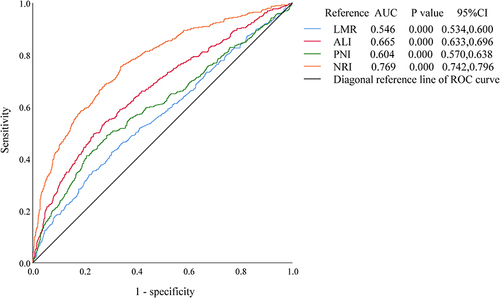
To evaluate their suitability as predictors for sarcopenia diagnosis, the values of derived inflammatory and nutritional biomarkers were plotted into the ROC curves. And in and , the Youden index was utilized to determine the diagnostic thresholds. and , illustrate the ROC curves predicting sarcopenia incidence based on higher or lower values of these biomarkers in all participants. Among all the biomarkers, only the NRI had an area under the curve (AUC) exceeding 0.7 (0.769, [95% CI, 0.742, 0.796], P < 0.001). The optimal NRI cutoff value, corresponding to the maximum Youden index (−0.583), with sensitivity of 75.6% and specificity of 66.1%, was determined to be 106.016.
Table 3 The AUC of ROC Curves for Derived Inflammatory and Nutritional Biomarkers
Table 4 Diagnostic Values of NRI to Predict the Incidence of Sarcopenia in Men and Women
Figure 2 ROC curves of derived inflammatory and nutritional biomarkers with higher values to predict the incidence of sarcopenia.
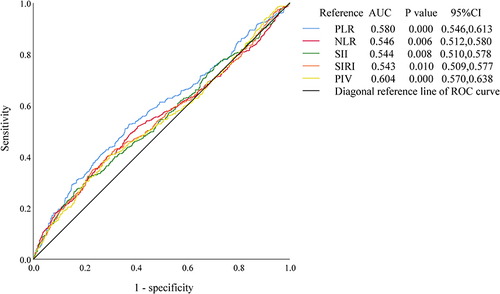
Figure 3 ROC curves of derived inflammatory and nutritional biomarkers with lower values to predict the incidence of sarcopenia.
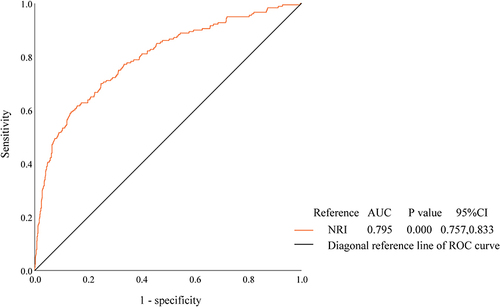
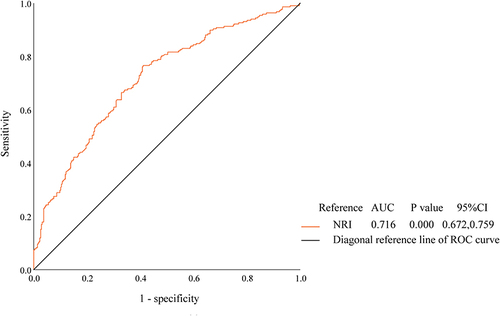
Given that the SMI threshold values from AWGS2019 differ by gender, ROC curves were plotted for the middle-aged and elderly populations of males and females without cancer, respectively. and , indicate that the diagnostic efficacy of NRI for sarcopenia appears to be more significant in the middle-aged and elderly males than females, with AUCs of 0.795 ([95% CI, 0.757, 0.833], P < 0.001) and 0.716 ([95% CI, 0.672, 0.759], P < 0.001), respectively. Meanwhile the cutoff value for NRI in men was 102.3415 corresponding to the maximum Youden index (−0.541, with sensitivity 61.7% and specificity 84.2%) and in women was 106.24 corresponding to the maximum Youden index (−0.644, with sensitivity 76.6% and specificity 59.0%).
Discussions
In our study, the associations between the values of derived inflammatory and nutritional biomarkers and sarcopenia in the hospitalized middle-aged and elderly, population without cancer in central China were investigated. We found significant differences in hemoglobin and lymphocyte counts between the sarcopenic and non-sarcopenic groups, while there were no significant differences in full blood count (WBC, neutrophil, platelet, and monocyte counts). Higher values of PLR, NLR, SII, SIRI, and PIV, along with lower values of LMR, ALI, and NRI suggest low-grade systemic inflammation and a high malnutrition risk in sarcopenia. However, the PNI was not statistically significant in either the sarcopenic group or non-sarcopenic group in Model 2 of logistic regression analysis. Previous studies have shown that hemoglobin levels are independently related to sarcopenia, with a higher prevalence of low hemoglobin in sarcopenia patients.Citation32–34 Reduced muscle mass in sarcopenia patients may be attributed to decreased lymphocyte activity on muscle satellite cells.Citation35 Studies have also indicated that higher values of PLR, NLR, SII, and SIRI, and lower LMR values are closely associated with higher prevalence of sarcopenia in populations aged 50 or older in China, as well as an increased SII value in a large population aged 20 years and over is associated with a higher risk of low muscle mass in the United States.Citation17,Citation36,Citation37
Our findings align with these previous studies; however, different diagnostic criteria for sarcopenia, may lead to different conclusions. For example, PLR, NLR, and LMR values were not associated with the risk of sarcopenia presence in Chinese community-dwelling older people.Citation38 To identify a biomarker that can effectively and efficiently screen for sarcopenia in the middle-aged and elderly population, we introduced inflammatory and nutritional biomarkers (PIV, ALI, PNI, and NRI/GNRI) that are widely used to evaluate therapeutic outcomes and predict prognosis in cancer patients with sarcopenia.Citation20,Citation22,Citation23,Citation39,Citation40 NRI, which combines albumin and weight loss, has been commonly utilized as an index of malnutrition in hospitalized adults. Compared to previous studies, our study provides a more comprehensive and scientifically rigorous analysis due to the relatively larger sample size and inclusion of indexes. Our main finding suggests that lower values of NRI could be an effective strategy in the screening and management of sarcopenia.
Numerous studies have demonstrated the importance of systemic inflammation in the pathogenesis of sarcopenia, osteoporosis, and obesity, collectively termed osteosarcopenic obesity (OSO) components.Citation17,Citation41 Systemic inflammatory cytokines contribute to an imbalance between muscle protein synthesis and catabolism breakdown, impacting multiple molecular pathways.Citation1,Citation7,Citation42 Recent interventions targeting sarcopenia have been shown to reduce levels of CRP, IL-6 and TNF-α.Citation9 In addition, the GNRI has been found to correlate significantly with SMI and overall survival in the middle-aged and elderly cancer patients with sarcopenia.Citation23,Citation40,Citation43 Similarly, the NRI has been identified as an independent predictor of sarcopenia presence and survival in cancer and surgery patients, suggesting it could serve as an alternative means of malnutrition assessment for populations with sarcopenia.Citation22,Citation44 Our study confirmed NRI as a clinical indicator for independently predicting sarcopenia incidence, with a good predictive value, especially in middle-aged and elderly males. Compared to other derived inflammatory and nutritional biomarkers, NRI appears to be more promising for further study.
China, with the world’s largest elderly population, is transitioning into an aging society.Citation45 The gradual loss of SMM starting around the age of 50 underscores the significance of sarcopenia as a health concern that cannot be overlooked.Citation1 Our study, focusing on sarcopenic populations, offers insights into promoting “healthy aging”, crucial for China in addressing the challenges of aging. The study enhances understanding of the relationship between inflammatory and nutritional biomarkers and sarcopenia, highlighting anti-inflammatory therapy and nutritional supplementation as potential preventive measures for middle-aged and elderly individuals with low muscle mass or sarcopenia. However, several limitations should be considered when interpreting our results: 1) Lack of baseline data on grip strength, CRP, IL-6, TNF-α; 2) The cross-sectional study design precludes establishing causality between these markers and sarcopenia; and 3) Further investigation is warranted on the role of inflammatory and nutritional biomarkers in OSO evaluation.
Conclusions
In conclusion, elevated levels of PLR, NLR, SII, SIRI, and PIV, as well as reduced levels of LMR, ALI, and NRI are closely associated with sarcopenia incidence in China’s middle-aged and elderly population. NRI, compared to BIA or DXA, demonstrates early, rapid, and effective sarcopenia screening capabilities, as evidenced in the middle-aged and elderly population without cancer in China. Its discriminative ability is more pronounced in men than in women. These results support the use of NRI monitoring in clinical practice to screen and identify individuals at high risk of sarcopenia, requiring management and follow-up. Furthermore, health education and appropriate interventions promoting patient self-management to maintain normal weight and nutritional status (albumin) can help reduce the risk of sarcopenia. Future high-quality, prospective, cohort studies focusing on inflammatory and nutritional biomarkers, particularly NRI, may provide deeper insights into sarcopenia and offer new avenues for therapeutic intervention to improve sarcopenia outcomes.
Ethics Approval and Consent to Participate
The study fully complied with the Declaration of Helsinki and was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (S045). The ethics committee of Tongji Medical College, Huazhong University of Science and Technology approved the request for a waiver of informed consent. Informed consent was not required because all medical data were retrospectively reviewed and analysed anonymously. The participants’ information was kept anonymous, was noninvasive, and will not cause harm to them.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Acknowledgments
We thank all the participants for their contributions to the study.
Data Sharing Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Additional information
Funding
References
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi:10.1016/s0140-6736(19)31138-9
- Beaudart C, Demonceau C, Reginster JY, et al. Sarcopenia and health-related quality of life: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(3):1228–1243. doi:10.1002/jcsm.13243
- Mayhew AJ, Amog K, Phillips S, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56. doi:10.1093/ageing/afy106
- Nie G, Wan J, Jiang L, Zhang M, Yan F, Peng W. Association of hyperuricemia combined with sarcopenia on ASCVD risk. BMC Cardiovasc Disord. 2023;23(1):325. doi:10.1186/s12872-023-03336-2
- Wiedmer P, Jung T, Castro JP, et al. Sarcopenia - Molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. doi:10.1016/j.arr.2020.101200
- Liu JC, Dong SS, Shen H, et al. Multi-omics research in sarcopenia: current progress and future prospects. Ageing Res Rev. 2022;76:101576. doi:10.1016/j.arr.2022.101576
- Jayawardena TU, Kim SY, Jeon YJ. Sarcopenia; functional concerns, molecular mechanisms involved, and seafood as a nutritional intervention - review article. Crit Rev Food Sci Nutr. 2023;63(14):1983–2003. doi:10.1080/10408398.2021.1969889
- Livshits G, Kalinkovich A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res Rev. 2019;56:100980. doi:10.1016/j.arr.2019.100980
- Byrne T, Cooke J, Bambrick P, McNeela E, Harrison M. Circulating inflammatory biomarker responses in intervention trials in frail and sarcopenic older adults: a systematic review and meta-analysis. Exp Gerontol. 2023;177:112199. doi:10.1016/j.exger.2023.112199
- Zhang FM, Wu HF, Shi HP, Yu Z, Zhuang CL. Sarcopenia and malignancies: epidemiology, clinical classification and implications. Ageing Res Rev. 2023;91:102057. doi:10.1016/j.arr.2023.102057
- Liaw FY, Huang CF, Chen WL, et al. Higher platelet-to-lymphocyte ratio increased the risk of sarcopenia in the community-dwelling older adults. Sci Rep. 2017;7(1):16609. doi:10.1038/s41598-017-16924-y
- Hu Q, Mao W, Wu T, et al. High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are associated with sarcopenia risk in hospitalized renal cell carcinoma patients. Front Oncol. 2021;11:736640. doi:10.3389/fonc.2021.736640
- Itami Y, Miyake M, Tatsumi Y, et al. Preoperative predictive factors focused on inflammation-, nutrition-, and muscle-status in patients with upper urinary tract urothelial carcinoma undergoing nephroureterectomy. Int J Clin Oncol. 2019;24(5):533–545. doi:10.1007/s10147-018-01381-y
- Song HN, Kim JY, Kim JM, et al. Sarcopenia using pectoralis muscle area and lymphocyte-to-monocyte ratio (LMR) are independent prognostic factors in patients for nonmetastatic breast cancer. Medicine. 2022;101(49):e32229. doi:10.1097/md.0000000000032229
- Karanth SD, Washington C, Cheng TD, et al. Inflammation in relation to sarcopenia and sarcopenic obesity among older adults living with chronic comorbidities: results from the National Health and Nutrition Examination Survey 1999–2006. Nutrients. 2021;13(11). doi:10.3390/nu13113957
- Liu YY, Ruan GT, Ge YZ, et al. Systemic inflammation with sarcopenia predicts survival in patients with gastric cancer. J Cancer Res Clin Oncol. 2023;149(3):1249–1259. doi:10.1007/s00432-022-03925-2
- Nie YZ, Yan ZQ, Yin H, Shan LH, Wang JH, Wu QH. Osteosarcopenic obesity and its components-osteoporosis, sarcopenia, and obesity-are associated with blood cell count-derived inflammation indices in older Chinese people. BMC Geriatr. 2022;22(1):532. doi:10.1186/s12877-022-03225-x
- Zhao M, Duan X, Han X, et al. Sarcopenia and systemic inflammation response index predict response to systemic therapy for hepatocellular carcinoma and are associated with immune cells. Front Oncol. 2022;12:854096. doi:10.3389/fonc.2022.854096
- Shimizu T, Miyake M, Hori S, et al. Clinical impact of sarcopenia and inflammatory/nutritional markers in patients with unresectable metastatic urothelial carcinoma treated with pembrolizumab. Diagnostics. 2020;10(5). doi:10.3390/diagnostics10050310
- Ruan GT, Ge YZ, Xie HL, et al. Association between systemic inflammation and malnutrition with survival in patients with cancer sarcopenia-a prospective multicenter study. Front Nutr. 2021;8:811288. doi:10.3389/fnut.2021.811288
- Zhao A, Hou C, Li Y, Liu Y. Preoperative low muscle mass and malnutrition affect the clinical prognosis of locally advanced gastric cancer patients undergoing radical surgery. Front Oncol. 2023;13:1156359. doi:10.3389/fonc.2023.1156359
- Lee SI, Choi CH, Park CH, Son KH. Nutritional indices for screening sarcopenia before adult cardiac surgery. J Thorac Dis. 2023;15(6):3307–3318. doi:10.21037/jtd-22-1865
- Hisada H, Tsuji Y, Obata M, et al. The impact of sarcopenia on short- and long-term outcomes of endoscopic submucosal dissection for early gastric cancer. J Gastroenterol. 2022;57(12):952–961. doi:10.1007/s00535-022-01923-2
- Ganapathy A, Nieves JW. Nutrition and sarcopenia-what do we know? Nutrients. 2020;12(6). doi:10.3390/nu12061755
- Abd Aziz NAS, Teng N, Abdul Hamid MR, Ismail NH. Assessing the nutritional status of hospitalized elderly. Clin Interv Aging. 2017;12:1615–1625. doi:10.2147/CIA.S140859
- Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–07.e2. doi:10.1016/j.jamda.2019.12.012
- Ding P, Lv J, Sun C, et al. Combined systemic inflammatory immunity index and prognostic nutritional index scores as a screening marker for sarcopenia in patients with locally advanced gastric cancer. Front Nutr. 2022;9:981533. doi:10.3389/fnut.2022.981533
- Hua X, Long ZQ, Huang X, et al. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr Probl Cancer. 2020;44(4):100560. doi:10.1016/j.currproblcancer.2020.100560
- Şahin AB, Cubukcu E, Ocak B, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep. 2021;11(1):14662. doi:10.1038/s41598-021-94184-7
- Cheng X, Dong Y, Lou F. The predictive significance of the Advanced Lung Cancer Inflammation Index (ALI) in patients with melanoma treated with immunotherapy as second-line therapy. Cancer Manag Res. 2021;13:173–180. doi:10.2147/cmar.S286453
- Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69(8):1151–1176. doi:10.1080/01635581.2017.1367947
- Tseng SH, Lee WJ, Peng LN, Lin MH, Chen LK. Associations between hemoglobin levels and sarcopenia and its components: results from the I-Lan longitudinal study. Exp Gerontol. 2021;150:111379. doi:10.1016/j.exger.2021.111379
- Bani hassan E, Vogrin S, Hernandez Viña I, Boersma D, Suriyaarachchi P, Duque G. Hemoglobin levels are low in sarcopenic and osteosarcopenic older persons. Calcif Tissue Int. 2020;107(2):135–142. doi:10.1007/s00223-020-00706-2
- Liu Q, You J, Zhong M, Wu Z, Geng Y, Huang C. Hemoglobin level is negatively associated with sarcopenia and its components in Chinese aged 60 and above. Front Public Health. 2023;11:1081843. doi:10.3389/fpubh.2023.1081843
- Huang SW, Xu T, Zhang CT, Zhou HL. Relationship of peripheral lymphocyte subsets and Skeletal Muscle Mass Index in sarcopenia: a cross-sectional study. J Nutr Health Aging. 2020;24(3):325–329. doi:10.1007/s12603-020-1329-0
- Zhao WY, Zhang Y, Hou LS, et al. The association between systemic inflammatory markers and sarcopenia: results from the West China Health and Aging Trend Study (WCHAT). Arch Gerontol Geriatr. 2021;92:104262. doi:10.1016/j.archger.2020.104262
- Shi L, Zhang L, Zhang D, Chen Z. Association between systemic immune-inflammation index and low muscle mass in US adults: a cross-sectional study. BMC Public Health. 2023;23(1):1416. doi:10.1186/s12889-023-16338-8
- Tang T, Xie L, Tan L, Hu X, Yang M. Inflammatory indexes are not associated with sarcopenia in Chinese community-dwelling older people: a cross-sectional study. BMC Geriatr. 2020;20(1):457. doi:10.1186/s12877-020-01857-5
- Yamanouchi K, Murakami S, Sato A, Ogawa S, Shinagawa H, Kamohara Y. Integrated evaluation of inflammatory, nutritional, and sarcopenia markers to predict survival in metastatic breast cancer patients. Vivo. 2023;37(2):811–817. doi:10.21873/invivo.13146
- Peng H, Tan X. The prognostic significance of sarcopenia and the neutrophil-to-lymphocyte ratio in elderly patients with esophageal squamous cell carcinoma. Cancer Manag Res. 2021;13:3209–3218. doi:10.2147/cmar.S302274
- Stefanaki C, Paltoglou G, Mastorakos G, Chrousos GP. Chronic Stress and steatosis of muscles, bones, liver, and pancreas: a review. Horm Res Paediatr. 2023;96(1):66–73. doi:10.1159/000522540
- Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17(11):647–661. doi:10.1038/s41574-021-00551-9
- Tan X, Peng H, Gu P, Chen M, Wang Y. Prognostic significance of the L3 skeletal muscle index and advanced lung cancer inflammation index in elderly patients with esophageal cancer. Cancer Manag Res. 2021;13:3133–3143. doi:10.2147/cmar.S304996
- Éb NB, Daly LE, Power DG, Cushen SJ, MacEneaney P, Ryan AM. Computed tomography diagnosed cachexia and sarcopenia in 725 oncology patients: is nutritional screening capturing hidden malnutrition? J Cachexia Sarcopenia Muscle. 2018;9(2):295–305. doi:10.1002/jcsm.12258
- Yang Y, Meng Y. Is China moving toward healthy aging? A tracking study based on 5 phases of CLHLS data. Int J Env Res Public Health. 2020;17(12). doi:10.3390/ijerph17124343