Abstract
The role of honey in wound healing continues to attract worldwide attention. This study examines the anti-inflammatory effect of four honeys on wound healing, to gauge its efficacy as a treatment option. Isolated phenolics and crude extracts from manuka (Leptospermum scoparium), kanuka (Kunzea ericoides), clover (Trifolium spp.), and a manuka/kanuka blend of honeys were examined. Anti-inflammatory assays were conducted in HEK-Blue™-2, HEK-Blue™-4, and nucleotide oligomerization domain (NOD)2-Wild Type (NOD2-WT) cell lines, to assess the extent to which honey treatment impacts on the inflammatory response and whether the effect was pathway-specific. Kanuka honey, and to a lesser extent manuka honey, produced a powerful anti-inflammatory effect related to their phenolic content. The effect was observed in HEK-Blue™-2 cells using the synthetic tripalmitoylated lipopeptide Pam3CysSerLys4 (Pam3CSK4) ligand, suggesting that honey acts specifically through the toll-like receptor (TLR)1/TLR2 signaling pathway. The manuka/kanuka blend and clover honeys had no significant anti-inflammatory effect in any cell line. The research found that kanuka and manuka honeys have an important role in modulating the inflammatory response associated with wound healing, through a pathway-specific effect. The phenolic content of honey correlates with its effectiveness, although the specific compounds involved remain to be determined.
Introduction
Honey has long been used as a natural treatment in wound repair and has increased in popularity with antibiotic resistance increase.Citation1–Citation4 Honey is effectual and cost-effective, and its healing properties are well documented.Citation5–Citation6 Honey can decrease healing time via a dual effect on the inflammatory response. It suppresses the production and proliferation of inflammatory cells at the wound site, to prevent a prolonged inflammatory response, and it stimulates proinflammatory cytokine production, enabling normal healing to occur.Citation7–Citation11 Wound healing is a tissue remodeling process, comprising a systematic progression of events involving multiple interactions that are regulated by biologically active cytokines, growth factors, and proteases.Citation12–Citation15 The transcription factor nuclear factor-kappa beta (NF-KB) is an important marker of inflammation.Citation16 It enhances proinflammatory activity, thereby contributing to an amplified inflammatory response, and activates genes encoding for proinflammatory cytokines – interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α).Citation8,Citation17 These proinflammatory cytokines stimulate nitric oxide production, an important mediator of inflammation. Nitric oxide production and NF-KB activation are inhibited by the flavonoids present in honey.Citation18 When healing is impaired, chronic wounds develop, characterized by proinflammatory cytokines and reactive oxygen species.Citation8,Citation19–Citation22
Honey’s effectiveness in wound care has been hypothesized to be largely due to its anti-inflammatory action.Citation8 The specific compounds and the mechanisms involved are largely undetermined.Citation23 However, it has been suggested that specific polyphenols, the flavonoids, and caffeic acid phenethyl ester, are important factors.Citation24–Citation26 The antioxidants found in honey are considered to be important determinants of its anti-inflammatory activity.Citation2 An elevated inflammatory response results from hydrogen peroxide oxygen radicals present at the wound site, triggering NF-KB to enhance the inflammatory response.Citation2,Citation8 New Zealand honeys have been suggested to display significant anti-inflammatory activity, particularly kanuka and manuka honeys, by reducing neutrophil superoxide production.Citation23 Manuka honey has been shown to specifically decrease the inflammatory response associated with ulcerative colitis, an inflammatory bowel disease characterized by an over-expression of inflammatory cells, possibly by increasing antioxidant activity.Citation27–Citation29
Several studies have investigated the anti-inflammatory activity of New Zealand honeys in treating topical wounds. This study further investigated the anti-inflammatory properties of manuka and kanuka honey, and demonstrates the effectiveness of these New Zealand honeys in reducing the inflammatory response associated with healing, independent of its known topical effect. Furthermore, specific signaling pathways, through which these honeys are effective, were observed.
Materials and methods
Preparation of honey extracts
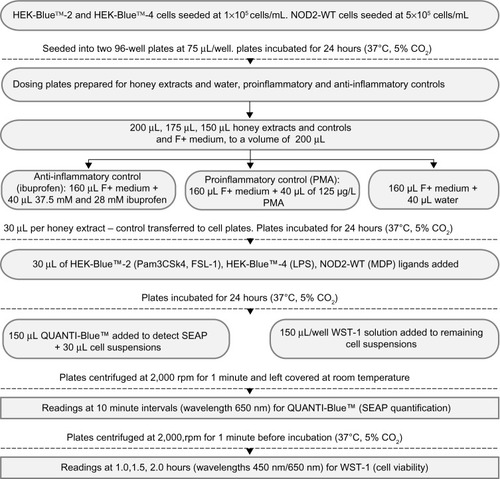
Four New Zealand honeys were used – manuka, kanuka, a manuka/kanuka blend (all supplied by Comvita New Zealand Ltd, Te Puke, NZ), and clover honey (supplied by Airborne Honey Ltd, Leeston, NZ). Honey extracts were fractionated by Dr Peter Brooks (University of the Sunshine Coast, Sippy Downs, QLD, Australia) to isolate their phenolic compounds. The percentages of phenolics were determined (manuka 59%, kanuka 39%, the manuka/kanuka blend 59%, and clover 40%). They were stored at 4°C. Both phenolic and crude extracts were tested. depicts the process fow for the preparation of the following materials and the accompanying observation steps.
Figure 1 Materials, preparation, and observation process flow chart.
Abbreviations: LPS, lipopolysaccharide; MDP, muramyl dipeptide; NOD2-WT, nucleotide oligomerization domain 2-Wild Type; PMA, phorbol 12-myristate 13-acetate; SEAP, secreted alkaline phosphatase; Pam3CSK4, Pam3CysSerLys4; FSL-1, Pam2CGDPKHPKSF.
Reagents
HEK-Blue™-2, HEK-Blue™-4, and nucleotide oligomerization domain (NOD)2-Wild Type (NOD2-WT) embryonic kidney cell lines were selected due to their accessibility and relatively high expression of matrix metalloproteinase (MMP)-1, -2, and -9. These were obtained from the Auckland Cancer Society Research Centre (Auckland NZ). Dulbecco’s Modified Eagle’s Medium (DMEM), an antibiotic mixture (penicillin, streptomycin, L-glutamine), and fetal calf serum (FCS) were obtained from Life Technologies Corp (Carlsbad CA, USA). Phorbol 12-myristate 13-acetate (PMA) and ibuprofen were purchased from Sigma-Aldrich Corp (St Louis, MO, USA). Lipopolysaccharide (LPS), Pam3CysSerLys4 (Pam3CSK4), and FSL-1 (Pam2CGDPKHPKSF, a synthetic diacylated lipoprotein), Muramyl dipeptide (MDP), Blasticidin, Zeocin™, HEK-Blue™ Selection, and QUANTI-Blue™ were from InvivoGen (San Diego, CA, USA). Cell Proliferation Reagent WST-1 was obtained from Roche Applied Science (Penzberg, Germany).
Cell culture
The HEK-Blue™-2 and HEK-Blue™-4 cells were maintained in DMEM high glucose supplemented with 10% FCS, 1% penicillin/streptomycin/glutamine, and HEK-Blue™ Selection. The NOD2 WT cells were maintained in DMEM supplemented with 10% FCS, 1% penicillin/streptomycin, 0.06% Blasticidin, and 0.1% Zeocin.
Anti-inflammatory assay
The HEK-Blue™-2 and HEK-Blue™-4 cells were seeded at 1 × 105 cells/mL and the NOD2 WT cells at 5 × 105 cells/mL, into 96-well plates, and incubated for 24 hours at 37°C, 5% CO2. The honey extracts, at a suitable dose range (5.3%–14.3%) as determined by preliminary half maximal inhibitory concentration (IC50) data, and the controls (75 mM Ibuprofen, 1 mg/mL PMA, and a solvent control) were added to the plate and further incubated for 24 hours. The appropriate ligand for each cell line was added (3.125 μg/mL LPS, 10 ng/mL Pam3CSK4, 10 ng/mL FSL-1, and 22.73 μg/mL MDP), and all were incubated for 24 hours. The secreted embryonic alkaline phosphatase (SEAP) production was measured using QUANTI-Blue, every 10 minutes for 50 minutes. Cell viability was determined by WST-1 after 60-, 90-, and 120-minute incubations. The results were normalized for cell viability and against a solvent control.
Statistical analysis
A generalized linear model was fitted to test the anti-inflammatory effect of eight honey extracts at five different concentrations (% honey) compared with untreated cells, in the presence of the specific ligand corresponding to the cell line. The means with standard error and estimates with 95% confidence interval along with P-value were calculated. provides the anti-inflammatory effect of the honey extracts at five different concentrations (% honey) compared with untreated cells, in the HEK-Blue™-4 cell line using the LPS ligand. provides the anti-inflammatory effect of the honey extracts at five different concentrations compared with untreated cells, in the HEK-Blue™-2 cell line using the FSL-1 ligand. provides the anti-inflammatory effect of the honey extracts at five different concentrations compared with untreated cells, in the HEK-Blue™-2 cell line using the Pam3CSK4 ligand. provides the anti-inflammatory effect of the honey extracts at five different concentrations compared with untreated cells, in the NOD2-WT cell line using the MDP ligand. provides the materials, preparation, and observation process fow. provides the anti-inflammatory effect of the four honey extracts when differentiated by honey phenolic and crude honey extract. The units were expressed as SEAP relative to the control (%). The higher the value, the higher was the level of SEAP, resulting in a lower anti-inflammatory effect. A P-value of less than 0.05 indicated that a significant anti-inflammatory effect was observed in those cells treated with honey compared with those cells that were not. All analyses were carried out using SAS® 9.3 (SAS Institute, Cary, NC, USA).
Figure 2 The anti-inflammatory effect of the four honey extracts when differentiated by honey phenolics and crude honey extracts.
Abbreviation: SEAP, secreted alkaline phosphatase.
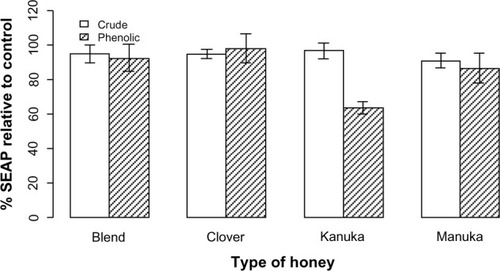
Table 1 The anti-inflammatory effect of honey extracts at five different concentrations (% honey) compared with untreated cells, in the HEK-Blue™-4 cell line using the LPS ligand
Table 2 The anti-inflammatory effect of honey extracts at five different concentrations (% honey) compared with untreated cells, in the HEK-Blue™-2 cell line using the FSL-I ligand
Table 3 The anti-inflammatory effect of honey extracts at five different concentrations (% honey) compared with untreated cells, in the HEK-Blue™-2 cell line using the Pam3CSK4 ligand
Table 4 The anti-inflammatory effect of honey extracts at five different concentrations (% honey) compared with untreated cells, in the NOD2-WT cell line using the MDP ligand
Results
Effect of honey extracts on inflammation in HEK-Blue™-4 cells
The honey extracts were analyzed in the HEK-Blue™-4 cell line in the presence of LPS. details their anti-inflammatory effect. No substantive effect was observed with treatment by any of the extracts. Following an increase in honey concentration, a noticeable anti-inflammatory effect was observed with the higher concentrations of the phenolic and crude kanuka extracts. The manuka phenolic extract produced a significant effect at the 7.1% (P=0.0131) and 14.3% (P=0.0024) concentrations. No significant difference was observed between both the manuka/kanuka blend and clover honeys and untreated cells at any concentration, with the exception of the 12.5% concentration of the phenolic clover extract (P=0.0360).
Effect of honey extracts on inflammation in HEK-Blue™-2 cells
Honey treatment was investigated in HEK-Blue™-2 cells in the presence of two ligands, FSL-1 and Pam3CSK4. FSL-1 is specific to TLR2 and TLR6, and Pam3CSK4 is recognized by TLR2 and TLR1. and detail the effect of the honey extracts on the inflammatory response in the HEK-Blue™-2 cells.
By examining honey treatment using two ligands, the specific pathway through which honey might act could be determined. Honey treatment at all concentrations had little impact, as compared with that in untreated cells with FSL-1, except for the 14.3% concentration of the phenolic kanuka extract (P=0.0390) and the 12.5% concentration of the crude manuka extract (P=0.0462). Stronger anti-inflammatory effects were observed in the presence of the Pam3CSK4 ligand, where the manuka and kanuka phenolics were particularly effective. At the highest concentrations, manuka honey significantly decreased the inflammatory response. With kanuka honey, all five concentrations significantly reduced the level of inflammation compared with that of no treatment. Treatment with the manuka/kanuka blend, the clover honey phenolics, and the crude extracts from all four honeys had no significant impact on the inflammatory response.
illustrates the anti-inflammatory effect observed using the HEK-Blue™-2 cell line when treated with the highest concentration (14.3%) of honey phenolics and crude extracts and stimulated with Pam3CSK4. A significant decrease in inflammation was observed for each extract compared with PMA (data not shown). Treatment with the phenolic and crude manuka/kanuka blend and clover extracts did not differ markedly from treatment with solvent. Treatment with the kanuka phenolic extract differed considerably from the solvent and the crude kanuka extract. The kanuka phenolic extract produced a comparable anti-inflammatory effect to that of ibuprofen (results not shown).
Effect of honey extracts on inflammation in NOD2-WT cells
details the anti-inflammatory effect of honey treatment in NOD2-WT cells. There was no significant effect by the honeys, at any concentration, on the inflammatory response when compared with no treatment, with the exception of the 12.5% concentration of the crude clover extract, where a significant anti-inflammatory effect was observed (P=0.0442).
Discussion
An inflammatory response was induced in HEK-Blue™-2, HEK-Blue™-4, and NOD2-WT cell lines using different ligands, to illustrate whether treatment with four honeys could produce an anti-inflammatory effect. The cell lines act through different signaling pathways (TLR2, TLR4, and NOD-like receptor [NLR] respectively). Thus, by being able to observe in which cell line(s) the honey treatment was effective, the pathway through which it produced an anti-inflammatory response could be demonstrated. The NOD2-WT cell line acts through the NLR signaling pathway and recognizes the MDP ligand. A substantial body of research exists to support the anti-inflammatory activity of a variety of honeys. This research, however, demonstrated that the honeys examined did not produce a significant anti-inflammatory effect via either the TLR4 or NLR signaling pathway, observing a noticeable but not significant anti-inflammatory activity with honey treatment.
Anti-inflammatory activity with honey treatment was observed in the HEK-Blue™-2 cell line and most significantly with kanuka honey. The kanuka phenolic extract was highly anti-inflammatory and had a greater effect than did the crude extract, indicating that a higher phenolic content correlates with its elevated anti-inflammatory activity. The manuka honey phenolics also had an anti-inflammatory effect in the HEK-Blue™-2 cells, although to a lesser extent than for the kanuka honey phenolics, with the highest concentrations producing a significant difference as compared with no treatment, thereby supporting the importance of polyphenols in the anti-inflammatory activity of honey. The anti-inflammatory effect by the kanuka and manuka honeys was strongest in the presence of the Pam3CSK4 ligand, indicating that the honeys act through the TLR1/TLR2 signaling pathway.Citation30 The anti-inflammatory activity of kanuka and manuka honeys is therefore pathway-specific. No significant effect was observed with honey treatment, at any concentration, in the NOD2-WT cell line, supporting the anti-inflammatory activity of honey being pathway-specific. A hypothesis for the means by which kanuka honey exhibits anti-inflammatory activity is through the downregulation of proinflammatory mediators, such as IL-1β and NF-KB.
In wound healing, the inflammatory response is one phase of repair that is fundamental for normal healing. An elevated or prolonged inflammatory response is associated with a delay in wound repair, an increase in tissue damage, and the development of nonhealing, chronic wounds. By demonstrating significant anti-inflammatory activity, kanuka honey has the potential to be an effective treatment in preventing chronic wounds. International studies have shown that honey has a significant effect on the inflammatory response and support the use of honey in wound healing.Citation18,Citation24,Citation31
Research has also shown a causal association between inflammatory diseases and treatment with honey.Citation27,Citation29,Citation32 This study sought to investigate and further advance these fndings. Anti-inflammatory assays were conducted using HEK-Blue™-2, HEK-Blue™-4, and NOD2-WT cell lines, acting through different signaling pathways. The results demonstrate that kanuka honey exhibits anti-inflammatory effects in a pathway-specific manner. Further investigation would help to discover the exact mechanisms of action by which honeys act.
HEK-Blue™-2 is a more sensitive cell line than is HEK-Blue™-4. The noticeable but not significant anti-inflammatory effect observed by the honey phenolics in the HEK-Blue™-4 cells contrasts with the significant impact in the HEK-Blue™-2 cells, suggesting a reduced sensitivity rather than no anti-inflammatory activity. Further investigation using larger volumes of honey would be required to determine whether more significant results could be obtained.
Conclusion
New Zealand honeys have a well-established anti-inflammatory effect in topical wound healing. However, less was known of their effect in vitro and of the signaling pathways through which they act. Treatment with kanuka and manuka honeys resulted in powerful anti-inflammatory effects in HEK-Blue™-2 cells, but not in the HEK-Blue™-4 or NOD2-WT cells. Specifically, the anti-inflammatory effect occurred via the TLR1/TLR2 signaling pathway. The effects suggest a correlation with the phenolic content of the honeys, with a higher phenolic content producing an elevated anti-inflammatory effect. Kanuka and manuka honeys therefore can have a positive impact on the inflammatory response associated with wound healing. Subsequent investigation is needed to determine the specific compounds present in the honeys that are agents responsible for their anti-inflammatory activity.
Acknowledgments
The authors express their appreciation to Comvita Innovation (Comvita New Zealand Ltd) for their assistance in conducting the research, and to the Department of Nutrition and the Auckland Cancer Society Research Centre laboratories (The University of Auckland) for their help and support, particularly Dr Nishi Karunasinghe and Noha Ahmed Nasef. Sincere thanks to Airborne Honey Ltd and to B Stevenson (Comvita New Zealand Ltd) for the provision of the New Zealand honey samples.
Disclosure
Victoria Tomblin received a research scholarship and funding for materials from Comvita New Zealand Ltd. Ralf Schlothauer is an employee of Comvita New Zealand Ltd. The authors report no other conflicts of interest in this work.
References
- LeeDSSinnoSKhachemouneAHoney and wound healing: an overviewAm J Clin Dermatol201112318119021469763
- MolanPCUsing honey in wound careIJCA2006322124
- MolanPCThe role of honey in the management of woundsJ Wound Care19998841541810808853
- MathewsKABinningtonAGWound management using honeyCompendium for the Continuing Education of the Practicing Veterinarian20022415360
- SubrahmanyamMHoney dressing versus boiled potato peel in the treatment of burns: a prospective randomized studyBurns19962264914938884013
- BogdanovSJurendicTSieberRGallmannPHoney for nutrition and health: a reviewJ Am Coll Nutr200827667768919155427
- KhanFRUl AbadinZRaufNHoney: nutritional and medicinal valueInt J Clin Pract200761101705170717877657
- MolanPCRe-introducing honey in the management of wounds and ulcers – theory and practiceOstomy Wound Manage20024811284012426450
- TonksAJDudleyEPorterNGA 5.8-kDa component of manuka honey stimulates immune cells via TLR4J Leukoc Biol20078251147115517675558
- MwipatayiBPAngelDNorrishJHamiltonMJScottASieunarineKThe use of honey in chronic leg ulcers: a literature reviewPrimary Intention2004123107108110112
- SteinhornGSimsIMCarnachanSMCarrAJSchlothauerRIsolation and characterisation of arabinogalactan-proteins from New Zealand kanuka honeyFood Chemistry20111284949956
- LundLRRomerJBuggeTHFunctional overlap between two classes of matrix-degrading proteases in wound healingEMBO J199918174645465610469644
- PilcherBKDuminJASudbeckBDKraneSMWelgusHGParksWCThe activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrixJ Cell Biol19971376144514579182674
- MajtanJKumarPMajtanTWallsAFKlaudinyJEffect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytesExp Dermatol2010198e73e7919845754
- SchultzGSMastBAMolecular analysis of the environments of healing and chronic wounds: cytokines, proteases and growth factorsPrimary Intention199971714
- LeeYKimHKimSKimKHChungJHActivation of toll-like receptors 2, 3 or 5 induces matrix metalloproteinase-1 and -9 expression with the involvement of MAPKs and NF-kappaB in human epidermal keratinocytesExp Dermatol2010198e44e4919758322
- ChungTWMoonSKChangYCNovel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanismFASEB J200418141670168115522912
- HämäläinenMNieminenRVuorelaPHeinonenMMoilanenEAnti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophagesMediators Inflamm200720074567318274639
- BrandnerJMZachejaSHoudekPMollILobmannRExpression of matrix metalloproteinases, cytokines, and connexins in diabetic and nondiabetic human keratinocytes before and after transplantation into an ex vivo wound-healing modelDiabetes Care200831111412017898090
- TrengoveNJStaceyMCMacAuleySAnalysis of the acute and chronic wound environments: the role of proteases and their inhibitorsWound Repair Regen19997644245210633003
- DiegelmannRFEvansMCWound healing: an overview of acute, fibrotic and delayed healingFront Biosci2004928328914766366
- van den BergAJvan den WormEvan UffordHCHalkesSBHoekstraMJBeukelmanCJAn in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honeyJ Wound Care200817417217417618494436
- LeongAGHerstPMHarperJLIndigenous New Zealand honeys exhibit multiple anti-inflammatory activitiesInnate Immun201218345946621978989
- KassimMAchouiMMustafaMRMohdMAYusoffKMEllagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activityNutr Res201030965065920934607
- KhalilMSulaimanSABoukraaLAntioxidant properties of honey and its role in preventing health disorderThe Open Nutraceuticals Journal201031616
- PyrzynskaKBiesagaMAnalysis of phenolic acids and flavonoids in honeyTrAC2009287893902
- MahgoubAAel-MedanyAHHagarHHSabahDMProtective effect of natural honey against acetic acid-induced colitis in ratsTrop Gastroenterol2002232828712632976
- MedhiBPrakashAAvtiPKSaikiaUNPandhiPKhandujaKLEffect of Manuka honey and sulfasalazine in combination to promote antioxidant defense system in experimentally induced ulcerative colitis model in ratsIndian J Exp Biol200846858359018814487
- PrakashAMedhiBAvtiPKSaikiaUNPandhiPKhandujaKLEffect of different doses of Manuka honey in experimentally induced inflammatory bowel disease in ratsPhytother Res200822111511151918688794
- UhlenMOksvoldPFagerbergLTowards a knowledge-based Human Protein AtlasNat Biotechnol201028121248125021139605
- Al-WailiNSBoniNSNatural honey lowers plasma prostaglandin concentrations in normal individualsJ Med Food20036212913312935324
- BilselYBugraDYamanerSBulutTCevikbasUTurkogluUCould honey have a place in colitis therapy? Effects of honey, prednisolone, and disulfiram on inflammation, nitric oxide, and free radical formationDig Surg2002194306311 discussion 311–31212207075
