Abstract
Purpose
In essential hypertensive patients, cardiac remodeling may be associated with the risk of renal damage in the future which can be reflected by the estimated glomerular filtration rate (eGFR). Through retrospective analysis, we evaluated the potential of cardiac remodeling based on echocardiographic measurements to predict the risk of renal damage in the future with hypertensive patients.
Methods
We retrospectively analyzed the relationship between the changes of left heart structure and function and renal damage for 510 patients with hypertension, who were diagnosed between 2016 to 2022. Demography data, clinical data, blood samples and echocardiographic variables were used for survival analysis, and the Cox proportional hazards regression model was used.
Results
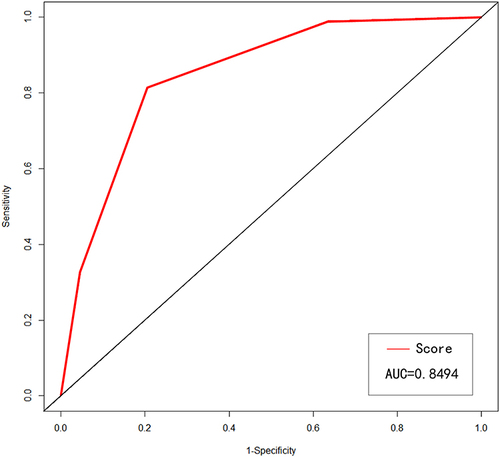
In our study, we found that age, serum creatinine (SCR), creatine kinase isoenzyme MB (CK MB), abnormal high-sensitivity troponin I (TNI), interventricular septum thickness (IVST) and left ventricular ejection fraction (LVEF) could be used as independent predictors in risk of renal impairment in hypertensive patients (p<0.05). Combined in a score where one point was given for the presence of each of the parameters above, this score could strongly predict renal function damage in the future (p<0.05). In receiver operating characteristics (ROC) curve analyses, the area under the curve of the risk factor score was 0.849 (P<0.001).
Conclusion
In essential hypertensive patients, LVEF and IVST can predict the risk of future adverse renal outcomes. Moreover, combining risk variables into a simplified score may enable to assess the risk of renal impairment in hypertensive patients at an early stage.
Background
Hypertension is a common chronic disease which affects about one-third of the world’s population, although medicine treatment has made considerable progress, it is still a major risk factor for cardiovascular and cerebrovascular disease.Citation1–3 It can damage the structure and function of important organs, such as the heart, brain and kidney, and ultimately lead to functional failure of these organs.Citation4
Hypertensive nephropathy is the second major cause of end-stage renal disease (ESRD) after diabetes, and the clinical manifestations are nocturia, proteinuria and decreased estimated glomerular filtration rate (eGFR).Citation5 In patients with hypertension, renal dysfunction is a relatively common condition but often ignored, for most of them are asymptomatic or only manifest whit mild albuminuria.Citation6,Citation7 Therefore, it is best to use simple parameters to routinely assess renal function in hypertensive patients in the early stage. Hypertensive heart disease refers to cardiac structural and functional disorders secondary to hypertension, which is usually characterized by left ventricular hypertrophy (LVH), left atrial enlargement, and left ventricular systolic and diastolic dysfunction.Citation8 The most accurate way to diagnose heart anatomy and function is by echocardiography, which can also be used to assess the prognosis and course of many cardiovascular diseases.Citation9,Citation10
Cardiorenal syndrome (CRS) is a disease involving the heart and kidneys, which interact with each other in an acute or chronic manner to produce dysfunction, and even death. In CRS, acute or chronic dysfunction of one organ can cause acute or chronic dysfunction of the other organCitation11. According to a study, alterations in heart structure were linked to subclinical renal impairment in hypertension patients.Citation12 Renal impairment was linked to ventricular remodeling and systolic dysfunction in a cohort of patients with heart failure with preserved ejection fraction.Citation13 Some researchers have found that renal insufficiency in individuals with acute myocardial infarction could be predicted by impaired systolic and diastolic function as determined by echocardiography.Citation14 However, the relationship between early structural and functional changes in the heart and subsequent renal damage in people with essential hypertension has not yet been thoroughly studied. In the current study, we performed a retrospective analysis to investigate the potential of cardiac remodeling based on echocardiographic measurements to predict future renal insufficiency in patients with essential hypertension.
Methods
Participants
We collected 510 patients with essential hypertension from the Department of Cardiovascular Medicine, Zhongnan Hospital, Wuhan University from January 2016 to September 2022. We determined the sample size through census sampling. The inclusion criteria for patients in this single-center, retrospective cohort study were: (a) patients who were followed up regularly for equal or more than 6 months, (b) patients who were equal or older than 18 years, (c) patients who met the international diagnostic criteria of essential hypertension, (d) the medical records were complete for all patients, (e) patients whose estimated glomerular filtration rate (eGFR) were equal or greater than 90 mL/min/1.73 m2. Patients were excluded from the study cohort if they met the following criteria: (a) patients with unscheduled outpatient follow-up or follow-up for less than 6 months, (b) patients who were younger than 18 years, (c) patients with moderate to severe valvular disease, acute heart failure, acute myocardial infarction, severe arrhythmia, congenital heart disease or primary cardiomyopathy and other organic heart diseases, known or suspected secondary hypertension, history of kidney or heart transplantation, acute or chronic renal dysfunction or maintenance dialysis (eGFR < 90 mL/min/1.73 m2), and (d) patients with two or more missing data in medical records.
Data Collection
The data were extracted from an electronic database which included clinical and laboratory data, including age, sex, history of diabetes, history of hyperlipidemia, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose, as well as electrocardiogram (ECG), serum creatinine (SCR), blood urea nitrogen (BUN), serum uric acid (SUA), blood calcium ion concentration (Ca2+), blood potassium ion concentration (K+), blood sodium ion concentration (Na+), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatine kinase (CK), creatine kinase isoenzyme MB (CK MB), lactate dehydrogenase (LDH), high-sensitivity troponin I (TNI) and N-terminal Pro-B-type natriuretic peptide (NT proBNP). According to the laboratory reference range of Zhongnan Hospital of Wuhan University, TNI > 26.2 pg/mL, NT proBNP > 900 pg/mL and SCR > 90umol/L were considered abnormal. The endpoint of the study was an eGFR less than 60mL/min/1.73 m2. We used eGFR < 60mL/min/1.73 m2 to reflect renal dysfunction or renal damage or renal event. And the “future renal damage” means the risk of renal damage in the future. The eGFR was estimated by the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) creatinine equation.Citation15 According to the International Society of Hypertension, hypertension was defined as ≥ 140mmHg in the office systolic blood pressure and/or ≥ 90mmHg in the office diastolic blood pressure after repeated blood pressure measurements on different days, with re-measurement intervals of 1–4 weeks, and at least 140/90mmHg for more than 2–3 times.Citation16
Echocardiographic Measurements
All echocardiographic studies were conducted using a Philips EPIQ7 ultrasound machine. All patients were placed in the left lateral decubitus position, and two-dimensional echocardiography, M-mode echocardiography and Doppler echocardiography were used to measure through the standard left parasternal window and apical window. Echocardiography-related parameters were performed by echocardiography readers blinded to study participants. The ascending aorta diameter (AAO diameter), left atrial diameter (LAD), left ventricular diameter at the end of diastole (LVDd), interventricular septum thickness (IVST) and left ventricular ejection fraction (LVEF) were measured according to recommendations from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. AAO diameter of > 34mm for men and > 31mm for women were defined as AAO widening, LAD of > 40mm for men and > 38mm for women were defined as left atrial enlargement, LVDd of > 58mm for men and > 52mm for women were defined as left ventricular enlargement, IVST of > 10mm for men and > 9mm for women were defined as IVS thickening and LVEF of < 52% for men and < 54% for women were suggestive of abnormal left ventricular systolic function.Citation17,Citation18 Echocardiographic data were obtained for all patients within 3 days of admission.
Statistical Analysis
SPSS (SPSS26.0) and R software (4.2.3) were used for data management and statistical analysis. The variables of normal distribution were expressed as the mean ± standard deviation and compared with t-test. Variables with skewed distribution were represented by median and quartile intervals, and compared by rank sum test. The categorical data was expressed as absolute frequencies and percentages, and compared with χ2 test. Pearson correlation coefficient and Spearman correlation coefficient were used to evaluate the correlation between research variables of normal distribution and non-normal distribution distribution. Cox proportional risk model was used for survival analysis. First, Cox regression univariate analysis was used to evaluate the association between general clinical data, echocardiographic parameters and study endpoints, and multivariate Cox regression analysis was conducted for variables with P < 0.05. Combining these independent predictive risk factor above in a simple score and the renal event-free survival curve was constructed by Kaplan-Meier method and compared with log rank test. The receiver operating characteristics (ROC) curve was used to evaluate the predictive ability of risk factor score on the risk of renal damage progression in hypertensive patients. Double-tailed P value<0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 510 individuals identified with confirmed hypertension were enrolled in this study, including 106 patients with diabetes and 93 patients with hyperlipidemia. Patients selection flow chart are shown in . The median follow-up time of patients was 650 days, including 245 males (48%) and 265 females (52%), with a median age of 67 years. According to the left ventricular structure and function as shown by echocardiography, patients were divided into normal left ventricular structure and function group and abnormal left ventricular structure and function group. Compared with normal group, patients with abnormal left ventricular structure and function seemed older, and the biochemical indexes (TNI, NT proBNP, SCR, BUN, SUA and LDH) and the incidence of and ST-segment elevation or depression were higher (P < 0.05). However, the other indicators were not significantly different between the two groups (P > 0.05). Full demographic data are shown in .
Table 1 Comparisons of Clinical Features Between Normal and Abnormal Left Cardiac Structure and Function with Hypertension Patients
Relationship Between AAO Diameter, LAD, LVDd, IVST, LVEF and eGFR After Follow-Up
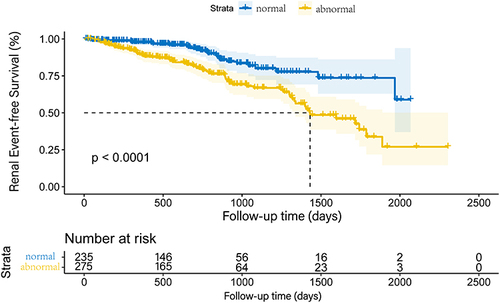
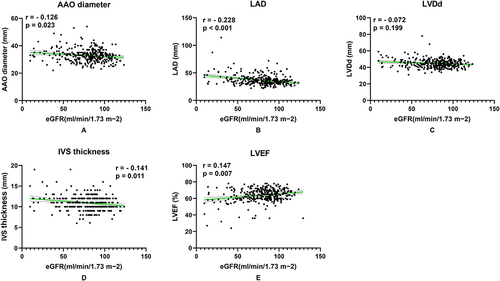
The relationship between AAO diameter, LAD, LVDd, IVST, LVEF and future renal function in hypertensive patients are shown in . After follow-up, eGFR was negatively correlated with AAO diameter, LAD and IVST, while positively correlated with LVEF. The Kaplan-Meier renal event-free survival curves of hypertensive patients according to whether left cardiac structure and function was abnormal are shown in , which indicated abnormal left heart structure and function was associated with the risk of renal damage in primary hypertensive patients.
Figure 2 Univariate correlations between the changes of left heart structure and function and renal damage. (A) eGFR was negatively related to AAO diameter. (B) eGFR was negatively related to LAD. (C) eGFR was negatively related to LVDd, but p > 0.05. (D) eGFR was negatively correlated with IVST. (E) eGFR was positively related to LVEF.
Role of AAO Diameter, LAD, LVDd, IVST and LVEF in Predicting Progression of Renal Damage in Patients with Hypertension
In univariate survival analysis, variables associated with the study endpoint (p < 0.05) included age, history of diabetes, incidence of ST-segment change, incidence of atrial fibrillation, SBP, DBP, SCR, BUN, SUA, Na+, CK, CK MB, incidence of abnormal TNI, incidence of abnormal NT proBNP, LAD, IVST and LVEF. In the multivariate survival analysis, the Cox proportional risk model was adjusted with the above factors which the p-value was less than 0.05 as confounding factors, and we obtained that the age, SCR, CK MB, TNI abnormal incidence, IVST and LVEF were independent risk factors for renal damage in hypertensive patients (age: HR 1.146, p < 0.001; SCR: HR 1.040, p < 0.001; CK MB: HR 1.101, p = 0.017; TNI abnormal incidence: HR 8.458, p = 0.012; IVST: HR 1.472, p = 0.027; LVEF: HR 0.896, p = 0.031). Complete data are shown in .
Table 2 Univariate and Multivariate Cox Regression Analysis of Renal Impairment After Follow-Up in Hypertensive Patients
Composite Factor Score
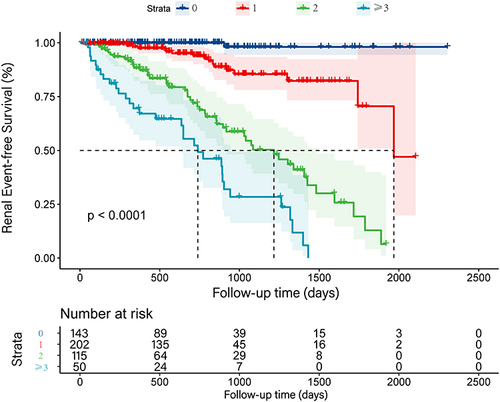
Multivariate analysis showed that increased age, increased SCR, increased CK MB, abnormal TNI, IVS thickening and LVEF declining were significantly associated with impaired renal function after follow-up. Each of the above risk factors was scored with 1 point for score statistics. We got 4 groups, namely 0, 1, 2 and ≥ 3 points. Group 0 (143, 28.0%) had no risk factors, group 1 (202, 39.6%) had one risk factor, group 2 (115, 22.6%) had two risk factors and group ≥ 3 (50, 9.8%) had ≥ 3 abnormal parameters. Among them, 1 point would be given if the patient is older than 67. With the increase of risk factor scores, the eGFR of hypertensive patients after follow-up gradually decreased (p < 0.001) (Supplementary Figure S1). In addition, compared with patients with none of these variables, there was a significant and graded increased hazard ratio of renal damage in patients with risk factors. (1 points: HR 12.560, p = 0.015; 2 points: HR 47.892, p < 0.001; ≥ 3 points: HR 68.866, p < 0.001) (). The Kaplan-Meier survival curve of the four groups did not cross, which indicated that there was no violation of proportional hazard. (p < 0.001) ()
Table 3 Hazard for Impaired Renal Function by Number of Significant Variables
Figure 4 Kaplan-Meier renal event-free survival curves with hypertension according to the risk factor score. P-value of the Log rank test for 0 point group, 1 point group, 2 points group and ≥ 3 points group were all < 0.001. The K-M survival curves of the four groups did not cross, indicating that there was no violation of proportional hazard.
Next, we made the ROC curve of this score on the prediction ability of patients’ renal damage, and found that the area under the curve (AUC) for the score was 0.849 (95% CI 0.809–0.892, p < 0.001), and the optimal cut-off value of score for predicting renal damage was 2 points. () (Supplementary Table S1)
Figure 5 The ROC curve of the risk factor score, and the AUC was 0.849 (95% CI 0.809–0.892, p < 0.001). The ROC curve indicated that the risk factor score can better predict the risk of renal dysfunction in hypertensive patients.
Discussion
The heart and kidneys are essential for maintaining cardiovascular homeostasis.Citation19 Hypertension can both cause changes in structure and function of the heart and renal dysfunction, and Primary disorders in one of these two organs often result in secondary dysfunction or damage in the other organ.Citation20 Therefore, there is an urgent need to monitor and manage heart and kidney damage at an early stage in patients with hypertension. However, the relationship between cardiac remodeling and renal damage in hypertensive patients is unclear, and it is unknown whether cardiac remodeling can be used to assess renal damage in patients with hypertension. It is important to solve these issues for the treatment of patients with hypertension. This study used statistical methods to retrospectively analyze the relationship between the changes of left cardiac structure and function reflected by echocardiography and the future eGFR in hypertensive patients. We scored every risk factor that might predict renal damage in hypertensive patients and found that the score obtained could better predict renal impairment in hypertensive patients.
The influence of hypertension on patients’ heart and kidney function and the relationship between heart and kidney have always been an interesting topic for clinical researchers. Left ventricular hypertrophy (LVH), left atrial enlargement, and left ventricular systolic and diastolic dysfunction are common in hypertension patients, especially LVH, which is a sign of hypertensive heart disease.Citation21,Citation22 It’s worth caring about that LVH and systolic left ventricular failure are significantly related to renal function.Citation23–25 In Japan, there was a cross-sectional study on the heart tissues of 334 patients. The study showed that in the autopsy samples of the general population, the lower eGFR was related to the greater myocardial wall thickness and the greater myocardial cell hypertrophy. Furthermore, participants with hypertension were more likely to show an association between cardiomyocyte hypertrophy and decreased eGFR.Citation24 Song et al retrospectively analyzed the relationship between cardiac remodeling, especially LVH, and eGFR decline in 265 patients with type 2 diabetes. They found that LVPW and IVST were independent risk factors for renal damage patients of type 2 diabetes.Citation26 However, it is unclear whether risk factors, such as abnormalities in cardiac structure and function as seen by echocardiography, can be used to predict renal damage in patients with essential hypertension because there are still few studies on the relationship between cardiac remodeling and impending renal damage in patients with hypertension in clinical data. In our study, through multivariate Cox regression analysis, we found that IVST and LVEF in hypertensive patients could be used to evaluate the risk of renal damage after follow-up (p < 0.05). As a result, medication therapy options for individuals who have increased IVST and lower LVEF in the early stages of hypertension need to be carefully chosen because they may be at an increased risk of renal impairment in the future.
In fact, early-stage hypertensive patients with abnormalities in cardiac structure and function, particularly those with IVH, may not manifest aberrant renal function. But as hypertension progresses as well as other risk factors take effect, individuals may experience renal function damage. Therefore, In the early stage, we’d better have easy-to-obtain evaluation indicators to predict the risk of renal function damage in patients with hypertension, so that relevant interventions can be carried out to reduce the incidence of hypertensive nephropathy. It is essential to address these issues for hypertensive patients’ treatment and prognosis.
In our study, we obtained risk factors other than IVST and LVEF through multivariate Cox regression analysis. We simultaneously calculated the risk factor scores and discovered that this score might be utilized to assess the potential abnormalities of renal function in hypertensive individuals. The probability of renal insufficiency increased with the risk factor score increasing. Although a significant number of clinical trials are needed for verification, the risk factor score we obtained may be used to predict the risk of renal impairment in the early stage of hypertension. Plenty of studies are also similar to our findings.Citation27–30 What’s more, some scholars also found that ABPM index, SUA level and microalbuminuria related to renal damage could be used as independent predictors of renal impairment in hypertensive patients.Citation31–33 Since our clinical data are limited, this may require additional researches with a larger sample size.
As hypertension progresses, patients suffer gradual damage to their cardiac structure and function. This may result in reduced cardiac output and secondary renal malperfusion. Inadequate renal blood flow or perfusion pressure can promote activation of the sympathetic nervous system (SNS) and the renin-angiotensin-aldosterone system (RAAS), prompting renin release by the juxtaglomerular cells, which will lead to the retention of sodium, increased vascular congestion, and lead to renal afferent arteriolar vasoconstriction and narrowing of the preglomerular microcirculation, and finally a continued decrease in renal blood flow and glomerular filtration rate will happen.Citation34 In addition, renal autoregulation disorder allows elevated systemic blood pressure to be transmitted to the glomerulus, followed by glomerular capillary hyperperfusion and hypertension, thereby facilitating glomerular structural injury and progressive loss of renal function.Citation35 We hypothesize that this may be one of the mechanisms by which LVEF and IVST might be used as independent predictive factors of renal damage in hypertension individuals. Interestingly, multiple studies showed that improving cardiac remodeling with angiotensin converting enzyme inhibitors or angiotensin receptor blockers could reduce proteinuria and delay deterioration of renal function, which supported our speculation.Citation36
The current study contains a few limitations. First, patients in our study recruited from the same hospital. And Since our study did not collect relevant data on treatment plans for patients with hypertension and study participants were treated with a variety of antihypertensive medications, which might have potential effects on the heart and kidney. Our conclusions need to be confirmed in additional, independent cohorts of hypertension patients. Second, because our study was retrospective, the clinical data we had was limited and it can be impacted by relevant confounding factors although we have tried our best to reduce its impact. Third, because our hospital was a general hospital, inpatient clinical data was collected. Our findings of study might not be representative of the entire hypertensive population. Fourth, this study found that blood pressure was related to patients’ future risk of renal damage in univariate Cox regression analysis, but no correlation was found in multivariate Cox regression analysis. It might due to the uncontrolled occurrence of antihypertensive regimen changes throughout time, the significant contribution of blood pressure to renal hemodynamic might have been understated. Fifth, Studies have shown that QT interval prolongation is a predictor of sudden cardiac death and also associated with LVH, and Electrocardiograms are low cost diagnostic tools for renal therapy centers.Citation37 But we did not consider the relationship between QT interval and renal damage outcomes in patients with hypertension, which requires further researches. Despite the limitations mentioned above, we conducted a preliminary exploration on the risk of long-term renal insufficiency caused by thickened IVS and decreased LVEF in hypertensive patients, and obtained meaningful results that could be used for clinical reference.
Conclusion
The abnormality of heart structure and function in patients with hypertension can not only reflect heart damage but also be used to evaluate the risk of kidney damage. Our study found that IVST and LVEF could be used as independent predictors of the risk of future renal damage in hypertensive patients. Through statistical risk factor scores, we found among the six risk factors of age≥67 years, elevated SCR, elevated CK MB, abnormal TNI, thickened IVST and reduced LVEF, the more risk factors suggested that patients were at higher risk of developing hypertensive renal dysfunction. Therefore, the recognition of alterations in left cardiac structure and function in hypertensive patients may help evaluate renal damage at an early stage.
Abbreviations
AAO, diameter Ascending aorta diameter; AUC, Area under curve; BUN, Blood urea nitrogen; Ca2+, Calcium ion concentration; CK, Clinical data of creatine kinase; CK MB, Creatine kinase isoenzyme MB; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CRS, Cardiorenal syndrome; DBP, Diastolic blood pressure; ECG, Electrocardiogram; eGFR, Estimated glomerular filtration rate; ESRD, End-stage renal disease; HDL, High-density lipoprotein; HR, Hazard ratio; IVS, thickness Interventricular septum thickness; K+, Potassium ion concentration; LAD, Left atrial diameter; LDL, Low-density lipoprotein; LDH, Lactate dehydrogenase; LVH, Left ventricular hypertrophy; LVDd, Left ventricular diameter at the end of diastole; LVEF, Left ventricular ejection fraction; Na+, Sodium ion concentration; NT proBNP, N-terminal pro-B-type natriuretic peptide; ROC, Receiver operating characteristics; SCR, Serum creatinine; SBP, Systolic blood pressure; SUA, Serum uric acid; TC, Total cholesterol; TDE, Two-dimensional echocardiography; TG Triglyceride; TNI, High-sensitivity troponin I.
Ethics Approval and Consent to Participate
The ethics committee of Zhongnan Hospital of Wuhan University approved this study, and the informed consent was obtained from all patients or their relatives or legally accepted representatives. All experiments were performed in accordance with the relevant guidelines and regulations according to the principles expressed in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article. All authors gave final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Fei Li, Feifei Yan, and Shengnan Liu are co-first authors for this study. We would like to gratefully acknowledge the support from Zhongnan Hospital of Wuhan University, study participants who participated in this work.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi:10.1161/CIR.0000000000000558
- Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi:10.1161/CIR.0000000000000757
- Zhou B, Carrillo-Larco RM, Danaei G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–980. doi:10.1016/S0140-6736(21)01330-1
- Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370(9587):591–603. doi:10.1016/S0140-6736(07)61299-9
- Saran R, Robinson B, Abbott KC, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6–A7. doi:10.1053/j.ajkd.2019.09.003
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a genoa investigation on complications. Hypertension. 1997;30(5):1135–1143. doi:10.1161/01.HYP.30.5.1135
- Ruilope LM, Salvetti A, Jamerson K, et al. Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol. 2001;12(2):218–225. doi:10.1681/ASN.V122218
- Santos M, Shah AM. Alterations in cardiac structure and function in hypertension. Curr Hypertens Rep. 2014;16(5):428. doi:10.1007/s11906-014-0428-x
- Jameel FA, Junejo AM, Khan Q, et al. Echocardiographic changes in chronic kidney disease patients on maintenance hemodialysis. Cureus. 2020;12(7):e8969. doi:10.7759/cureus.8969
- Kirkpatrick JN, Vannan MA, Narula J, Lang RM. Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol. 2007;50(5):381–396. doi:10.1016/j.jacc.2007.03.048
- Ronco C, Chionh CY, Haapio M, Anavekar NS, House A, Bellomo R. The cardiorenal syndrome. Blood Purif. 2009;27(1):114–126. doi:10.1159/000167018
- Ratto E, Viazzi F, Bonino B, et al. Left ventricular dilatation and subclinical renal damage in primary hypertension. J Hypertens. 2015;33(3):605–611, 611. doi:10.1097/HJH.0000000000000430
- Gori M, Senni M, Gupta DK, et al. Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3442–3451. doi:10.1093/eurheartj/ehu254
- Ersbøll M, Valeur N, Hassager C, Søgaard P, Køber L. The association between renal impairment and cardiac structure and function in patients with acute myocardial infarction. Am Heart J. 2014;167(4):506–513. doi:10.1016/j.ahj.2013.12.029
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004. doi:10.1097/HJH.0000000000002453
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367. doi:10.1016/S0894-7317(89)80014-8
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi:10.1093/ehjci/jev014
- Boudoulas KD, Triposkiadis F, Parissis J, Butler J, Boudoulas H. The cardio-renal interrelationship. Prog Cardiovasc Dis. 2017;59(6):636–648. doi:10.1016/j.pcad.2016.12.003
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–1539. doi:10.1016/j.jacc.2008.07.051
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–2219. doi:10.1093/eurheartj/eht151
- Georgiopoulou VV, Kalogeropoulos AP, Raggi P, Butler J. Prevention, diagnosis, and treatment of hypertensive heart disease. Cardiol Clin. 2010;28(4):675–691. doi:10.1016/j.ccl.2010.07.005
- Amann K, Rychlík I, Miltenberger-Milteny G, Ritz E. Left ventricular hypertrophy in renal failure. Kidney Int Suppl. 1998;68:S78–S85. doi:10.1046/j.1523-1755.1998.06818.x
- Izumaru K, Hata J, Nakano T, et al. Reduced estimated GFR and cardiac remodeling: a population-based autopsy study. Am J Kidney Dis. 2019;74(3):373–381. doi:10.1053/j.ajkd.2019.02.013
- Shamseddin MK, Parfrey PS. Sudden cardiac death in chronic kidney disease: epidemiology and prevention. Nat Rev Nephrol. 2011;7(3):145–154. doi:10.1038/nrneph.2010.191
- Song H, Hu H, Liao D, et al. Left ventricular hypertrophy predicts the decline of glomerular filtration rate in patients with type 2 diabetes mellitus. Int Urol Nephrol. 2018;50(11):2049–2059. doi:10.1007/s11255-018-1942-6
- Wu Y, Ma G, Sun H, Zhang S, Li X, Pelusi D. Prediction of renal function damage in patients with essential hypertension based on stepwise regression equation scanning by AASI. Scanning. 2022;2022:4728921. doi:10.1155/2022/4728921
- Yamada T, Ishihara M, Ichikawa K, Hiramatsu K. Proteinuria and renal function during antihypertensive treatment for essential hypertension. J Am Geriatr Soc. 1980;28(3):114–117. doi:10.1111/j.1532-5415.1980.tb00243.x
- Pang L, Wang Z, Zhao ZL, et al. Associations between estimated glomerular filtration rate and cardiac biomarkers. J Clin Lab Anal. 2020;34(8):e23336. doi:10.1002/jcla.23336
- Al BW, Mukherjee D, Kline-Rogers E, et al. Clinical association between renal insufficiency and positive troponin I in patients with acute coronary syndrome. Cardiology. 2004;102(4):215–219. doi:10.1159/000081013
- Leng WX, Zhang M, Cui H, Zeng LH, Hu YX. Correlations between indices of dynamic components of ambulatory blood pressure and renal damage in elderly Chinese male with essential hypertension. Blood Press Monit. 2020;25(6):303–309. doi:10.1097/MBP.0000000000000470
- Zhang L, Wang F, Wang X, Liu L, Wang H. The association between plasma uric acid and renal function decline in a Chinese population-based cohort. Nephrol Dial Transplant. 2012;27(5):1836–1839. doi:10.1093/ndt/gfr597
- Sakata S, Kimura G. [Microalbuminuria in hypertension]. Nihon Rinsho. 2004;62(1):97–102. Japanese.
- Kumar U, Wettersten N, Garimella PS. Cardiorenal syndrome: pathophysiology. Cardiol Clin. 2019;37(3):251–265. doi:10.1016/j.ccl.2019.04.001
- Ruiz-Hurtado G, Ruilope LM. Microvascular injury and the kidney in hypertension. Hipertens Riesgo Vasc. 2018;35(1):24–29. doi:10.1016/j.hipert.2017.03.002
- Russo D, Pisani A, Balletta MM, et al. Additive antiproteinuric effect of converting enzyme inhibitor and losartan in normotensive patients with IgA nephropathy. Am J Kidney Dis. 1999;33(5):851–856. doi:10.1016/S0272-6386(99)70416-6
- Mozos I. Laboratory markers of ventricular arrhythmia risk in renal failure. Biomed Res Int. 2014;2014:509204. doi:10.1155/2014/509204