Abstract
Background
Pulmonary infections are significant global health burdens, and conventional diagnostic methods (culture and polymerase chain reaction), are often limited by slow results and low sensitivity. Metagenomic next-generation sequencing (mNGS) offers a rapid, comprehensive alternative for identifying diverse pathogens, including rare and mixed infections. Thus, we assessed the diagnostic performance of mNGS in pulmonary infections, compared the findings with those of traditional pathogen detection methods, and explored its potential to enhance clinical diagnostics and patient care.
Methods
We collected samples from 125 immunocompromised patients diagnosed with pulmonary infection at the Department of Respiratory Medicine of Shenzhen Longgang Central Hospital from March 2020 to July 2022. We compared the rate of pathogen positivity and pathogen distribution between conventional pathogen detection methods and mNGS using samples including sputum, blood, and bronchoalveolar lavage fluid.
Results
Among the 125 cases of unexplained pulmonary infection, 82 (65.6%) and 40 (32.0%) tested positive for pathogens using mNGS and routine culture, respectively (P < 0.05). Both methods of pathogen detection were positive in 28 (22.4%) cases (complete match, 9; complete mismatch, 13; partial match, 6). However, 43.2% of cases only tested positive using mNGS, 9.4% only tested positive using routine tests, and 24.8% tested negative using both methods. A viral infection was present in 55.2% of cases. The detection rate of mycobacteria using mNGS (12.8%) was higher than that using conventional pathogen detection methods (5.6%).
Conclusion
mNGS technology enhances pathogen detection in unexplained pulmonary infections, enabling targeted antimicrobial therapy and consequently helping to reduce broad-spectrum antibiotic use, aligning treatments more closely with the causative pathogens. Thus, mNGS offers significant clinical value by improving treatment efficacy and potentially reducing antibiotic resistance in pulmonary infection cases.
Introduction
Pulmonary infection is the most common type of infectious disease. It is caused by a single or mixed infection of microorganisms, including bacteria, fungi, and viruses. Over the past few decades, the gradual increase in the aging Chinese population has led to increased year-on-year mortality due to pulmonary infection.Citation1 The etiology of most pulmonary infections is unclear.Citation2 Additionally, the current abuse of antibiotics in China is a critical issue, placing a great burden on healthcare resources.Citation3 It is difficult to use targeted drugs to treat an infection when the causative pathogen is uncertain. This aggravates the patient’s condition and may even lead to death. Therefore, accurate and timely diagnosis of the cause of infection and the reduction of unnecessary antibiotic use are crucial for treating pulmonary infection.Citation4
Although conventional microbiological tests (CMTs), including microbial culture, immunology,Citation5 and polymerase chain reaction (PCR),Citation6,Citation7 can identify the pathogens of some pulmonary infectious diseases, they are time-consuming and have a low detection rate. Furthermore, the use of antibiotics prior to sample collection reduces the rate of pathogen-positive cultures. More importantly, viruses, parasites, Mycoplasma, Chlamydia, and other atypical pathogens are difficult to culture and are vulnerable to the interference of bacteria that colonize the respiratory tract.Citation8 Immunological testing and PCR cannot detect unknown pathogens.Citation6,Citation7 Thus, the diversity of pathogens, the emergence of multidrug-resistant pathogens, and the occurrence of mixed infections mean that conventional etiological tests are unable to meet clinical needs.Citation9,Citation10
Metagenomic next-generation sequencing (mNGS) has the advantages of high throughput, unbiased detection, and a short detection time.Citation11 Kawabata et alCitation12 found that mNGS detected pathogens quickly and provided clear guidance on antibiotic treatment. mNGS is capable of detecting thousands of pathogens simultaneously, which may greatly improve diagnostic efficiency. Furthermore, mNGS results are not influenced by antibiotic use.Citation11,Citation13,Citation14 Therefore, mNGS is a potential comprehensive diagnostic tool to help identify causative pathogens of pulmonary infection,Citation15 and further studies are warranted.
Thus, we analyzed 125 patients with pulmonary infection to compare mNGS with CMTs and explore the clinical impact of mNGS detection of bacteria, viruses, fungi, parasites, and atypical pathogens (Mycobacterium, Mycoplasma, Chlamydia, etc.) in the diagnosis of unexplained pulmonary infection.
Materials and Methods
Study Participants
The study cohort comprised 133 patients diagnosed with pulmonary infection from March 2020 to July 2022 at the Department of Respiratory and Critical Care Medicine of Shenzhen Longgang Central Hospital. shows the patient selection process. Patients were included if they showed the following indications of unexplained pulmonary infection: (1) fever ≥38°C), (2) imaging features of pulmonary infection, (3) total white blood cell count decreased or returned to normal or lymphocyte count decreased during the early stage of the disease, and (4) no significant improvement or progressive worsening of the condition after 3–5 days of standardized antibiotic treatment Additionally, the patients had to be capable of withstanding fiberoptic bronchoscopy examination and volunteer to participate in our study. Patients were excluded if lung shadows on imaging were caused by noninfectious factors, such as interstitial lung disease, lung tumors, etc., and if bronchoscopy was contraindicated or if they were unwilling to undergo bronchoscopy. We collected the clinical information of all patients and recorded underlying diseases.
This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Shenzhen Longgang Central Hospital (2023ECPJ035). All patients gave informed consent.
Sample Collection and Processing
We collected samples of patients during hospitalization, including sputum, bronchoalveolar fluid (BALF), and blood. For sputum collection, individuals were instructed to deeply cough into a sterile container, ideally in the morning prior to consuming any food or beverages to ensure the sample accurately represented lower respiratory tract secretions. Prior to sputum culture, the quality of the sputum specimen was evaluated, with <10 squamous epithelial cells/LP and >25 multinucleated neutrophils/LP as the general criteria for eligibility. BALF was obtained using fiberoptic bronchoscopy conducted under local anesthesia and mild sedation. Briefly, a bronchoscope was inserted via the mouth or nose into the bronchi to specifically target areas highlighted in earlier imaging studies. Saline was then infused through the instrument and subsequently retrieved, carrying with it cells and fluids from the airways and alveoli of the lungs. Blood was drawn adhering to aseptic practices to avoid contamination. All specimens were promptly transported to the laboratory under strictly controlled conditions to maintain sample integrity.
All samples underwent CMTs and concurrent mNGS. Routine CMTs included smears and cultures, as well as real-time PCR for Mycobacterium tuberculosis (MTB) complex detection.
A patient was considered immunocompromised if suffering from autoimmune diseases, primary immunodeficiency diseases, or hematological malignancies or if receiving cancer chemotherapy, antirheumatic drugs, or other immunosuppressive drugs (including cyclosporin, hydroxychloroquine, and methotrexate).Citation16,Citation17 Additionally, patients suffering from diabetes, chronic lung disease, liver disease, kidney disease, or other common diseases, as well as those who were old and weak, were considered immunocompromised.
DNA Extraction and Sequencing
We used a Magen HiPure Bacterial DNA Kit to extract DNA from BALF and blood samples. The resultant DNA was ultrasonically broken into small fragments (200–300 bp). Adapter sequences were added to the spliced sequences, which were finally cyclized to form a single-stranded circular structure. Then, rolling circle amplification was used to generate DNA nanoballs, and a DNA library was prepared following subsequent processing. The library quality was checked using an Agilent 2100 Bioanalyzer and quantified using a Qubit 4.0 fluorometer. Sequencing was performed on an Illumina sequencing platform.
Data Analysis
To obtain high-quality sequencing data, we removed low-quality and small fragments (<35 bp), compared the sequences using Burrows Wheel Aligner (http://bio-bwa.sourceforge.net/), and removed human (host) sequences. We classified microorganisms by comparison with genomic databases of viruses, bacteria, fungi, and parasites. The classified reference database can be downloaded from the National Biotechnology Center for Biotechnology Information (ftp://ftp.ncbi.nlm.nih.gov/genomes/).
Criteria for Positive CMTs
For bacteria, cultures were considered positive if the number of colony-forming units was >104/mL. Smears were considered positive if gram-positive or gram-negative bacteria were detected under microscopy. For fungal culture and smear analysis, host factors, clinical characteristics, and microbiological evidence were all considered. Samples showing growth on MTB culture media or smears positive for acid-fast bacilli were considered positive for tuberculosis.
Criteria for Positive mNGS Results
When the coverage rate of a pathogen (including bacteria, viruses, and parasites but excluding MTB and fungi) detected by mNGS was >10 times that of other pathogens, it was considered a positive result.Citation18 Because of the low proportion of fungi, a coverage rate >5 times that of other pathogenic fungi detected by mNGS was considered positive.Citation19,Citation20 Samples were considered MTB-positive if ≥1 read was detected.Citation21,Citation22
Statistical Analysis
SPSS v22.0 statistical software was used to analyze all data. We used the t-test to determine the variance of normally distributed data. P-values < 0.05 were considered statistically significant.
Results
Clinical Characteristics and Sample Types
We enrolled 133 patients who were hospitalized in the Department of Respiratory Medicine at Shenzhen Longgang Central Hospital from March 2020 to July 2022 and underwent mNGS testing. We collected relevant clinical data from all patients. However, due to the loss of raw data (n = 5) and key clinical data (n = 3), only 125 cases were included in the final study cohort (). There were 77 males and 48 females, with an average age of 55 years. The elderly accounted for 36.0% (n = 45), and some patients had underlying diseases, such as hypertension (n = 32), diabetes (n = 22), chronic kidney disease (n = 3), coronary heart disease (n = 5) and hematologic disease (n = 8). Most patients had received antibiotic treatment before sample collection (107/125, 85.6%). 21 (16.8) patients received immunosuppressive drugs treatment. The average hospital stay was 13.2 days.
Table 1 Clinical Characteristics of All Patients (N = 125)
The sample types included tissue (n = 2), blood (n = 3), cerebrospinal fluid (n = 1), and BALF (n = 119).
CMT results for Pathogen Detection
All 30 blood cultures tested negative. We cultured pharyngeal swabs from 5 cases, all of which remained negative after 3 days. Of the 70 BALF samples, 7 were positive for MTB complex culture, and 9 were negative for acid-fast bacilli staining. Of the 115 sputum samples that underwent culture and smear examination, 40 were positive for pathogens (). Furthermore, there were 18 cases of mixed pathogenic infections, including 11 cases of fungal and bacterial coinfection, 4 cases of bacterial and viral coinfection, 2 cases of bacterial and parasitic coinfection, and 1 case of bacterial and Mycoplasma coinfection. The detected pathogens included Candida albicans, C. parapsilosis, C. lusitaniae, Penicillium, Aspergillus, Pseudomonas aeruginosa, Mycobacterium, and Klebsiella.
Table 2 Results of Traditional Culture Methods
Comparison Between mNGS and CMT Detection of Pathogens
In this study, 82/125 (65.6%) cases tested positive using mNGS, and 40 (32.0%) tested positive using traditional culture. There were 28 (22.4%) cases with positive results for both mNGS and traditional culture, meaning that 54 (43.2%) cases tested positive only with mNGS, 12 (9.6%) cases tested positive only with traditional culture, and 31 cases (24.8%) tested negative ( and ). Importantly, of the 28 cases that tested positive using both methods, 9 were completely matched, 13 completely mismatched, and the remaining 6 partially matched, indicating overlapping of at least one microbial pathogen.
Figure 2 Detection of pathogens using mNGS and CMTs. (A) Positive mNGS and culture results in 125 patients with pulmonary infection. (B) mNGS and traditional culture methods were positive for 82 and 40 patients, respectively. Among them, 28 cases were positive for both methods, 9 (7%) of which were completely matched.

Pathogen Types Detected by mNGS
mNGS of all 125 cases revealed that Pseudomonas was the most frequently identified (63 cases, 50.4%), followed by Haemophilus (18, 14.4%), Klebsiella (13, 10.4%, Achromobacter (5, 4%), and Oligotrophomonas (5, 4%). Other detected pathogens are presented in (). Haemophilus and Klebsiella were common in patients with fungal and bacterial infections. In a patient with chronic obstructive pulmonary disease and comorbidities of hypertension and coronary heart disease, the blaOXA-51 resistance gene was detected by mNGS, as well as pathogens such as Acinetobacter, Pneumocystis, and lymphocryptovirus. On the basis of these results, we used cefoperazone sodium, sulbactam sodium, and imipenem/cilastatin sodium intravenous for treatment, as well as oral compound sulfamethoxazole. After 12 days of hospitalization, the patient improved and was discharged.
Figure 3 Spectrum of pathogens detected by mNGS. (A) Distribution of the top 30 pathogens detected in the 125 cases of unexplained pulmonary infection. (B) Distribution of the top 30 pathogens detected in the 18 cases of mixed infection.
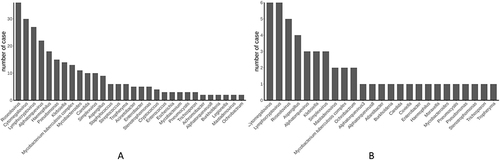
() presents the distribution of pathogens in the 18 patients with mixed infections, including Aspergillus (n = 4), Klebsiella (n = 3), Burkholderia (n = 1), Enterobacter (n = 1), Haemophilus (n = 1), Moraxella (n = 1), Stenotrophomonas (n = 1), and Pseudomonas (n = 1). Notably, the detection rate of cytomegalovirus and lymphocryptovirus in patients with mixed infection was also very high, accounting for 33.3% of mixed infections. Additionally, among the fungal and bacterial coinfections, 33.3% (n = 6) of patients with fungal infections were coinfected with 1 bacterial species, 27.8% (n = 5) with 2 bacterial species, and 38.9% (n = 7) with ≥3 bacterial species (Supplementary Table 1).
Distribution of Microorganisms in Different Groups
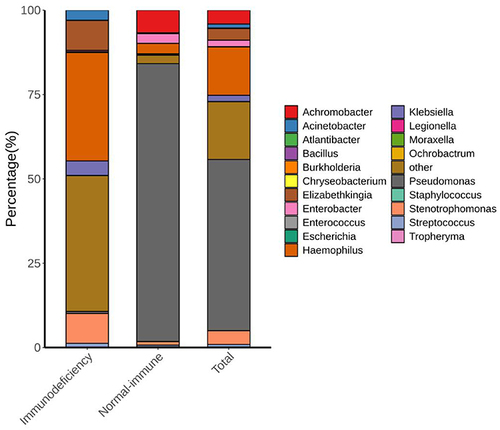
We classified the patients into two groups based on their clinical information: the immunocompromised group (n = 50) and the normal immune function group (n = 75). The pathogens detected in each group differed. The most common pathogens in the normal immune function group were Pseudomonas, Arcobacter, Haemophilus, Enterobacter, Stenotrophomonas, and Streptococcus. Haemophilus, Elizabethkingia, Stenotrophomonas, Klebsiella, Acinetobacter, Streptococcus, and Pseudomonas were the most common in the immunocompromised group. Notably, four pathogens (Alternaria, Elizabethkingia, Enterococcus, and Moraxella) were only detected in the immunocompromised group, and four different pathogens (Achromobacter, Bacillus, Legionella, and Chryseobacterium) were only detected in the normal immune function group ().
Pathogen Analysis of MTB-Positive Population
We statistically analyzed the pathogen lineage of MTB-positive patients. CMTs identified 7 (5.6%) MTB-positive cases, while mNGS identified 16 (12.8%) MTB-positive cases. The sensitivity of mNGS for detecting MTB was more than twice that of CMTs. Moreover, 6 cases tested positive for MTB using both methods and 9 cases only tested positive using mNGS. Coinfection in the MTB-positive population included roseolovirus (28.6%), Enterobacter (14.3%), Klebsiella (14.3%), Aspergillus (14.3%), lymphocryptovirus (14.3%), Burkholderia (7.1%), Enterococcus (7.1%), Staphylococcus (7.1%), Tropheryma (7.1%), and Trichosporon (7.1%). The 2 cases of Aspergillus coinfection were consistent with routine clinical testing results and diagnosis. All patients with MTB in this study had good treatment outcomes.
Virus Detection by mNGS
The rate of virus detection by mNGS was 55.2%. Among them, roseolovirus, belonging to the subfamily betaherpesvirinae, was detected in 36 cases (28.8%), followed by lymphocryptovirus (34 cases, 27%), cytomegalovirus (30 cases, 24.0%), alphatorquevirus (28 cases, 22.4%), simplexvirus (13 cases, 10.4%), and mastadenovirus (3 cases, 2.4%). Alphatorquevirus was detected in 1 blood sample. Roseolovirus, alphatorquevirus, and cytomegalovirus were detected in a tissue sample from a patient who had not received antibiotic treatment. Adenovirus was detected in 2 patients in the immunocompromised group but not in any patients in the normal immune function group, and this was consistent with clinical symptoms. One patient suffered from a severe autoimmune disease.
Discussion
This study explored the diagnostic performance of mNGS in unexplained pulmonary infection. Pathogen identification is extremely important for accurate treatment. CMTs (microbial culture, immunological methods, and PCR) have significant limitations for pathogen detection.Citation5–7 In contrast, mNGS is an unbiased sequencing method that detects all pathogenic bacteria in a sample, including commensal bacteria, pathogenic bacteria, and bacterial contaminants.
We used mNGS to analyze samples from 125 cases of pulmonary infection and compared the results with those of CMTs. The positivity rate was higher for mNGS than for CMTs, and this was statistically significant. Thus, mNGS has substantial advantages, which is consistent with previous studies. Tang et al analyzed 16 patients with immune deficiency using mNGS, revealing that mNGS detected more viruses and bacteria than traditional methods.Citation23 Fang et al used mNGS to analyze BALF from 72 patients with ventilator-associated pulmonary infection, reporting that the sensitivity for bacterial detection was 97.1% and that mNGS was more advantageous for detecting infections with multiple pathogens and infections with viruses.Citation24
Mackie et al reported that the number of fungi detected by mNGS was higher than that detected by traditional culture (27.8% vs 20.7%), indicating that mNGS was superior to traditional culture methods for detecting fungi.Citation25 However, in our study, the number of fungi detected by mNGS and traditional culture methods was similar (24.3% vs 24.0%). This may be because most patients in our study had hematological malignancies and were immunocompromised, so they were more likely to have received broad-spectrum antibiotics, which makes bacterial culture difficult. In our study, the types of fungi detected by mNGS included Candida, Aspergillus, Cryptococcus, Pneumocystis, Trichosporon, Cunninghamella, Fusarium, Mucor, and Rhizopus, while the only fungus detected by traditional culture was Candida. Therefore, our results indicate that mNGS detection of pathogens is superior to traditional culture for identifying multiple fungus types. Patients with severe fungal infections are often infected with multiple pathogens, and it is difficult to simultaneously detect multiple pathogens using traditional culture methods. There is a consensus among experts recommending the use of mNGS for the detection of multiple pathogens in critically ill patients.Citation26 In our study, the rate of detection of mixed infections using mNGS was significantly higher than using traditional culture (74.4% vs 14.4%; P < 0.05), confirming that mNGS is superior to traditional culture for the detection of mixed infections.
In our study, 12.8% and 5.6% of cases tested positive for MTB complex using mNGS and CMTs, respectively. A diagnosis of pulmonary infection due to nontuberculous mycobacteria is inadequate, and mNGS may be an effective method for precise pathogen identification in such cases.Citation27,Citation28 In our study, one sample was MTB-positive using CMTs but was not detected using mNGS. This may be due to the relatively thick cell wall of MTB, which requires a more aggressive wall-breaking process to release nucleic acids, resulting in a lower quality of extracted DNA.Citation25
Immunocompromised patients have an increased risk of infection with atypical and opportunistic pathogens,Citation29 and this was verified by the detection of Atlantic, Elizabethkingia, Enterococcus, and Moraxella in the immunocompromised group only. Moraxella has been confirmed to cause chronic respiratory tract infections, and Moraxella catarrhalis infection often occurs in patients with primary lung diseases.Citation30
An important consideration in the adoption of new diagnostic technologies is cost-effectiveness. Although mNGS provides a comprehensive spectrum of pathogen detection and a high degree of sensitivity, it may initially appear costlier than traditional culture-based methods. The direct costs of mNGS include the sequencing process and data analysis, which are generally higher than processes for traditional cultures. Nevertheless, the reduction in unnecessary broad-spectrum antibiotic use due to precise pathogen identification using mNGS can result in significant savings. This is particularly relevant in the treatment of pulmonary infections, where inappropriate therapy leads to extended hospital stays and additional treatments due to ineffective initial care. Indirect costs saved by using mNGS include reduced infection transmission, particularly in hospital settings, by enabling quicker and more accurate diagnoses. Moreover, faster diagnostic turnaround times will decrease the duration of hospitalization and time spent in intensive care units, substantially lowering healthcare costs. In the long term, the use of mNGS will contribute to decreased antimicrobial resistance—a major global health threat and economic burden. By reducing antibiotic misuse, mNGS will not only help to maintain the efficacy of existing antibiotics but also potentially decrease the need for developing new antibiotics, which is a costly and lengthy process. Further research is necessary to comprehensively evaluate the cost-effectiveness of mNGS, particularly compared with less invasive methods, like sputum analysis, and more traditional methods, such as BALF analysis and culture. Future studies should consider both the direct and indirect healthcare costs and savings, providing a holistic view of the economic impact of mNGS technologies in clinical practice.
This study has several limitations that warrant consideration in future research. First, the heterogeneity of the disease spectrum due to patients having various underlying conditions (diabetes, chronic obstructive pulmonary disease, interstitial lung disease, hypertension, and autoimmune diseases) may affect the generalizability of our findings. Second, the sample size was relatively small, limiting the robustness and statistical power of our conclusions. Third, obtaining BALF samples from all patients is unfeasible, particularly from those who are not critically ill or are at a higher risk from invasive procedures. Forth, in terms of pathogen detection, although mNGS enhances bacterial identification, it does not address undetectable pathogens that often require empirical treatment, particularly in patients with nonspecific symptoms or when infections are suspected but not confirmed by standard tests. This gap highlights a significant limitation of our study, which only focuses on detectable pathogens, potentially overlooking the broader spectrum of clinical realities where empirical treatments are common. Looking forward, it is crucial for future studies to include a larger and more diverse sample population to better validate our findings. Furthermore, future studies should emphasize comparative analyses between mNGS results from BALF and sputum samples to clearly define their diagnostic accuracies and clinical utilities. This approach will not only help the optimization of mNGS in clinical practice but also clarify the potential of mNGS as a supplement to or replacement for empirical treatment methods in scenarios where the use of CMTs for pathogen detection is challenging.
Conclusions
This study conclusively demonstrates the efficacy of mNGS for clinical diagnostics. mNGS considerably enhances pathogen detection rates and is capable of identifying rare and emerging pathogens with high accuracy and without bias. Given its comprehensive detection capabilities, mNGS holds substantial clinical value for the etiological diagnosis of pulmonary infections, suggesting its potential as a standard diagnostic method in clinical microbiology.
Abbreviations
mNGS, Metagenomic next-generation sequencing; CMT, Conventional microbiological test; MTB, Mycobacterium tuberculosis; PCR, Polymerase chain reaction; BALF, Bronchoalveolar lavage fluid.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Shenzhen Longgang Central Hospital (2023ECPJ035). Informed consent was obtained from all individual participants included in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Consent for Publication
Not applicable.
Disclosure
The authors declare that they have no competing interests in this work.
Acknowledgments
The authors would like to thank all patients who participated in this research for their support in publishing this article.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Sun Y, Li H, Pei Z, et al. Incidence of community-acquired pneumonia in urban China: a national population-based study. Vaccine. 2020;38(52):8362–8370. doi:10.1016/j.vaccine.2020.11.004
- Zhu YG, Tang XD, Lu YT, Zhang J, Qu JM. Contemporary situation of community-acquired pneumonia in China: a systematic review. J Transl Int Med. 2018;6(1):26–31. doi:10.2478/jtim-2018-0006
- Jiang N, Li R, Bao J, et al. Incidence and disease burden of community-acquired pneumonia in southeastern China: data from integrated medical resources. Hum Vaccin Immunother. 2021;17(12):5638–5645. doi:10.1080/21645515.2021.1996151
- Hardak E, Avivi I, Berkun L, et al. Polymicrobial pulmonary infection in patients with hematological malignancies: prevalence, co-pathogens, course and outcome. Infection. 2016;44(4):491–497. doi:10.1007/s15010-016-0873-3
- Loeffelholz M, Chonmaitree T. Advances in diagnosis of respiratory virus infections. Int J Microbiol. 2010;2010:126049. doi:10.1155/2010/126049
- Maartens G, Griesel R, Dube F, Nicol M, Mendelson M. Etiology of pulmonary infections in human immunodeficiency virus-infected inpatients using sputum multiplex real-time polymerase Chain reaction. Clin Infect Dis. 2020;70(6):1147–1152. doi:10.1093/cid/ciz332
- Gadsby NJ, Russell CD, McHugh MP, et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis. 2016;62(7):817–823. doi:10.1093/cid/civ1214
- Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10(2):299–306. doi:10.1038/mi.2016.108
- Zhang Y, Shou S. Pathogens and drug-resistance of hospital-acquired pneumonia in an EICU in Tianjin, China. Int J Biochem Mol Biol. 2021;12(2):49–54.
- Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021;10(10):1310. doi:10.3390/pathogens10101310
- Zhou X, Wu H, Ruan Q, et al. Clinical evaluation of diagnosis efficacy of active mycobacterium tuberculosis complex infection via metagenomic next-generation sequencing of direct clinical samples. Front Cell Infect Microbiol. 2019;9:351. doi:10.3389/fcimb.2019.00351
- Kawada J, Okuno Y, Torii Y, et al. Identification of viruses in cases of pediatric acute encephalitis and encephalopathy using next-generation sequencing. Sci Rep. 2016;6:33452. doi:10.1038/srep33452
- Chiu CY, Coffey LL, Murkey J, et al. Diagnosis of fatal human case of st. louis encephalitis virus infection by metagenomic sequencing, California, 2016. Emerg Infect Dis. 2017;23(10):1964–1968. doi:10.3201/eid2310.161986
- Murkey JA, Chew KW, Carlson M, et al. Hepatitis E virus-associated meningoencephalitis in a lung transplant recipient diagnosed by clinical metagenomic sequencing. Open Forum Infect Dis. 2017;4(3):ofx121. doi:10.1093/ofid/ofx121
- Chen X, Ding S, Lei C, et al. Blood and bronchoalveolar lavage fluid metagenomic next-generation sequencing in pneumonia. Can J Infect Dis Med Microbiol. 2020;2020:6839103. doi:10.1155/2020/6839103
- Ramirez JA, Musher DM, Evans SE, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. 2020;158(5):1896–1911. doi:10.1016/j.chest.2020.05.598
- Chen Y, Feng W, Ye K, et al. Application of metagenomic next-generation sequencing in the diagnosis of pulmonary infectious pathogens from bronchoalveolar lavage samples. Front Cell Infect Microbiol. 2021;11:541092. doi:10.3389/fcimb.2021.541092
- Langelier C, Zinter MS, Kalantar K, et al. Metagenomic sequencing detects respiratory pathogens in hematopoietic cellular transplant patients. Am J Respir Crit Care Med. 2018;197(4):524–528. doi:10.1164/rccm.201706-1097LE
- Schlaberg R, Chiu CY, Miller S, et al. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch Pathol Lab Med. 2017;141(6):776–786. doi:10.5858/arpa.2016-0539-RA
- Bittinger K, Charlson ES, Loy E, et al. Improved characterization of medically relevant fungi in the human respiratory tract using next-generation sequencing. Genome Biol. 2014;15(10):487. doi:10.1186/s13059-014-0487-y
- Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi:10.1093/cid/cix881
- Doughty EL, Sergeant MJ, Adetifa I, Antonio M, Pallen MJ. Culture-independent detection and characterisation of Mycobacterium tuberculosis and M. africanum in sputum samples using shotgun metagenomics on a benchtop sequencer. PeerJ. 2014;2(e585):e585. doi:10.7717/peerj.585
- Tang W, Zhang Y, Luo C, et al. Clinical application of metagenomic next-generation sequencing for suspected infections in patients with primary immunodeficiency disease. Front Immunol. 2021;12:696403. doi:10.3389/fimmu.2021.696403
- Fang X, Mei Q, Fan X, et al. Diagnostic value of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in ventilator-associated pneumonia patients. Front Microbiol. 2020;11:599756. doi:10.3389/fmicb.2020.599756
- Mackie SL, Dejaco C, Appenzeller S, et al. British society for rheumatology guideline on diagnosis and treatment of giant cell arteritis: executive summary. Rheumatology. 2020;59(3):487–494. doi:10.1093/rheumatology/kez664
- Society CT. Consensus of clinical pathways of metagenomic next-generation sequencing test in diagnosis of lower respiratory tract infections in China. Zhonghua Jie He He Hu Xi Za Zhi. 2023;46(4):322–335. doi:10.3760/cma.j.cn112147-20220701-00553
- Shi CL, Han P, Tang PJ, et al. Clinical metagenomic sequencing for diagnosis of pulmonary tuberculosis. J Infect. 2020;81(4):567–574. doi:10.1016/j.jinf.2020.08.004
- Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. doi:10.1183/13993003.00535-2020
- Azoulay E, Mokart D, Kouatchet A, Demoule A, Lemiale V. Acute respiratory failure in immunocompromised adults. Lancet Respir Med. 2019;7(2):173–186. doi:10.1016/S2213-2600(18)30345-X
- Okada F, Ando Y, Nakayama T, et al. Pulmonary thin-section CT findings in acute Moraxella catarrhalis pulmonary infection. Br J Radiol. 2011;84(1008):1109–1114. doi:10.1259/bjr/42762966