Abstract
Many large trials in the past 15 years have proven an increased risk of vascular complications in women using oral, mostly non-bioidentical, hormone therapy. The risk of vascular complications depends on the route of administration (oral versus transdermal), age, duration of administration, and type of hormones (bioidentical versus non-bioidentical). Acquired and/or hereditary thrombophilias (eg, factor V Leiden, prothrombin mutation G20210A, and others) lead to a further increase of risk for venous thromboembolism, stroke, or myocardial infarction. Therefore, bioidentical hormone therapy via the transdermal route seems to be the safest opportunity for hormone replacement therapy, although large trials for bioidentical hormone therapy are needed.
Introduction
Five major classes of human steroid hormones are known: estrogens, progestogens, androgens, mineralocorticoids, and glucocorticoids. The term “progestogen” refers to both the natural progesterone and synthetic compounds that have progestogenic activity similar to that of progesterone. The term “progestin” generally refers to synthetic progestogens. The term “progesterone” refers to the naturally occurring human molecule.Citation1–Citation3 Estrogens and progestogens are most commonly prescribed for the treatment of perimenopausal and menopausal symptoms such as hot flashes, night sweats, emotional lability, poor concentration, and sleep disturbance.
The endogenous estrogens found in humans include estradiol (E2), estriol (E3), estrone (E1), and their conjugates. The human ovary produces E2 and E1, whereas E3 is formed through 16α-hydroxylation of E1 and E2. Before menopause, the predominant estrogen in circulation is E2, secreted by the ovaries. E1 is found in highest concentration after menopause and is converted from E2 and adrenal androstenedione in adipose tissue. E3 is short-acting and the least potent estrogen, and it is not converted, unlike E1, into E2. E2 has the highest affinity for both estrogen receptors (alpha and beta); E1 binds only to estrogen receptor alpha (which is located in breast cancer cells and the endometrium); and E3 binds weakly to both receptors.
Progesterone in a nonpregnant woman is secreted by the ovaries and adrenal glands. Progestogens are needed in hormone replacement therapy (HRT) to prevent endometrial hyperplasia or neoplasia when estrogen is administered. In “classic” HRT, mostly synthetic progestins, such as medroxyprogesteronacetate (MPA), are used.Citation1 These synthetic progestins have different affinities for the progesterone receptor and they may also activate non-progesterone receptor steroid receptors in different tissues.Citation4
Because of this, the most physiological way to apply HRT is to give bioidentical hormones transdermally without first-pass mechanism in the liver to avoid unphysiological changes and actions of the hormones, as described in the Oral non-bioidentical HRT and the risk of venous thromboembolism (VTE), stroke, and coronary heart disease (CHD) section.
What types of sex hormones are used in HRT?
One should keep in mind that there are many different types of sex hormones in use for HRT, including those that are partially synthetic, semisynthetic, derived from animal sources, or bioidentical (which means identical to the naturally occurring human hormones, bioidentical human hormones) (see ).
Table 1 Types of sex hormones used in hormone replacement
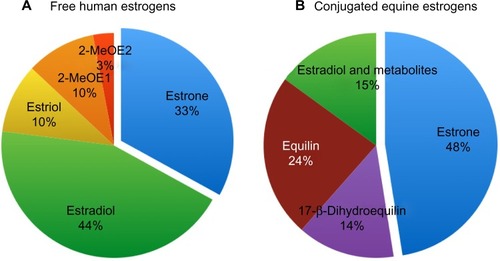
The synthetic hormones, or those from animal sources, that are used in HRT do not contain the physiological amounts of E1, E2, and E3. The physiological proportion of E1, E2, and E3 in human blood is about 33% E1, 45% E2, 10% E3, and about 10% metabolites of E1 and E2.Citation5 E2 is the most potent human estrogen. The distribution of estrogens in non-bioidentical estrogens differs significantly from human estrogens; this is especially true for conjugated equine estrogens (CEE) (see ).
Figure 1 Distribution of estrogens in women and horses.
Abbreviations: 2-MeOE1, 2-methoxyestrone; 2-MeOE2, 2-methoxyestradiol.
Progestins are synthetic progestogens, which are mostly used in HRT. The only bioidentical progestogen is progesterone (see ).
Both estrogens and progestins/progesterone can be used orally, transdermally, intranasally, or intramuscularly.
Oral non-bioidentical HRT and the risk of venous thromboembolism (VTE), stroke, and coronary heart disease (CHD)
shows the absolute risk of VTE in women with and without HRT.
Table 2 Absolute risk of VTE in peri- and postmenopausal women with and without HRT
Many large trials in the past 15 years, for example, the Women’s Health Initiative (WHI) trial in 2002Citation6,Citation7 and the Women’s International Study of long Duration Oestrogen after Menopause (WISDOM) trial,Citation8 showed a marked increase in the risk of VTE in women using oral non-bioidentical HRT. Of note, most of the women in these trials used oral CEE + MPA.
In the WHI trial, the risk of VTE in HRT patients was double that of patients in the placebo group;Citation6 in the Heart and Estrogen/progestin Replacement Study (HERS), the VTE risk was nearly threefold;Citation9 and, in the ESTHER study, the risk was fourfoldCitation10 (see ). VTE risk is much higher in women taking CEE than in women taking oral esterified estrogens.Citation11
Table 3 Risk for VTE, stroke, and CHD in women using oral, non-bioidentical hormone replacement therapy
The risk of VTE while taking CEE + MPA increases with age. Compared with women between the ages of 50–59 years who were taking placebo, the risk associated with HRT was higher with age: hazard ratio (HR) of 4.28 for women aged 60–69 years and 7.46 for women aged 70–79 years. Compared with women who were of normal weight and taking placebo, the risk associated with taking estrogen + progestin was increased among overweight (HR 3.8) and obese women (HR5.61), respectively.Citation12 The VTE risk in oral HRT users is highest in the first year of use,Citation13,Citation14 then declines, but remains on a higher level compared to nonusers. There is no elevated risk for past users of HRT after 6 weeks of stopping HRT.Citation15
The risk of VTE recurrence is lower in women who developed VTE on estrogen replacement and then stopped the HRT. This shows that, in most women with VTE on HRT, the HRT was the main risk factor.Citation16
The use of oral non-bioidentical HRT is associated with an overall 29% increase in the risk of ischemic stroke. The severity of stroke (poor functional outcome, death, disability, or dependency) increased with oral non-bioidentical HRT, with a nonsignificant increase of fatal stroke.Citation17 Tibolone, an oral synthetic steroid hormone similar to norethisterone and with estrogenic, progestogenic, and androgenic effects, also leads to an excess risk of stroke (odds ratio [OR] 2.18).Citation18
Oral CEE in combination with MPA was associated with an HR of 1.24 for CHD and not a protection against cardiac diseases, as was initially expected.Citation19 In postmenopausal women who had survived a myocardial infarction, the oral use of 2 mg E2 valerate did not reduce the overall risk for further cardiac events.Citation20 Women,who initiated hormone therapy (CEE) after hysterectomy closer to menopause tended to have reduced CHD risk compared with the increase in CHD risk among women more distant from menopause, but this trend test did not meet our criterion for statistical significance. The risk for stroke was increased in this study regardless of years since menopause.Citation21 In a group of women aged 45–58 years who were treated with either 2 mg oral E2 + norethisterone acetate, or in cases who had undergone hysterectomy with oral E2 alone, there was a significantly reduced risk of mortality, heart failure, and myocardial infarction.Citation22
Given the above results, the type of oral HRT, for example CEE + MPA versus E2 (which is a natural hormone) + progestogen of different types might be very important in terms of the risk of vascular complications. Of note, HRT with oral CEE + MPA did not have clinically meaningful effects on health-related quality of life.Citation23
The main reason for the increased risk for vascular complications in oral HRT is primarily the estrogen component; the progestin only modifies this risk, as is already known to be the case with oral contraception.Citation24 The amount of coagulation activation also seems to be dependent on the estrogen dosage used.Citation25
High endogenous levels of sex hormones of E2 and testosterone in the general population are not associated with increased risk of VTE.Citation26
Only one study of testosterone application in women and cardiovascular disease exists, and no increased risk of cardiovascular disease could be found in women prescribed testosterone tablets, injections, or implants.Citation27
Transdermal HRT and the risk of VTE
Sex hormones can be given easily via a transdermal route as a patch or gel/cream.
Both estrogens and progestogens have very good bioavailability when given transdermally. Another advantage of giving estrogens transdermally is the avoidance of the so-called first-pass mechanism in the liver. In the first-pass mechanism, which occurs only when steroid hormones are given orally, the structure of the hormones can change due to metabolizing effects, leading to formation of unphysiological molecules and activation of receptors of other steroid hormones. One possible consequence, among others, is a hypercoagulable state and a sometimes greatly reduced concentration of the drug, with the necessity of much higher doses needed orally in comparison to transdermal administration. With oral HRT, coagulation activation occurs (eg, higher levels of coagulation factor VII, greater thrombin generation peak levels, higher endogenous thrombin potential, higher level of prothrombin fragment 1.2)Citation28 and anticoagulants decrease (eg, antithrombin, protein C, protein S, tPA);Citation29,Citation30–Citation32 an acquired APC resistance phenotype can also occur.Citation33 Thrombin generation is significantly increased in women who use HRT orally (). This may be mediated by the first-pass metabolism of E1, the main metabolite of E2, because plasma E1 levels are higher in women taking oral estrogen.Citation34 The level of high-density lipoprotein cholesterol can increaseCitation35 and the level of lipoprotein(a) decreaseCitation36 while patients are on oral HRT, but this has obviously no clinical protective effect against arterial complications, as shown in the Oral non-bioidentical HRT and the risk of venous thromboembolism (VTE), stroke, and coronary heart disease (CHD) section.
Table 4 Changes in coagulation and metabolic parameters in users of oral versus transdermal hormone replacement therapy (HRT)
In a multicenter case-control study in postmenopausal women in France, oral use of estrogen (E2, mean dose 1.5 mg) was associated with a fourfold risk of VTE, especially when combined with norpregnane derivatives, whereas E2 via the transdermal route showed an OR of 0.9 for VTE.Citation10,Citation37
Roach et al showed that non-oral HRT did not increase the risk of VTE, but oral HRT did, by fourfold.Citation38 Another nested case-control study came to the same conclusion, with a relative risk of VTE of about 1.5 for oral estrogen use and no increase of VTE risk for transdermal use of estrogen.Citation39 The same was true in another study from 2011 involving around 54,000 women and comparing transdermal with oral estrogen use. The incidence ratio for VTE in transdermal users was 0.72.Citation40
Intranasal application of E2 + norethisterone is also not associated with an activation of the coagulation system.Citation41
Thrombophilia and HRT
For the absolute risk of VTE in women over 49 years, see .Citation12
Table 5 Thrombophilias and risk of VTE
The condition of thrombophilia can be hereditary (eg, factor V Leiden (FVL) mutation; prothrombin mutation G20210A [PTM]; deficiencies of antithrombin, protein C, or protein S; elevated lipoprotein[a]; non-O blood groupCitation42); acquired (obesity, smoking, varicosis, chronic inflammatory bowel disease, rheumatic diseases, intake of corticosteroids, antiphospholipid syndrome, hyperhomocysteinemia, elevated factor VIII levels, surgery, cast, etc);Citation43 or, often, a combination of both.
In a case-control study in women aged 45–64 years, the relative risk of idiopathic VTE in HRT users showed a significant association with APC resistance (OR 4.06), low antithrombin levels (OR 3.33), low protein C levels (OR 2.93), or high D-dimer levels (OR 3.84). D-dimer levels rose in patients on oral HRT,Citation44 but transdermal HRT had no effect in this regard.Citation30 Carriers of APC resistance/FVL who used oral HRT had a 13-fold increase of VTE risk.Citation45 Two other studies found similar results, with an OR of about 14 for women with FVL + oral HRT compared to women assigned to placebo.Citation46,Citation47
In another study in postmenopausal women with idiopathic VTE, the combination of either FVL or PTM and oral estrogen was associated with a 25-fold increased risk of VTE compared with non-users without mutation.
FVL or PTM alone without oral estrogen use showed ORs of 3.4 and 4.8, respectively for VTE; however, the risk for women with prothrombotic mutation using transdermal estrogen was similar to that of women with a prothrombotic mutation who were not using estrogen.Citation48
For the other known hereditary thrombophilias, such as deficiencies of antithrombin, protein C, and protein S or elevation of lipoprotein(a), no data exist for the VTE risk in HRT users; the same is true for the acquired thrombophilic disorder antiphospholipid syndrome. This may be due to the rarity of these conditions (see ).
Combined hereditary risk factors, eg, the combination of non-O blood group and either FVL or PTM, further increases the risk of VTE and myocardial infarction.Citation49
In a study from 2001, in which postmenopausal women received mainly esterified oral estrogens, the risk of nonfatal myocardial infarction was increased eleven-fold in women with PTM compared with women without HRT and the wild-type genotype.Citation50
There are no further data regarding the roles of specific thrombophilias and HRT on the risk of CHD or stroke.
Bioidentical HRT (BHR)
The finding that the CCE + MPA arm, in particular, of the WHI study showed more risks than benefits for the patients led to a dramatically changed prescribing practice of HRT all over the world.Citation51 In USA, prescription of CEE + MPA has decreased by 63% between 2002 and now. A substantial number of patients expressed a loss of trust in information about HRT and in their physicians after publication of the WHI Trial. Furthermore, many women desire a “natural” alternative medication for treating menopausal symptoms. BHR may be one such alternative.
Endocrinologists define bioidentical hormones as compounds that have exactly the same chemical and molecular structure as hormones that are produced in the human body. This is only true for a few estrogens and for the only natural progestogen (progesterone) (see ). The bioidentical hormones are usually derived from plant sources. Progesterone is available as oral micronized progesterone in oil or for vaginal use as gel or capsules. The micronized form of progesterone improves absorption of oral progesterone. The most common combinations for BHR include endogenous estrogen (mostly E2, E1, E3) and progesterone, preferably transdermally. Sometimes other ingredients, such as testosterone, are added. In Europe in particular, several bioidentical formulations for transdermal application are approved, for example E2 (oral 1–2 mg, or as a patch [25–100 μg/24 h]) and E3 (0.5–2 mg) for oral and transdermal or vaginal use. Natural micronized progesterone (100 mg capsule for oral or vaginal use) is also approved in most countries. Some small studies have shown favorable effects of BHR on myocardial ischemia and cardiovascular biomarkers.Citation52–Citation54
A recently published study showed a higher, doubled risk of VTE and possibly myocardial infarction in users of oral CEE compared to oral E2.Citation55 Another study showed a 2.5-fold risk for VTE in users of CEE, and the risk was much higher for women with hereditary thrombophilia (OR 9.1). In this study, the use of esterified estrogen was not associated with a higher risk for VTE without thrombophilia.Citation56
BHR has also been demonstrated to cure typical menopausal symptomsCitation57–Citation59 while lowering lipid levels.Citation60
BHR, usually applied transdermally, seems to be a more physiological and safer alternative to classic HRT (eg, CEE + MPA), but large clinical studies are needed to confirm this.
Conclusion
Many different types of HRT exist (synthetic versus bioidentical, oral versus transdermal, etc), so the results, benefits, and risks of a particular type of HRT should not be assumed of other types of HRT.
The highest risk for vascular complications is associated with oral, non-bioidentical HRT, especially with oral CEE + MPA or with oral estrogens + synthetic progestins. Oral HRT shows an increased risk of vascular complications, while transdermal applications do not. Women with hereditary and/or acquired risk factors or a history of vascular complications should use transdermal and not oral HRT.
Transdermal BHR is possibly the best choice for any woman wishing to use HRT, but further studies on this option are needed.
Acknowledgments
I would like to thank my colleague Dr Guether Kapper for the creation of .
Disclosure
The author reports no conflicts of interest in this work.
References
- FilesJAKoMGPruthiSBioidentical hormone therapyMayo Clin Proc201186767368021531972
- ConawayEBioidentical hormones: an evidence-based review for primary care providersJ Am Osteopath Assoc2011111315316421464264
- MoskowitzDA comprehensive review of the safety and efficacy of bioidentical hormones for the management of menopause and related health risksAltern Med Rev200611320822317217322
- HoltorfKThe bioidentical hormone debate: are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy?Postgrad Med20091211738519179815
- XuXRomanJMIssaqHJKeeferLKVeenstraTDZieglerRGQuantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometryAnal Chem200779207813782117848096
- RossouwJEAndersonGLPrenticeRLWriting Group for the Women’s Health Initiative InvestigatorsRisks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trialJAMA2002288332133312117397
- AndersonGLLimacherMAssafARWomen’s Health Initiative Steering CommitteeEffects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trialJAMA2004291141701171215082697
- VickersMRMacLennanAHLawtonBWISDOM groupMain morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal womenBMJ2007335761323917626056
- HulleySGradyDBushTRandomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research GroupJAMA199828076056139718051
- CanonicoMOgerEPlu-BureauGEstrogen and Thromboembolism Risk (ESTHER) Study GroupHormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER studyCirculation2007115784084517309934
- SmithNLHeckbertSRLemaitreRNEsterified estrogens and conjugated equine estrogens and the risk of venous thrombosisJAMA2004292131581158715467060
- CushmanMKullerLHPrenticeRWomen’s Health Initiative InvestigatorsEstrogen plus progestin and risk of venous thrombosisJAMA2004292131573158015467059
- Pérez GutthannSGarcía RodríguezLACastellsagueJDuque OliartAHormone replacement therapy and risk of venous thromboembolism: population based case-control studyBMJ199731470837968009081000
- HøibraatenEQvigstadEArnesenHLarsenSWickstrømESandsetPMIncreased risk of recurrent venous thromboembolism during hormone replacement therapy – results of the randomized, double-blind, placebo-controlled estrogen in venous thromboembolism trial (EVTET)Thromb Haemost200084696196711154141
- CanonicoMFournierACarcaillonLPostmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort studyArterioscler Thromb Vasc Biol201030234034519834106
- EischerLEichingerSKyrlePAThe risk of recurrence in women with venous thromboembolism while using estrogens: a prospective cohort studyJ Thromb Haemost201412563564024548536
- BathPMGrayLJAssociation between hormone replacement therapy and subsequent stroke: a meta-analysisBMJ2005330748734215640250
- FormosoGPerroneEMaltoniSShort and long term effects of tibolone in postmenopausal womenCochrane Database Syst Rev20122CD00853622336846
- MansonJEHsiaJJohnsonKCWomen’s Health Initiative InvestigatorsEstrogen plus progestin and the risk of coronary heart diseaseN Engl J Med2003349652353412904517
- CherryNGilmourKHannafordPESPRIT teamOestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trialLancet200236093502001200812504395
- RossouwJEPrenticeRLMansonJEPostmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopauseJAMA2007297131465147717405972
- SchierbeckLLRejnmarkLToftengCLEffect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trialBMJ2012345e640923048011
- HaysJOckeneJKBrunnerRLWomen’s Health Initiative InvestigatorsEffects of estrogen plus progestin on health-related quality of lifeN Engl J Med2003348191839185412642637
- LidegaardØNielsenLHSkovlundCWSkjeldestadFELøkkegaardERisk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–2009BMJ2011343d642322027398
- KohKKShinMSSakumaIEffects of conventional or lower doses of hormone replacement therapy in postmenopausal womenArterioscler Thromb Vasc Biol20042481516152115166013
- HolmegardHNNordestgaardBGSchnohrPTybjaerg-HansenABennMEndogenous sex hormones and risk of venous thromboembolism in women and menJ Thromb Haemost201412329730524329981
- van StaaTPSprafkaJMStudy of adverse outcomes in women using testosterone therapyMaturitas2009621768019108962
- BlondonMvan Hylckama VliegAWigginsKLDifferential associations of oral estradiol and conjugated equine estrogen with hemostatic biomarkersJ Thromb Haemost201412687988624628832
- van BaalWMEmeisJJvan der MoorenMJKesselHKenemansPStehouwerCDImpaired procoagulant-anticoagulant balance during hormone replacement therapy? A randomised, placebo-controlled 12-week studyThromb Haemost2000831293410669150
- PostMSChristellaMThomassenLGEffect of oral and transdermal estrogen replacement therapy on hemostatic variables associated with venous thrombosis: a randomized, placebo-controlled study in postmenopausal womenArterioscler Thromb Vasc Biol20032361116112112730085
- ScarabinPYAlhenc-GelasMPlu-BureauGTaisnePAgherRAiachMEffects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women: a randomized controlled trialArterioscler Thromb Vasc Biol19971711307130789409295
- TeedeHJMcGrathBPSmolichJJPostmenopausal hormone replacement therapy increases coagulation activity and fibrinolysisArterioscler Thromb Vasc Biol20002051404140910807761
- OgerEAlhenc-GelasMLacutKSARAH InvestigatorsDifferential effects of oral and transdermal estrogen/progesterone regimens on sensitivity to activated protein C among postmenopausal women: a randomized trialArterioscler Thromb Vasc Biol20032391671167612869355
- BagotCNMarshMSWhiteheadMThe effect of estrone on thrombin generation may explain the different thrombotic risk between oral and transdermal hormone replacement therapyJ Thromb Haemost2010881736174420553380
- [No authors listed]Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI TrialJAMA199527331992087807658
- SomaMROsnago-GaddaIPaolettiRThe lowering of lipoprotein[a] induced by estrogen plus progesterone replacement therapy in postmenopausal womenArch Intern Med199315312146214688390232
- ScarabinPYOgerEPlu-BureauGEStrogen and THromboEmbolism Risk Study GroupDifferential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism riskLancet2003362938242843212927428
- RoachRELijferingWMHelmerhorstFMCannegieterSCRosendaalFRvan Hylckama VliegAThe risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapyJ Thromb Haemost201311112413123136837
- RenouxCDell’AnielloSSuissaSHormone replacement therapy and the risk of venous thromboembolism: a population-based studyJ Thromb Haemost20108597998620230416
- LalibertéFDeaKDuhMSKahlerKHRolliMLefebvrePDoes the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapyMenopause201118101052105921775912
- HemelaarMRosingJKenemansPThomassenMCBraatDDvan der MoorenMJLess effect of intranasal than oral hormone therapy on factors associated with venous thrombosis risk in healthy postmenopausal womenArterioscler Thromb Vasc Biol20062671660166616645152
- GuimarãesDAdos SantosMSGomesKBInteraction between oral estrogen plus progestogen therapy and ABO blood groups on coagulation activation in postmenopausal womenMenopause201219333934522089178
- BergendalABremmeKHedenmalmKRisk factors for venous thromboembolism in pre-and postmenopausal womenThromb Res2012130459660122704078
- SuminoHIchikawaSSawadaYEffects of hormone replacement therapy on blood coagulation and fibrinolysis in hypertensive and normotensive postmenopausal womenThromb Res2005115535936615733968
- LoweGWoodwardMVesseyMRumleyAGoughPDalyEThrombotic variables and risk of idiopathic venous thromboembolism in women aged 45–64 years. Relationships to hormone replacement therapyThromb Haemost200083453053510780311
- HerringtonDMVittinghoffEHowardTDFactor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary diseaseArterioscler Thromb Vasc Biol20022261012101712067913
- WuORobertsonLLanghornePOral contraceptives, hormone replacement therapy, thrombophilias and risk of venous thromboembolism: a systematic review. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) StudyThromb Haemost2005941172516113779
- StraczekCOgerEYon de Jonage-CanonicoMBEstrogen and Thromboembolism Risk (ESTHER) Study GroupProthrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administrationCirculation2005112223495350016301339
- SodeBFAllinKHDahlMGyntelbergFNordestgaardBGRisk of venous thromboembolism and myocardial infarction associated with factor V Leiden and prothrombin mutations and blood typeCMAJ20131855E229E23723382263
- PsatyBMSmithNLLemaitreRNHormone replacement therapy, prothrombotic mutations, and the risk of incident nonfatal myocardial infarction in postmenopausal womenJAMA2001285790691311180734
- HershALStefanickMLStaffordRSNational use of postmenopausal hormone therapy: annual trends and response to recent evidenceJAMA20042911475314709575
- StephensonKNeuenschwanderPFKurdowskaAKThe effects of compounded bioidentical transdermal hormone therapy on hemostatic, inflammatory, immune factors; cardiovascular biomarkers; quality-of-life measures; and health outcomes in perimenopausal and postmenopausal womenInt J Pharm Compd2013171748523627249
- RosanoGMWebbCMChierchiaSNatural progesterone, but not medroxyprogesterone acetate, enhances the beneficial effect of estrogen on exercise-induced myocardial ischemia in postmenopausal womenJ Am Coll Cardiol20003672154215911127455
- HillebrandUHausbergMStockC17beta-estradiol increases volume, apical surface and elasticity of human endothelium mediated by Na+/H+ exchangeCardiovasc Res200669491692416412402
- SmithNLBlondonMWigginsKLLower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogensJAMA Intern Med20141741253124081194
- SmithNLHeckbertSRLemaitreRNConjugated equine estrogen, esterified estrogen, prothrombotic variants, and the risk of venous thrombosis in postmenopausal womenArterioscler Thromb Vasc Biol200626122807281216973976
- SimonJABouchardCWaldbaumAUtianWZborowskiJSnabesMCLow dose of transdermal estradiol gel for treatment of symptomatic postmenopausal women: a randomized controlled trialObstet Gynecol2007109358859617329509
- BachmannGASchaefersMUddinAUtianWHLowest effective transdermal 17beta-estradiol dose for relief of hot flushes in postmenopausal women: a randomized controlled trialObstet Gynecol2007110477177917906008
- KicovicPMCortes-PrietoJMilojevićSHaspelsAAAljinovicAThe treatment of postmenopausal vaginal atrophy with Ovestin vaginal cream or suppositories: clinical, endocrinological and safety aspectsMaturitas1980242752826785553
- TakahashiKManabeAOkadaMKuriokaHKanasakiHMiyazakiKEfficacy and safety of oral estriol for managing postmenopausal symptomsMaturitas200034216917710714912
