Abstract
Purpose
Hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is a critical condition associated with unfavorable survival rates. Recent studies have indicated that the platelet-to-monocyte ratio (PMR) is considered an effective prognostic marker in several diseases. However, there has been no study to evaluate the prognostic value of PMR in HBV-ACLF patients. Therefore, this study aimed to investigate the association between PMR and 28-day survival in these patients.
Methods
In this retrospective study, data, including clinical and laboratory parameters, were collected for 184 HBV-ACLF patients. Disease severity was assessed using the Model for End-Stage Liver Disease (MELD) score. Logistic regression analyses were conducted to identify predictors influencing 28-day survival. Receiver-operating characteristic curve (ROC) analyses were performed to assess the predictive abilities of the identified predictors.
Results
During the 28-day follow-up period, 56 (30.4%) HBV-ACLF patients died. PMR was significantly lower in non-survivors than in survivors (P <0.001). Logistic regression demonstrated that PMR (Odds ratio, 0.983; 95% Confidence interval, 0.976–0.990; P=0.001) and MELD score (Odds ratio, 1.317; 95% Confidence interval, 1.200–1.446; P <0.001) were independent risk factors for mortality in HBV-ACLF patients. The area under ROC curve for PMR was 0.760 (sensitivity=0.840, specificity=0.620, P=0.001) at a cut-off value of 140.6, and the area under ROC curve for MELD score was 0.819 (sensitivity=0.700, specificity=0.860, P=0.001) at a cut-off value of 23.1. PMR and MELD score exhibited similar predictive performances (Z=1.229; P=0.219). Furthermore, the combined use of PMR and MELD score further increased the area under the ROC curve to 0.858, which more accurate prognosis prediction than use of either factor alone (both P< 0.05).
Conclusion
The PMR could serve as a reliable tool for predicting mortality in HBV-ACLF patients. Additionally, combining the PMR with the MELD score could improve prognostic accuracy for predicting 28-day mortality in these patients. However, further and larger studies are needed to confirm our findings.
Introduction
Acute-on-chronic liver failure (ACLF) is characterized by the presence of multiple organ failures, poor treatment effects, and poor survival.Citation1,Citation2 The short-term mortality of patients with ACLF can reach as high as 50–90%.Citation3,Citation4 In China, hepatitis B virus (HBV) infection accounts for approximately 80% of ACLF cases due to its high prevalence.Citation4,Citation5 Liver transplantation remains the most effective treatment for HBV-ACLF; however, its feasibility is limited by organ shortages. To date, several predictive scoring systems have been developed to evaluate the prognosis of patients with ACLF, including the Model for End-stage Liver Disease (MELD) score, the Chronic Liver Failure Consortium Organ Failure score (CLIF-C OFs), the CLIF-C ACLFs, and the Chinese Group on the Study of Severe Hepatitis B score (COSSH-ACLFs). However, the MELD score, CLIF-C OFs, and CLIF-C ACLFs were all established in Western countries, where the main causes of disease are hepatitis C virus and alcohol, rather than HBV. Consequently, using these scoring systems to predict HBV-ACLF prognosis remains challenging.Citation6,Citation7 Although the COSSH-ACLFs is a predictive scoring model based on HBV infection, it involves multiple parameters and requires complex organ failure assessments Therefore, there is an urgent need to identify an accurate and convenient marker for HBV-ACLF prognosis and disease monitoring, which are imperative for delivering effective treatment and reducing mortality.
Persistent inflammation and immune dysregulation have been shown to play central roles in progression of ACLF,Citation8,Citation9 and can determine the severity of tissue damage and patient prognosis. Numerous studies have indicated that inflammation has impacts on blood cell levels and associated parameters, such as white blood cells, neutrophils, lymphocytes, platelets, and red cell distribution width. Consequently, a series of blood cell-based indices, including neutrophil-to-lymphocyte ratio, red cell distribution width-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and platelet-to-white blood cell ratio, may have the potential to serve as prognostic indicators for end-stage liver disease.Citation10,Citation11 Monocytes play a vital role in innate immune responses, by serving as the initial defense against pathogens. These cells act as central mediators in immune responses and have an important role in ACLF pathogenesis. Studies have revealed the presence of monocyte dysfunction and immune abnormalities in patients with ACLF.Citation12,Citation13 Meanwhile, a decreased platelet count has been associated with liver fibrosis in patients with chronic hepatitis B,Citation14,Citation15 and thrombocytopenia is one of the most common complications in liver diseases. Its severity is often correlated with the severity of the liver condition.Citation16 Thus, the combination of monocytes and platelets may serve as a prognostic indicator for HBV-ACLF patients. Recently, platelet-to-monocyte ratio (PMR) has been shown to be correlated with prognosis in various clinical scenarios. For example, Dahlen et alCitation17 found that PMR was associated with cardiac function deterioration, as well as a poor prognosis. Similarly, Zhou et alCitation18 identified PMR as an independent predictor for 30-day mortality in decompensated cirrhosis, while Nikiforuk et alCitation19 demonstrated its utility in screening of patients for hepatitis C virus through diagnostic tests on complete blood samples. However, no studies have investigated the correlation between PMR and prognosis in HBV-ACLF. Given this context, the present study aimed to investigate the prognostic value of PMR in patients with HBV-ACLF.
Methods
Participants
Patients diagnosed with HBV-ACLF and admitted to the Department of Hepatology between March 2020 and April 2022 were included in the study. The retrospective study was performed according to the Declaration of Helsinki and was approved by the Ethics Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region (approval number: 2023–187). The requirement for obtaining informed consent from patients was waived because of the retrospective nature of the study. ACLF was defined as international normalized ratio (INR) ≥1.5 and total bilirubin ≥171.0 µmol/L in accordance with the Asian Pacific Association for the Study of the Liver guideline.Citation4 The inclusion criteria were: (1) age between 18 and 75 years; and (2) ACLF caused by HBV infection. The exclusion criteria were: (1) patients diagnosed with hepatocellular carcinoma; (2) patients with concurrent hepatitis C virus or human immunodeficiency virus infection; (3) patients currently receiving steroid therapy; (4) patients with hematological disorders; and (5) patients with underlying liver diseases, including autoimmune, alcohol-related, or drug-related liver conditions. Ultimately, 184 patients were enrolled in the study (). The outcome was the survival status at 28 days.
Demographic, Hematological, and Biochemical Parameters
The following data were obtained from the medical records of the patients on admission: demographic variables and laboratory test results, including white blood cells and their subpopulations (lymphocytes, neutrophils, monocytes), platelets, and hemoglobin. Liver function parameters (total bilirubin, alanine transaminase, aspartate transaminase, total protein, albumin), coagulation parameters (INR), and kidney function indicators (serum creatinine, blood urea nitrogen) were also recorded. Survival status was noted at 28 days after admission. Baseline PMR was calculated by dividing the platelet count by the monocyte count. Severity of liver failure and disease prognosis were assessed using the MELD score.Citation20
Statistical Analysis
Data were presented as median (25–75 percentiles) or number. Differences between the two groups were assessed using the Mann–Whitney U-test and the chi-square test. The Spearman test was employed to explore the relationships between variables. Logistic regression analyses were conducted to identify independent predictors for 28-day mortality. Receiver-operating characteristic (ROC) curve analyses were performed to assess the predictive accuracy for mortality. The discriminatory ability was estimated using the area under the ROC curve (AUROC). Survival rates were estimated by the Kaplan–Meier method. Values of P<0.05 were considered statistically significant. All statistical analyses were conducted using SPSS 22 software and MedCalc 12.7.0 software.
Results
Basic Clinical Data and Characteristics
A total of 184 patients were enrolled in the study. The patients comprised 166 (90%) men and 18 (10%) women, with a median age of 48.0 years (range: 37.5–57.0 years). The median PMR was 138.5 (25–75 percentiles: 102.1–202.2) on admission. Correlation analysis revealed a negative relationship between PMR and MELD score (r=−0.347; P=0.001).
The 184 patients were categorized into survivors and non-survivors based on their outcomes at 28 days. The baseline characteristics of the two groups are summarized in . Non-survivors exhibited higher levels of total bilirubin, blood urea nitrogen, INR, white blood cells, neutrophils, monocytes, and MELD score, but lower levels of platelets and PMR compared with survivors.
Table 1 Characteristics of the Survivors and Non-Survivors Among the HBV-ACLF Patients
Predictors of Survival
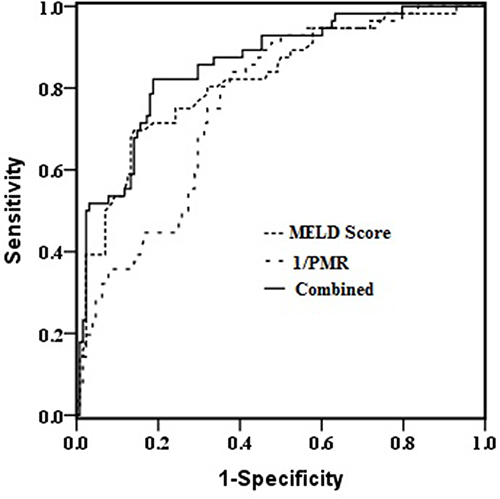
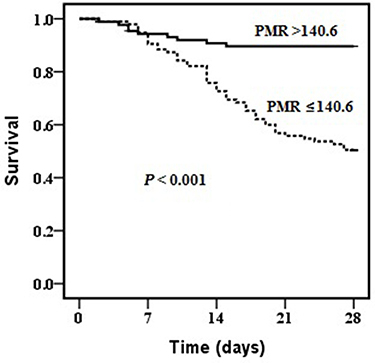
The univariate and multivariate analyses identified PMR and MELD score as independent predictors of mortality in HBV-ACLF patients (). The predictive performances of PMR and MELD score for 28-day survival were evaluated by ROC curve analyses. PMR was changed to 1/PMR by inverse transformation. The AUC for 1/PMR was 0.760, with a cut-off point of 140.6, a sensitivity of 0.840, and a specificity of 0.620. For MELD score, the AUC was 0.819, with a cut-off point of 23.1, a sensitivity of 0.700, and a specificity of 0.860. The prognostic accuracy of PMR was comparable to that of MELD score (Z=1.229; P=0.219). Combination of PMR with MELD score improved the prognostic accuracy (AUC: 0.858), compared with the use of each factor alone (both P<0.05; ). Moreover, patients with PMR >140.6 had a higher 28-day survival rate than patients with PMR ≤140.6 (P<0.001; ).
Table 2 Univariate and Multivariate Analyses of Risk Factors Associated with 28-Day Mortality in the HBV-ACLF Patients
Discussion
Accurate and early prediction of prognosis is crucial for the management of HBV-ACLF patients. In the present study, we investigated the association of PMR with 28-day outcomes in HBV-ACLF patients. We found that decreased PMR was associated with increased mortality, and that PMR has the potential to be a useful and relatively simple tool for risk stratification and management decision-making. The MELD score is considered the most useful scoring system for predicting the prognosis of liver disease patients, and is calculated using three key components: total bilirubin, creatinine, and INR. However, many studies have shown that the MELD score has limitations for accurate prediction of short-term mortality in ACLF patients.Citation21–23 This may arise because the MELD score does not consider the existence of all extrahepatic organ failures, such as cerebral failure, coagulation failure, renal failure, and lung failure. In addition, this scoring system require complex calculation and are inconvenient for routine practice.Citation20 In the present study, we found that non-survivors had a lower PMR than survivors. Furthermore, PMR was identified as a novel predictor of 28-day mortality in the multivariate regression analysis. According to the Results of the ROC curve, the AUC of PMR is 0.760 (acceptable) at a cut-off value of 140.6. This result indicates that a PMR higher than 140.6 may be a good predictor for excluding mortality in HBV-ACLF patients. In comparison, the AUC of the MELD score is 0.810 (excellent) at a cut-off value of 23.1. We found that the predictive ability of PMR is only slightly lower than that of the MELD score. PMR, which requires measurement of just two routine laboratory parameters, is more easily calculated and cost-effective than the MELD score. Previous studies have found correlations between prognosis and several hematological indices, including neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and red cell distribution width in end-stage liver disease.Citation24–26 The present findings suggest that PMR may serve as an effective predictive biomarker for identifying ACLF patients at high risk of mortality. Importantly, the combination of PMR and MELD score showed improved prognosis prediction compared with the use of each factor alone.
The mechanism underlying the association between low PMR and high mortality in HBV-ACLF remains to be elucidated. To date, HBV-ACLF has been recognized as a complex disease marked by excessive inflammation and immune dysfunction. In the present study, the decreased PMR in non-survivors was mainly attributed to lower platelets and higher monocytes compared with survivors. Monocytes, which play a crucial role as immune response initiators, have significant functions in phagocytosis, bacterial elimination, antigen presentation, cytokine production, immune cell recruitment to infection sites, and immune effector cell activation.Citation27 Shi et alCitation28 demonstrated that the inflammatory response can trigger the release of monocytes from the bone marrow into the peripheral blood, where these cells secrete pro-inflammatory molecules. The resulting pro-inflammatory molecules have the potential to exacerbate the inflammatory response, leading to increased tissue damage and potentially impacting the prognosis of HBV-ACLF patients. Previous studies have also provided evidence for monocyte dysfunction and immune irregularities in ACLF patients.Citation24,Citation25 For example, monocytes derived from ACLF patients tended to exhibit polarization toward an immunotolerant state, characterized by reduced human leucocyte antigen-DR expression, decreased factor-α/interleukin-6 production, elevated IL-10 production, and compromised phagocytic capacity. Moreover, monocyte dysfunction has been linked to increased susceptibility to secondary infections and higher mortality in ACLF patients.Citation13,Citation29 Therefore, the observed alterations in monocytes among HBV-ACLF patients likely arise as a combined result of the inflammatory response and immune dysfunction, and contribute to the progression of the disease.
Thrombocytopenia is commonly observed in patients with liver diseases, and the underlying reasons for thrombocytopenia include hypersplenism, increased platelet destruction, decreased platelet production, and antiplatelet antibodies.Citation30,Citation31 It is widely acknowledged that platelets play a significant role in the progression of liver diseases. Studies have shown that platelets not only contribute to liver protection but also promote angiogenesis and hepatic regeneration.Citation32,Citation33 Moreover, platelets can serve as a prognostic factor in ACLF patients.Citation34,Citation35 In the present study involving 184 HBV-ACLF patients, 92 (50%) patients had thrombocytopenia (platelet count: <100×109/L). Although monocytes and platelets were identified as risk factors for mortality in the univariate analyses, neither cell type was found to be independently predictive of mortality in the multivariate analysis. The present findings may be attributed to the composite nature of PMR, which reflects inflammation, immune activity, and liver injury. As a composite biomarker, PMR offers multiple prognostic values in HBV-ACLF patients, providing greater objectivity and comprehensiveness compared with individual parameters on their own. We further observed a significant negative correlation between PMR and MELD score, with lower PMR indicating poorer survival. Therefore, we propose that PMR holds promise as a useful prognostic indicator for HBV-ACLF patients.
Our study has some limitations. Firstly, the study design is retrospective, and complete avoidance of selection bias is not possible. Thus, the findings require validation through clinical studies conducted by independent research groups. Secondly, the PMR was recorded only once at admission, limiting the observation of dynamic changes over time. In future research endeavors, we intend to increase the sample size and analyze patients undergoing various treatments to elucidate the clinical value of PMR. Thirdly, we did not assess whether PMR correlates with other blood parameters, such as the lymphocyte-to-monocyte ratio, neutrophil-to-platelet ratio, and red blood cell distribution width in HBV-ACLF patients. Lastly, as a simple predictive indicator, PMR exhibits unsatisfactory specificity, possibly due to numerous interfering factors when used as a single indicator. Currently, none of the scoring systems are flawless, necessitating further studies to ensure the broader applicability and reliability of PMR as a prognostic indicator in HBV-ACLF patients.
In summary, the PMR is a readily available and accurate indicator that may be useful for predicting mortality in HBV-ACLF patients. It should be considered together with traditional models for evaluating prognosis in these patients and is conducive to clinical practice. However, a comprehensive understanding of the significance of PMR requires prospective multicenter studies in the future.
Abbreviations
ACLF, Acute-on-chronic liver failure; AUCs, Areas under the curve; CI, Confidence interval; HBV, Hepatitis B virus; MELD score, Model for End-stage liver disease score; PMR, Platelet-to-monocyte ratio; ROC, Receiver operating characteristic.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Sharing Statement
The data that has been used is confidential.
References
- Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382(22):2137–2145. doi:10.1056/NEJMra1914900
- Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. 2015;386(10003):1576–1587. doi:10.1016/S0140-6736(15)00309-8
- Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20(3):252–261. doi:10.1159/000047017
- Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67(12):2181–2191. doi:10.1136/gutjnl-2017-314641
- Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353–390. doi:10.1007/s12072-019-09946-3
- Wiesner R, Edwards E, Freeman R, et al. United network for organ sharing liver disease severity score committee. model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi:10.1053/gast.2003.5001
- Jalan R, Saliba F, Pavesi M, et al. CANONIC study investigators of the EASL-CLIF consortium. development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–1047. doi:10.1016/j.jhep.2014.06.012
- Jung MC, Pape GR. Immunology of hepatitis B infection. Lancet Infect Dis. 2002;2(1):43–50. doi:10.1016/s1473-3099(01)00172-4
- Khanam A, Kottilil S. Acute-on-chronic liver failure: pathophysiological mechanisms and management. Front Med. 2021;8:752875. doi:10.3389/fmed.2021.752875
- Mao T, Zhang B, Yang T, Qian Y, Zhou C, He C. Evaluation of five lymphocyte-based scores for prediction of mortality in hepatitis B virus-associated decompensated cirrhosis. Heliyon. 2023;9(8):e18556. doi:10.1016/j.heliyon.2023.e18556
- Mao W, Wu J. Haematologic indices in hepatitis B virus-related liver disease. Clin Chim Acta. 2020;500:135–142. doi:10.1016/j.cca.2019.10.007
- Bernsmeier C, Pop OT, Singanayagam A, et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology. 2015;148(3):603–615.e14. doi:10.1053/j.gastro.2014.11.045
- Bernsmeier C, Triantafyllou E, Brenig R, et al. CD14+ CD15− HLA-DR− myeloid-derived suppressor cells impair antimicrobial responses in patients with acute-on-chronic liver failure. Gut. 2018;67(6):1155–1167. doi:10.1136/gutjnl-2017-314184
- Nwokediuko SC, Ibegbulam O. Quantitative platelet abnormalities in patients with hepatitis B virus-related liver disease. Gastroenterology Res. 2009;2(6):344–349. doi:10.4021/gr2009.12.132
- Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol. 2000;95(10):2936–2939. doi:10.1111/j.1572-0241.2000.02325.x
- Lisman T, Luyendyk JP. Platelets as Modulators of Liver Diseases. Semin Thromb Hemost. 2018;44(2):114–125. doi:10.1055/s-0037-1604091
- Dahlen B, Schulz A, Göbel S, et al. The impact of platelet indices on clinical outcome in heart failure: results from the MyoVasc study. ESC Heart Fail. 2021;8(4):2991–3001. doi:10.1002/ehf2.13390
- Zhou J, Li X, Wang M, Gu C, Liu J. Platelet-to-monocyte ratio as a novel promising agent for the prognosis of hepatitis B virus-associated decompensated cirrhosis. Can J Gastroenterol Hepatol. 2023;2023:6646156. doi:10.1155/2023/6646156
- Nikiforuk AM, Karim ME, Patrick DM, Jassem AN. Influence of chronic hepatitis C infection on the monocyte-to-platelet ratio: data analysis from the national health and nutrition examination survey (2009-2016). BMC Public Health. 2021;21(1):1388. doi:10.1186/s12889-021-11267-w
- Kamath PS, Kim WR, Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD. Hepatology. 2007;45(3):797–805. doi:10.1002/hep.21563
- Wu D, Sun Z, Liu X, et al. HINT: a novel prognostic model for patients with hepatitis B virus-related acute-on-chronic liver failure. Aliment Pharmacol Ther. 2018;48(7):750–760. doi:10.1111/apt.14927
- Zhang Y, Nie Y, Liu L, Zhu X. Assessing the prognostic scores for the prediction of the mortality of patients with acute-on-chronic liver failure: a retrospective study. PeerJ. 2020;8:e9857. doi:10.7717/peerj.9857
- Li H, Zheng J, Chen L, Cai J, Zhang M, Wang G. The scoring systems in predicting short-term outcomes in patients with hepatitis B virus-related acute-on-chronic liver failure. Ann Palliat Med. 2020;9(5):3048–3058. doi:10.21037/apm-20-608
- Kuo NR, Hou MC, Chu WC, et al. Low lymphocyte-to-monocyte ratio, calcitriol level, and CD206 level predict the development of acute-on-chronic liver failure in patients cirrhosis with acute decompensation. J Chin Med Assoc. 2023;86(3):265–273. doi:10.1097/JCMA.0000000000000867
- Cai YJ, Dong JJ, Dong JZ, et al. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment Pharmacol Ther. 2017;45(11):1413–1426. doi:10.1111/apt.14046
- Lou Y, Wang M, Mao W. Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B. PLoS One. 2012;7(5):e37644. doi:10.1371/journal.pone.0037644
- Geng A, Flint E, Bernsmeier C. Plasticity of monocytes and macrophages in cirrhosis of the liver. Front Netw Physiol. 2022;2:937739. doi:10.3389/fnetp.2022.937739
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi:10.1038/nri3070
- Berry PA, Antoniades CG, Carey I, et al. Severity of the compensatory anti-inflammatory response determined by monocyte HLA-DR expression may assist outcome prediction in cirrhosis. Intensive Care Med. 2011;37(3):453–460. doi:10.1007/s00134-010-2099-7
- Landolfi R, Leone G, Fedeli G, Storti S, Laghi F, Bizzi B. Platelet-associated IgG in acute and chronic hepatic diseases. Scand J Haematol. 1980;25(5):417–422. doi:10.1111/j.1600-0609.1981.tb01423.x
- Karasu Z, Tekin F, Ersoz G, et al. Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Dig Dis Sci. 2007;52(6):1535–1539. doi:10.1007/s10620-006-9144-y
- Jie Y, Gong J, Xiao C, et al. Low platelet to white blood cell ratio indicates poor prognosis for acute-on-chronic liver failure. Biomed Res Int. 2018;2018:7394904. doi:10.1155/2018/7394904
- Hisakura K, Murata S, Takahashi K, et al. Platelets prevent acute hepatitis induced by anti-fas antibody. J Gastroenterol Hepatol. 2011;26(2):348–355. doi:10.1111/j.1440-1746.2010.06334.x
- Lin W, Zhang J, Liu X, et al. A dynamic model for predicting outcome in patients with hbv related acute-on-chronic liver failure. Ann Hepatol. 2018;17(3):392–402. doi:10.5604/01.3001.0011.7383
- Shi X, Zhu P, Yan G, et al. Clinical characteristics and long-term outcome of acute kidney injury in patients with HBV-related acute-on-chronic liver failure. J Viral Hepat. 2016;23(11):920–929. doi:10.1111/jvh.12566